Abstract
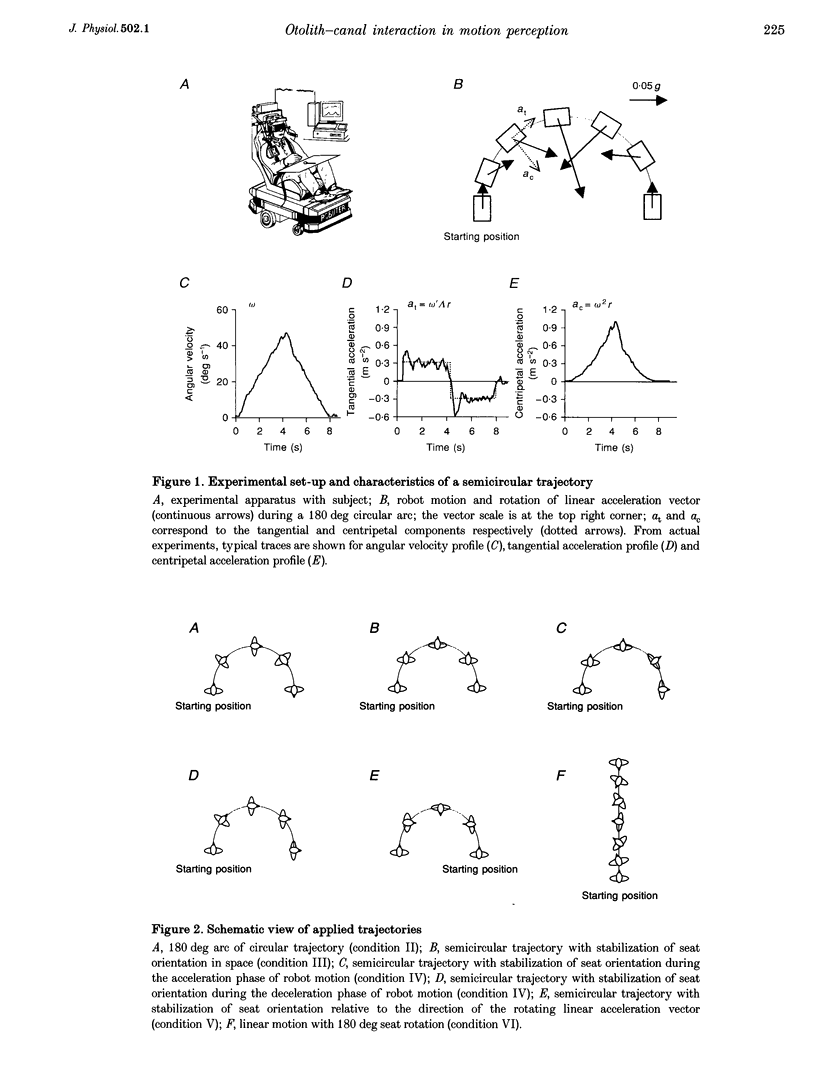
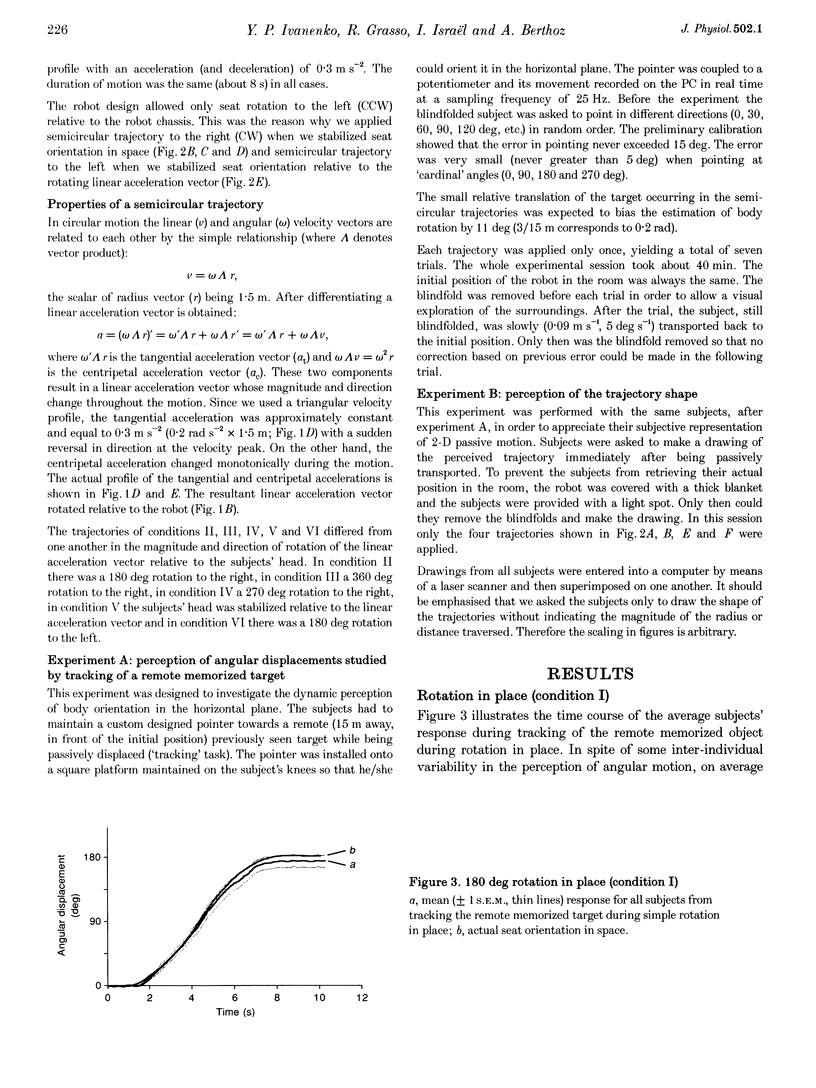
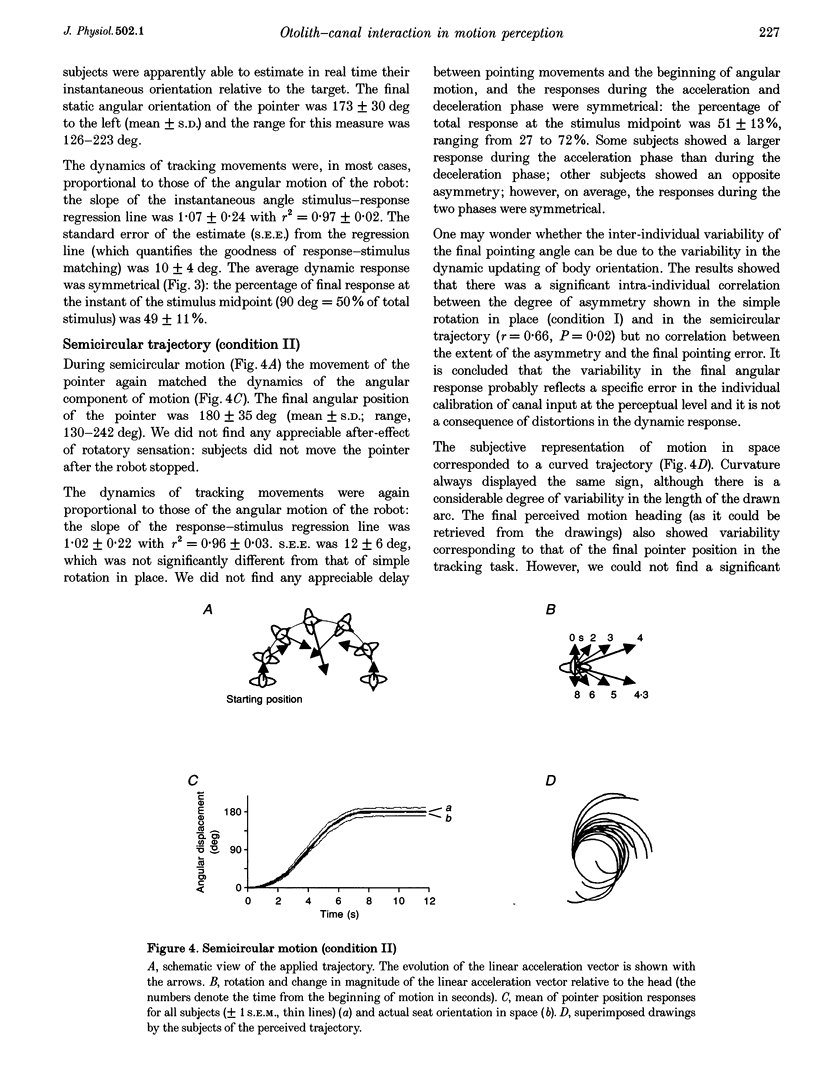
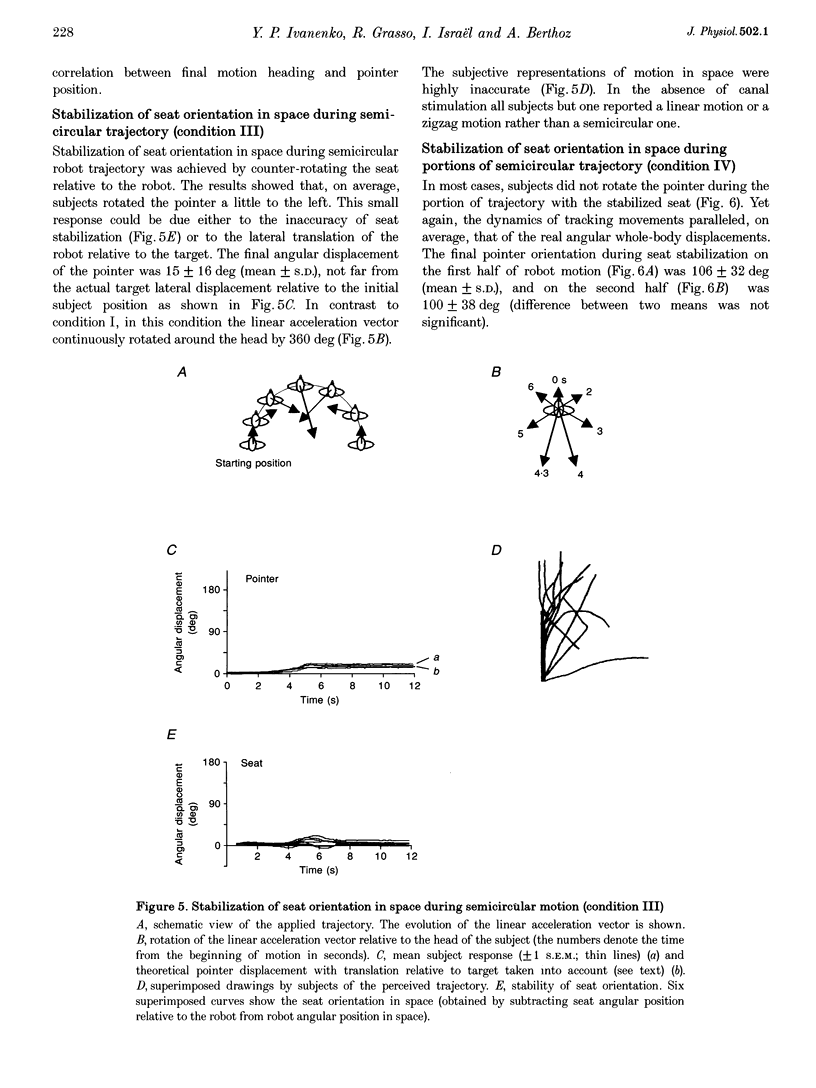
1. Perception of two-dimensional (2-D) whole-body passive motion in the horizontal plane was studied in twelve blindfolded healthy volunteers: pure rotation in place (180 deg), linear motion (4.5 m) and a semicircular trajectory (radius, 1.5 m; angular acceleration, 0.2 rad s-2) were applied in random sequence by means of a remote-controlled robot equipped with a racing-car seat. The seat orientation in the horizontal plane was controlled by the experimenter, independent of the robot trajectory. Thus different degrees of otolith-canal interaction were obtained. The maximal linear acceleration during the semicircular trajectory was 0.1 g; however, the linear acceleration vector was complex as it rotated relative to the subject's head. 2. In the first of two sessions, subjects were instructed to maintain an angular pointer oriented towards a remote (15 m) previously seen target during the passive movements. In the second session they had to make a drawing of the path of the perceived trajectory, after the movement was finished. 3. The results showed that, on average, the movement of the pointer matched the dynamics of the rotatory component of the 2-D motion well. This suggests that, in the range of linear accelerations used in this study, no appreciable influence of otolith input on canal-mediated perception of angular motion occurred. 4. The curvature of the drawn paths was mostly explained by the input to the semicircular canals. Subjects' reconstruction of motion did not account for the directional dynamics of the input to the otoliths occurring during passive motion. 5. This finding proves that reconstructing trajectory in space does not imply a mathematically perfect transformation of the linear and angular motion-related inputs into a Cartesian or polar 2-D representation. Physiological constraints on the interaction between motion direction and change of heading play an important role in motion perception.
Full text
PDF

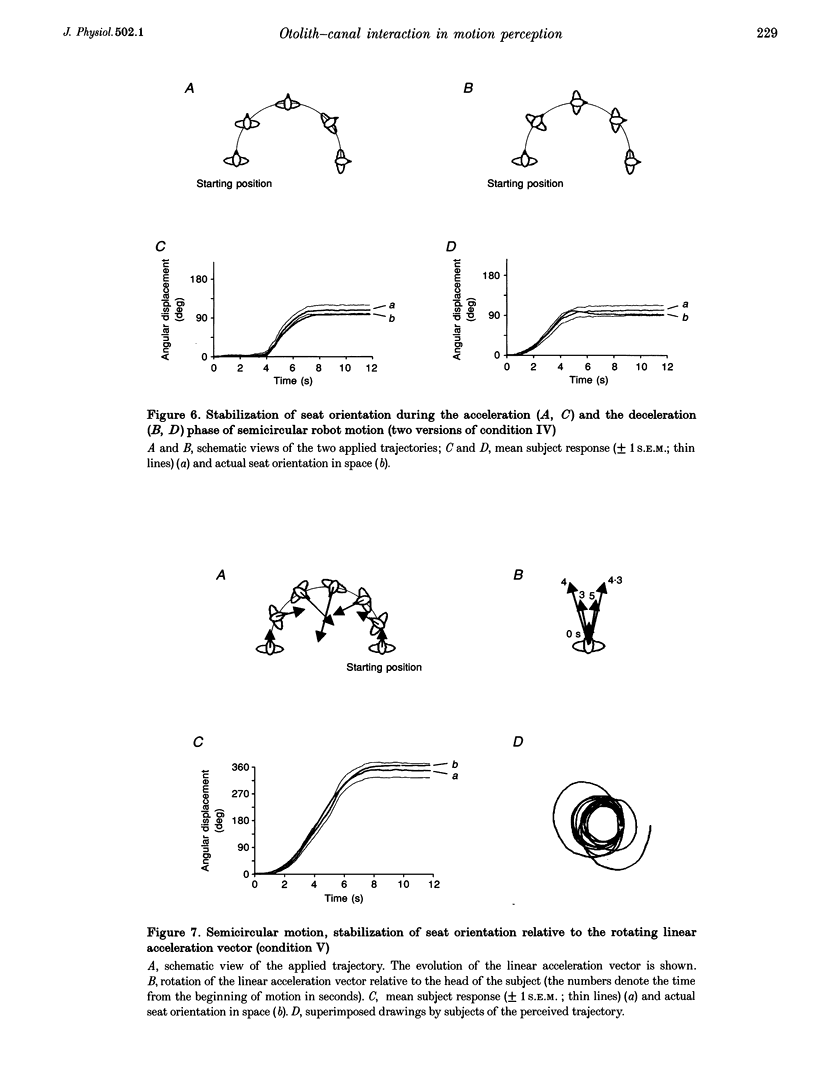
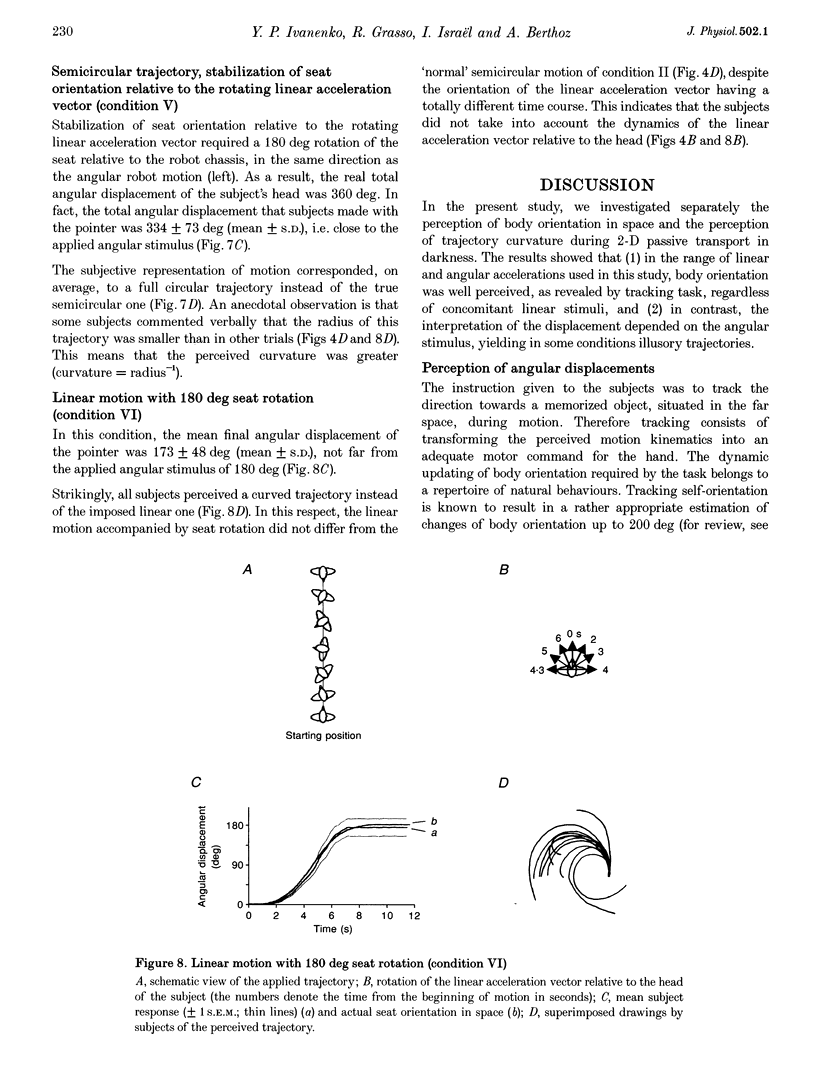



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow J. S. Inertial navigation as a basis for animal navigation. J Theor Biol. 1964 Jan;6(1):76–117. doi: 10.1016/0022-5193(64)90067-0. [DOI] [PubMed] [Google Scholar]
- Benson A. J., Bodin M. A. Interaction of linear and angular accelerations on vestibular receptors in man. Aerosp Med. 1966 Feb;37(2):144–154. [PubMed] [Google Scholar]
- Benson A. J., Diaz E., Farrugia P. The perception of body orientation relative to a rotating linear acceleration vector. Fortschr Zool. 1975;23(1):264–274. [PubMed] [Google Scholar]
- Benson A. J., Spencer M. B., Stott J. R. Thresholds for the detection of the direction of whole-body, linear movement in the horizontal plane. Aviat Space Environ Med. 1986 Nov;57(11):1088–1096. [PubMed] [Google Scholar]
- Berthoz A., Israël I., Georges-François P., Grasso R., Tsuzuku T. Spatial memory of body linear displacement: what is being stored? Science. 1995 Jul 7;269(5220):95–98. doi: 10.1126/science.7604286. [DOI] [PubMed] [Google Scholar]
- Bles W., de Jong J. M., de Wit G. Somatosensory compensation for loss of labyrinthine function. Acta Otolaryngol. 1984 Mar-Apr;97(3-4):213–221. doi: 10.3109/00016488409130982. [DOI] [PubMed] [Google Scholar]
- Bloomberg J., Melvill Jones G., Segal B. Adaptive modification of vestibularly perceived rotation. Exp Brain Res. 1991;84(1):47–56. doi: 10.1007/BF00231761. [DOI] [PubMed] [Google Scholar]
- Cohen M. M. Disorienting effects of aircraft catapult launchings: III. Cockpit displays and piloting performance. Aviat Space Environ Med. 1977 Sep;48(9):797–804. [PubMed] [Google Scholar]
- Darlot C., Denise P., Droulez J., Cohen B., Berthoz A. Eye movements induced by off-vertical axis rotation (OVAR) at small angles of tilt. Exp Brain Res. 1988;73(1):91–105. doi: 10.1007/BF00279664. [DOI] [PubMed] [Google Scholar]
- Denise P., Darlot C., Droulez J., Cohen B., Berthoz A. Motion perceptions induced by off-vertical axis rotation (OVAR) at small angles of tilt. Exp Brain Res. 1988;73(1):106–114. doi: 10.1007/BF00279665. [DOI] [PubMed] [Google Scholar]
- Fetter M., Heimberger J., Black R., Hermann W., Sievering F., Dichgans J. Otolith-semicircular canal interaction during postrotatory nystagmus in humans. Exp Brain Res. 1996 Mar;108(3):463–472. doi: 10.1007/BF00227269. [DOI] [PubMed] [Google Scholar]
- Glasauer S., Amorim M. A., Vitte E., Berthoz A. Goal-directed linear locomotion in normal and labyrinthine-defective subjects. Exp Brain Res. 1994;98(2):323–335. doi: 10.1007/BF00228420. [DOI] [PubMed] [Google Scholar]
- Gordon C. R., Fletcher W. A., Melvill Jones G., Block E. W. Adaptive plasticity in the control of locomotor trajectory. Exp Brain Res. 1995;102(3):540–545. doi: 10.1007/BF00230658. [DOI] [PubMed] [Google Scholar]
- Guedry F. E., Rupert A. H., McGrath B. J., Oman C. M. The dynamics of spatial orientation during complex and changing linear and angular acceleration. J Vestib Res. 1992 Winter;2(4):259–283. [PubMed] [Google Scholar]
- Israël I., Berthoz A. Contribution of the otoliths to the calculation of linear displacement. J Neurophysiol. 1989 Jul;62(1):247–263. doi: 10.1152/jn.1989.62.1.247. [DOI] [PubMed] [Google Scholar]
- Israël I., Chapuis N., Glasauer S., Charade O., Berthoz A. Estimation of passive horizontal linear whole-body displacement in humans. J Neurophysiol. 1993 Sep;70(3):1270–1273. doi: 10.1152/jn.1993.70.3.1270. [DOI] [PubMed] [Google Scholar]
- Israël I., Sievering D., Koenig E. Self-rotation estimate about the vertical axis. Acta Otolaryngol. 1995 Jan;115(1):3–8. doi: 10.3109/00016489509133338. [DOI] [PubMed] [Google Scholar]
- Merfeld D. M., Young L. R., Oman C. M., Shelhamer M. J. A multidimensional model of the effect of gravity on the spatial orientation of the monkey. J Vestib Res. 1993 Summer;3(2):141–161. [PubMed] [Google Scholar]
- Mergner T., Siebold C., Schweigart G., Becker W. Human perception of horizontal trunk and head rotation in space during vestibular and neck stimulation. Exp Brain Res. 1991;85(2):389–404. doi: 10.1007/BF00229416. [DOI] [PubMed] [Google Scholar]
- Metcalfe T., Gresty M. Self-controlled reorienting movements in response to rotational displacements in normal subjects and patients with labyrinthine disease. Ann N Y Acad Sci. 1992 May 22;656:695–698. doi: 10.1111/j.1749-6632.1992.tb25246.x. [DOI] [PubMed] [Google Scholar]
- Mittelstaedt H., Glasauer S., Gralla G., Mittelstaedt M. L. How to explain a constant subjective vertical at constant high speed rotation about an earth-horizontal axis. Acta Otolaryngol Suppl. 1989;468:295–299. doi: 10.3109/00016488909139064. [DOI] [PubMed] [Google Scholar]
- Mittelstaedt M. L. Influence of centrifugal force on angular velocity estimation. Acta Otolaryngol Suppl. 1995;520(Pt 2):307–309. doi: 10.3109/00016489509125257. [DOI] [PubMed] [Google Scholar]
- Mittelstaedt M. L., Mittelstaedt H. The influence of otoliths and somatic graviceptors on angular velocity estimation. J Vestib Res. 1996 Sep-Oct;6(5):355–366. [PubMed] [Google Scholar]
- O'Mara S. M., Rolls E. T., Berthoz A., Kesner R. P. Neurons responding to whole-body motion in the primate hippocampus. J Neurosci. 1994 Nov;14(11 Pt 1):6511–6523. doi: 10.1523/JNEUROSCI.14-11-06511.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube J. S., Muller R. U., Ranck J. B., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci. 1990 Feb;10(2):420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORCHEL P. The role of the vestibular organs in space orientation. J Exp Psychol. 1952 Jul;44(1):4–10. doi: 10.1037/h0058791. [DOI] [PubMed] [Google Scholar]


