Abstract
The association between nonalcoholic fatty liver disease (NAFLD) and sarcopenia has been suggested. We investigated sarcopenia’s impact on NAFLD severity and its relationship with cardiometabolic risk in adolescents. We conducted a retrospective study on 122 patients aged 13–18 years and diagnosed with both NAFLD and sarcopenia by laboratory tests, abdominal ultrasound (US), and multifrequency bioelectrical impedance analysis. Sarcopenia was stratified into tertiles based on the skeletal muscle-to-fat ratio (MFR), NAFLD severity was established by the US, and cardiometabolic risk was assessed by the triglyceride–glucose (TyG) index and the atherogenic index of plasma (AIP). Compared with the other patients, those in the lower MFR tertiles exhibited a greater severity of NAFLD (p < 0.001) and significantly higher TyG index and AIP. The independent effect of MFR was observed to have a negative correlation with the severity of NAFLD (p < 0.001). Based on the aforementioned results, the degree of sarcopenia can be considered as one of the risk factors of severe NAFLD and might be an indicator of cardiometabolic risk in adolescents. Weight training to reach the amount of muscle mass could be included in the treatment strategies to improve or prevent NAFLD in adolescents with sarcopenia.
Keywords: nonalcoholic fatty liver disease, skeletal muscle mass, sarcopenia, triglyceride and glucose index, atherogenic index of plasma
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) has emerged as a significant health concern worldwide, particularly among children and adolescents, along with the rise in obesity and metabolic syndrome (MetS) [1,2,3,4]. The well-known risk factors that play a significant role have been central obesity and insulin resistance [5,6,7], but recent attention has been paid to sarcopenia as an additional risk factor for NAFLD [8,9]. Sarcopenia, which is characterized by the loss of skeletal muscle mass (SMM), strength, and function, is a fragile condition that is associated with an increased risk of poor health outcomes. It was traditionally considered to affect only the elderly secondary to age-related changes in body composition, including decreased muscle mass and diminished resting metabolic rate. Studies in adults have linked sarcopenia with diabetes mellitus, MetS, and cardiovascular disease (CVD) [10,11,12]. However, sarcopenia has been recently observed in relatively young individuals, especially as the obesity rates increase [13,14]. Sarcopenic obesity (SO) is defined as the coexistence with increased fat mass [15].
In a previous study, we demonstrated that reduced SMM was an independent risk factor of pediatric NAFLD [16]. Building upon this, our aim in this present study was to further evaluate the impact of sarcopenia degree on the severity of NAFLD in adolescents. In addition, we sought to elucidate the relationship between sarcopenia degree and cardiometabolic risk in this population. To achieve these, we used the triglyceride–glucose (TyG) index and the atherogenic index of plasma (AIP), which are recognized optimal indicators of insulin resistance and CVD [17,18,19,20,21]. By incorporating these indices into our analysis, we aimed to gain a more comprehensive understanding of the association among sarcopenia, NAFLD severity, and cardiometabolic risk factors in adolescents.
2. Materials and Methods
2.1. Subjects
A retrospective study was conducted on patients who visited the pediatric gastrointestinal clinic at two general hospitals (CHA Bundang Medical Center and Chungnam National University Sejong Hospital) from February 2020 to December 2023 because of abnormal liver function tests. Adolescents aged 13–18 years who were finally diagnosed with NAFLD; had comprehensive data, including laboratory tests, abdominal ultrasound (US), and multifrequency bioelectrical impedance analysis (BIA) (InBody720 or Inbody570; Biospace, Seoul, Republic of Korea); and were defined as having sarcopenia were included for analysis. Patients with other confirmed diseases that cause liver function test elevation (e.g., hepatotropic virus infections, systemic infections, autoimmune hepatitis, Wilson disease, alcohol consumption, and other toxic hepatitis) and chronic diseases, such as immunosuppression or malignancy, were excluded. Patients with comorbid pathological obesity secondary to endocrine disorders, genetic diseases, and central nervous system disorders were also excluded.
This study was approved by the institutional review boards of CHA University (No. 2023-03-001) and Chungnam University (No. 2023-07-002). Written informed consent documents were obtained from the parents or guardians of all participating adolescents.
2.2. Anthropometric Measurements
To assess body composition, we performed a BIA test on each patient, who was asked to maintain a stance with legs apart on the machine for approximately 2 min, with arms slightly separated from the trunk, and while wearing light indoor clothes and no shoes. The patients were instructed to hold the handles of the analyzer to achieve contact of each limb with the electrodes. Various parameters, including height (cm), weight (kg), body mass index (BMI in kg/m2), body fat mass (kg), segmental lean muscle mass (kg), and percentage of body fat and lean muscle mass (%), were automatically measured.
2.3. Definition of Skeletal Muscle Mass Values and Sarcopenia
The BIA technique has been employed in recent studies to estimate appendicular SMM (ASM, kg) because it has a strong correlation with dual-energy X-ray absorptiometry and has been validated for the assessment of body composition [16,22]. Using the BIA results, we calculated ASM as the sum of the SMM of the four limbs under the assumption that all nonfat and nonbone tissues were skeletal muscles [16,23].
Skeletal muscle-to-fat ratio (MFR) was calculated, according to McCarthy et al. [23], by dividing ASM by body fat mass. Based on previous studies, the MFR cutoff value for sarcopenia in this study was defined as 1.155 for boys and 0.723 for girls and was calculated as follows: mean value −1 SD of the MFR for the third BMI quintile of Korean children and adolescents aged 10–18 years [16,23].
2.4. Clinical and Laboratory Assessments
Each subject completed a past medical history questionnaire and underwent anthropometric assessment and laboratory tests. Venous samples were obtained after 8 h of fasting. The laboratory tests included serum alanine aminotransferase (ALT), aspartate aminotransferase, and gamma-glutamyltransferase; total cholesterol, triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C); fasting glucose; hepatitis B surface antigen and antibodies to hepatitis A, B, and C virus; ceruloplasmin; and antinuclear antibody. Blood samples were collected in separator tubes containing silica and a gel clot (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), centrifuged, and analyzed within 2 h. All laboratory tests were performed using standard methods.
2.5. Sonographic Evaluation of Nonalcoholic Fatty Liver Disease and Fatty Liver Severity
Experienced pediatric radiologists, who were blinded to the clinical and laboratory results of the patients, performed US to diagnose fatty liver. The sonographic diagnosis was established based on characteristics such as bright liver, which indicated diffusely increased liver parenchymal echogenicity, in comparison with the adjacent kidney and spleen, without focal lesions, increased attenuation of the US beam, or diminished sonographic visualization of the portal and hepatic veins [24,25,26]. In accordance with the criteria published by Saadeh et al., NAFLD severity was semiquantitatively graded as mild (grade 1), moderate (grade 2), or severe (grade 3) [8,27].
2.6. Calculation and Reference for the Triglyceride–Glucose Index and Atherogenic Index of Plasma
The TyG index was calculated as follows: ln [(fasting TG (mg/dL) × fasting glucose (mg/dL)/2] [28,29]. The reference values for the TyG index were derived from a population-based study on Korean children and adolescents aged 10–18 years by Yoon et al., who reported a median TyG index of 8.07 for boys and 8.14 for girls [20]. The AIP was calculated as follows: log (TG/HDL-C) [30,31]. Based on previous studies, CVD risk was assessed based on the AIP range, as follows: low risk (−0.3–0.1), medium risk (0.1–0.24), or high risk (>0.24) [17,18,19].
2.7. Grouping of the Study Population, According to Sarcopenia Status
Patients were stratified into tertiles according to sarcopenia status on MFR for each sex. The group with the lowest MFR was defined as tertile 1 or severe sarcopenia group, whereas the group with the highest MFR was defined as tertile 3 or mild sarcopenia group.
2.8. Statistical Analysis
The data were analyzed using descriptive statistics and were presented as mean and standard deviation or proportion. Intergroup comparisons of the mean values of the continuous variables were conducted by analysis of variance. The chi-square test was employed to compare categorical variables, which were expressed as percentages. The relationship between US-graded NAFLD severity and clinical variables was examined using Kendall’s and Spearman’s rank correlation analyses. The association between NAFLD severity and MFR was assessed by ordinary regression analysis, which included calculations of β coefficients, standard errors, and 95% confidence interval. p values ≤ 0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics 25.0 (IBM® SPSS® Statistics Server, Armonk, NY, USA).
3. Results
3.1. Comparison of Baseline Characteristics of the Study Population, According to Sarcopenia Status
A total of 122 adolescents who were concurrently diagnosed with sarcopenia and NAFLD were enrolled in this study. Table 1 summarizes the baseline demographic and clinical characteristics of the three groups. The three groups showed no marked differences in sex distribution and mean age; the mean MFR was 0.56 for tertile 1 (0.59 for men, 0.46 for women) and 0.88 for tertile 3 (0.94 for men, 0.66 for women). Compared with the other groups, the tertile 1 group had significantly higher ALT levels and lower HDL-C levels. Serum TG, LDL-C, and glucose levels did not show a clear trend; however, the tertile 3 group had a significantly lower serum TG level.
Table 1.
Baseline characteristics of the study population according to MFR tertiles.
| Variables | Tertile 1 (Lowest, n = 41) |
Tertile 2 (n = 40) |
Tertile 3 (Highest, n = 41) |
p Value |
|---|---|---|---|---|
| Demographics | ||||
| Age (year) | 14.54 ± 1.45 | 14.95 ± 1.68 | 15.00 ± 1.34 | 0.310 |
| Gender, Male (%) | 32 (78.0) | 31 (77.5) | 32 (78.0) | 0.998 |
| Anthropometrics | ||||
| BMI (kg/m2) | 30.63 ± 5.41 | 30.89 ± 3.85 | 28.27 ± 2.98 | 0.010 |
| ASM (kg) | 20.48 ± 5.86 | 22.73 ± 4.32 | 23.79 ± 5.45 | 0.038 |
| MFR | 0.56 ± 0.07 | 0.70 ± 0.09 | 0.88 ± 0.15 | <0.001 |
| Male | 0.59 ± 0.05 | 0.74 ± 0.05 | 0.94 ± 0.10 | <0.001 |
| Female | 0.46 ± 0.04 | 0.56 ± 0.04 | 0.66 ± 0.03 | <0.001 |
| Biochemistry | ||||
| ALT (IU/L) | 128.00 ± 134.14 | 76.00 ± 53.74 | 70.00 ± 53.32 | 0.007 |
| TG (mg/dL) | 140.08 ± 61.16 | 145.63 ± 66.87 | 102.44 ± 6.87 | 0.002 |
| LDL-C (mg/dL) | 109.82 ± 31.15 | 110.72 ± 25.23 | 104.53 ± 21.14 | 0.566 |
| HDL-C (mg/dL) | 45.61 ± 6.40 | 45.80 ± 5.67 | 46.08 ± 8.29 | 0.954 |
| Glucose (mg/dL) | 99.46 ± 11.79 | 93.80 ± 11.64 | 97.51 ± 14.20 | 0.126 |
Data are presented as mean ± SD or number (percent). Abbreviation: SD, standard deviation; BMI, body mass index; ASM, appendicular skeletal muscle mass; MFR, skeletal muscle-to-body fat ratio; ALT, alanine aminotransferase; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
3.2. Association Between Nonalcoholic Fatty Liver Disease Severity and Sarcopenia Status
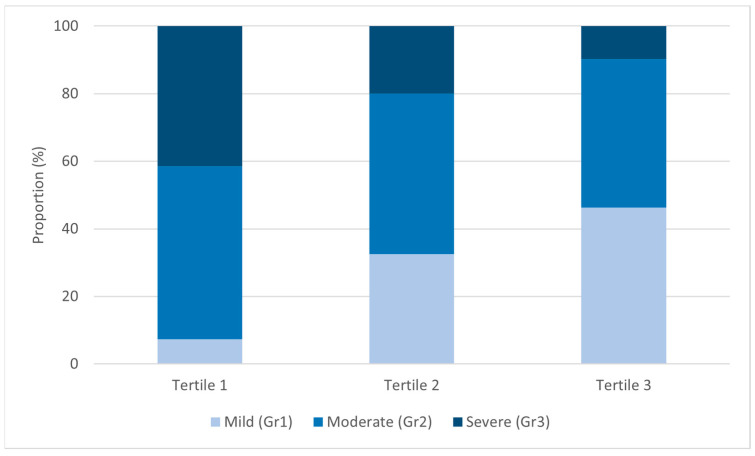
The severity of NAFLD was significantly higher in the patients in the lower MFR tertiles (p < 0.001), with the proportion of severe NAFLD being 41.5% in tertile 1, 20% in tertile 2, and 9.8% in tertile 3 (Figure 1). In the correlation analysis, NAFLD severity was significantly correlated with BMI (Kendall’s Tau b = 0.141, p = 0.046, Spearman’s rho = 0.186, p = 0.040) and MFR (Kendall’s Tau b = −0.283, p < 0.001, Spearman’s rho = −0.363, p < 0.001) (Table 2).
Figure 1.
US-graded severity of NAFLD according to MFR tertiles.
Table 2.
Correlation between US-graded severity of NAFLD and clinical variables.
| NAFLD Grade | Age | Gender | BMI | MFR | ALT |
|---|---|---|---|---|---|
| Kendall’s Tau-b | −0.016 | 0.059 | 0.141 | −0.283 | 0.093 |
| p value | 0.833 | 0.490 | 0.046 | <0.001 | 0.192 |
| TG | LDL-C | HDL-C | Glucose | Insulin | |
| Kendall’s Tau-b | 0.087 | 0.003 | −0.004 | 0.033 | 0.034 |
| p value | 0.223 | 0.970 | 0.954 | 0.645 | 0.716 |
| Age | Gender | BMI | MFR | ALT | |
| Spearman’s rho | −0.09 | 0.063 | 0.186 | −0.363 | 0.120 |
| p value | 0.833 | 0.492 | 0.040 | <0.001 | 0.186 |
| TG | LDL-C | HDL-C | Glucose | Insulin | |
| Spearman’s rho | 0.115 | 0.002 | −0.006 | 0.040 | 0.040 |
| p value | 0.210 | 0.985 | 0.945 | 0.661 | 0.742 |
Abbreviation: US, ultrasonography; NAFLD; nonalcoholic fatty liver disease; BMI, body mass index; MFR, skeletal muscle-to-body fat ratio; ALT, alanine aminotransferase; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
To verify the independent effect of sarcopenia on NAFLD severity, ordinal logistic regression analysis was performed. The results (Table 3) showed a significant negative correlation between the severity of NAFLD and MFR, while BMI is not significant; for each unit increase in the MFR, the probability of having severe NAFLD significantly decreased by 4.154 times (p = 0.001).
Table 3.
Ordinary regression regression analysis of the association between NAFLD severity and MFR or BMI.
| Severity of NAFLD (n = 122) | ||||
|---|---|---|---|---|
| ß | SE | 95% CI | p Value | |
| MFR | −4.154 | 1.296 | −6.694–−1.615 | 0.001 |
| BMI | 0.082 | 0.045 | −0.006–0.171 | 0.069 |
Abbreviation: NAFLD; nonalcoholic fatty liver disease; ß, beta regression coefficient; SE, standard error; CI, confidence interval; MFR, skeletal muscle-to-body fat ratio; BMI, body mass index.
3.3. Correlation of Cardiometabolic Risk with Sarcopenia Status
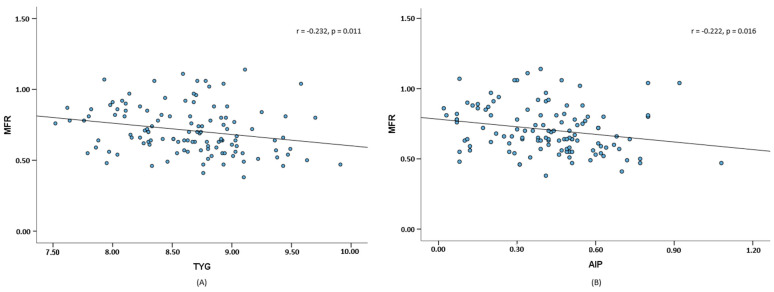
Compared with the other groups, the tertile 1 group had a significantly higher TyG index and AIP (Table 4). Compared with Korean adolescents [20], all patients in this study had higher median TyG index and AIP values associated with high CVD risk [17,18,19]. Moreover, as shown in Figure 2, the MFR had significant negative correlations with the TyG index (r = −0.232, p = 0.011) and AIP (r = −0.222, p = 0.016).
Table 4.
TyG index and AIP of the study population according to MFR tertiles.
| Parameters | Tertile 1 (Lowest, n = 41) |
Tertile 2 (n = 40) |
Tertile 3 (Highest, n = 41) |
p Value |
|---|---|---|---|---|
| TyG index | 8.75 ± 0.48 | 8.71 ± 0.53 | 8.45 ± 0.41 | 0.009 |
| AIP | 0.46 ± 0.22 | 0.45 ± 0.19 | 0.33 ± 0.19 | 0.007 |
Data are presented as mean ± SD. Abbreviation: SD, standard deviation; TyG, triglyceride and glucose; AIP, atherogenic index of plasma.
Figure 2.
Scatter plot and fitted lines of MFR, TyG (A), and AIP (B).
4. Discussion
Our study revealed significant insights into the relationship among sarcopenia, NAFLD severity, and cardiometabolic risk factors in adolescents. First, we found a significant association between sarcopenia status and NAFLD severity on the US. Compared with adolescents with higher MFR, those with lower MFR (i.e., severe sarcopenia) demonstrated a greater severity of NAFLD. This inverse relationship persisted even after accounting for potential confounding factors, such as BMI, highlighting the independent impact of sarcopenia status on NAFLD severity. Similarly, previous research on Korean adults indicated that compared with individuals without sarcopenia, those with sarcopenia were more likely to have severe NAFLD and that lower quartiles of ASM percentage were linked with greater NAFLD severity [8]. Another Korean nationwide survey indicated that compared with nonsarcopenic individuals, those with lower skeletal muscle index were more prone to advanced fibrosis [13].
Second, we explored the relationship between sarcopenia status and cardiometabolic risk using the TyG index and AIP. The TyG index is recognized as a reliable indicator of insulin resistance and has significant associations with type 2 diabetes mellitus, NAFLD, and MetS in adults [20]. Although research in children and adolescents had been limited, a population-based study on individuals aged 10–18 years in Korea in 2021 highlighted the clinical significance of the TyG index, which showed a stable distribution, regardless of age, sex, and BMI, with median values of 8.07 for boys and 8.14 for girls [20]. Another Korean study [32] suggested that among adolescents aged 10–18 years who had MetS, according to three sets of criteria by Cook et al. [33], de Ferranti et al. [34], and the International Diabetes Federation [35], the cutoff TyG indices were 8.48 (men 8.48, women 8.48); 8.41 (men 8.40, women 8.38); and 8.66 (men 8.66, women 8.61), respectively. In our study, the mean TyG index was high among patients with severe sarcopenia, surpassing the cutoff value for MetS in tertiles 1 and 2. This result was similar to the findings in a previous study, which showed that the TyG index was independently and negatively associated with a low skeletal muscle index among Korean adults [36]. Similarly, AIP has been used as an optimal indicator of the risks of atherosclerosis and CVD [17]. In this present study, all patients had an AIP of >0.24, which indicated high risk of atherosclerosis and CVD, with a notable increase in tertile 1. To the best of our knowledge, this finding was novel, because there had been no direct investigations on the relationship between AIP and sarcopenia.
Sarcopenia, which is caused by adverse muscle changes during the lifespan of an individual, significantly impedes daily activities, mobility, and overall quality of life [37,38,39,40] and has been linked with respiratory, cardiac, and cognitive issues [37,41,42,43]. It is a relatively novel concept in pediatrics because it has been traditionally associated with aging and the elderly [44]. Moreover, research on sarcopenia in children has been hampered by the lack of uniform definitions and limited studies on its potential impact on clinical outcomes. However, the development of sarcopenia is now recognized to begin earlier in life, especially with the rising incidence of SO in children. Obesity worsens sarcopenia by promoting fat infiltration into muscle and reducing physical function; in fact, SO is defined as reduced lean body mass in the context of excess adiposity [37,45,46]. During the COVID-19 pandemic, the rate of sarcopenia and SO in children and adolescents naturally increased because of limited activity levels.
Although the precise underlying mechanism of their close association remains incompletely explored, sarcopenia and NAFLD share several risk factors, including insulin resistance; chronic inflammation; myokines, such as myostatin; dysregulation of adiponectin; physical deconditioning; and deficiencies of nutrients, such as vitamin D [16,47,48]. Given that both the liver and skeletal muscle are target organs for insulin, insulin resistance is believed to be the primary contributing factor [48]. Decreased SMM can induce insulin resistance, thereby promoting adipokine-induced liver damage through heightened inflammation, oxidative stress, mitochondrial dysfunction, and increased ectopic fat accumulation secondary to increased free fatty acids and decreased lipid β oxidation [16,48]. Excessive secretion of cytokines, such as the commonly implicated inflammatory markers interleukin 6 and tumor necrosis factor α [16,48,49,50], further exacerbates chronic inflammation and oxidative stress, resulting in worsened dysregulation of the muscle–liver axis and driving both muscle mass loss and NAFLD progression. Myostatin, which is a critical myokine for regulating muscle mass, was reported to promote protein breakdown, inhibit skeletal muscle growth, and correlate with obesity and insulin resistance [9]. Deletion of myostatin in mice was found to increase muscle mass, reduce adiposity, enhance insulin sensitivity and glucose uptake, and protect against hepatic steatosis [51,52]. Low SMM contributes to physical disability and increases obesity risk, forming a vicious cycle that leads to liver steatosis [16]. Lastly, recent research indicated that vitamin D insufficiency or disrupted signaling pathways might contribute to metabolic disorders that affect both muscle and liver function [9,53]. Further research is necessary to validate and organize these hypotheses on the relationship between low SMM and NAFLD.
In this study, the MFR cutoff value for sarcopenia was based on our previous study [16] and another study on Korean children and adolescents [23]. Muscle mass was measured by BIA, which is known for its good correlation with dual-energy X-ray absorptiometry and offers several advantages of simplicity, speed, and noninvasiveness in pediatric patients [16,22,54].
This study has certain limitations that should be acknowledged. The recruitment of patients meeting the specific criteria for NAFLD, obesity, and sarcopenia was challenging, which limited our sample size. Additionally, we recognize that the cross-sectional design restricts our ability to infer causal relationships. Furthermore, the regional scope of the data, being limited to a specific area in Korea, may affect the generalizability of our findings to other populations. Finally, due to missing data, we could not adjust for all confounding factors, such as insulin levels, which are closely associated with both sarcopenia and NAFLD. Future longitudinal studies with a larger cohort will be necessary to validate these findings and enhance their applicability to a broader population.
In this study that comprehensively evaluated sarcopenia status in terms of its independent impact on NAFLD severity and its relationship with cardiometabolic risk, we aimed to contribute to the growing body of evidence and highlighted the importance of considering skeletal muscle health in the assessment and management of NAFLD in adolescents. Understanding the relationship between sarcopenia and NAFLD severity could have significant clinical implications in terms of the development of novel therapeutic targets and interventions, such as weight training, to mitigate the progression of liver disease in affected adolescents. Exploring the association between sarcopenia and cardiometabolic risk could offer valuable insights into the complex interplay between muscle health and metabolic health in adolescents. This knowledge could ultimately improve targeted strategies for preventing and managing NAFLD and its associated complications, potentially improving the long-term health outcomes and quality of life of this population.
5. Conclusions
The degree of sarcopenia can be considered as one of the risk factors of severe NAFLD and might be an indicator of cardiometabolic risk in adolescents. The increasing prevalence of abdominal obesity in this population, which is associated with sarcopenia, is a growing concern, underscoring the crucial role of physical activity education in addressing this issue. Incorporating weight training to enhance muscle mass should be considered as part of treatment strategies aimed at improving or preventing NAFLD and cardiometabolic diseases in adolescents with sarcopenia.
Acknowledgments
We are grateful to all subjects and investigators who participated in this study.
Author Contributions
Conceptualization, Y.K. and S.J.J.; methodology, J.A.C., Y.J.C., Y.K., and S.J.J.; software, Y.K. and J.A.C.; validation, Y.M.L., S.Y.C., I.H.Y., and T.H.K.; formal analysis, Y.K. and J.A.C.; investigation, Y.K., J.A.C., and Y.J.C.; resources, Y.K., and S.J.J.; data curation, Y.M.L. and S.Y.C.; writing—original draft preparation, Y.K. and J.A.C.; writing—review and editing, S.J.J.; visualization, Y.K.; supervision, S.J.J.; project administration, S.J.J.; funding acquisition, S.J.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the institutional review boards of CHA University (No. 2023-03-001, approval date: 2023-03-10) and Chungnam University (No. 2023-07-002, approval date: 2023-07-10).
Informed Consent Statement
Written informed consent documents were obtained from the parents or guardians of all participating adolescents.
Data Availability Statement
The original data shown in the publication are openly available on GitHub at https://github.com/YOOWON-KWON/Sarcopenia_NAFLD_Rawdata.git (accessed on 16 October 2024). This dataset includes raw experimental data, and further inquiries are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HR22C1605).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Anderson E.L., Howe L.D., Jones H.E., Higgins J.P., Lawlor D.A., Fraser A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS ONE. 2015;10:e0140908. doi: 10.1371/journal.pone.0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spiezia C., Di Rosa C., Fintini D., Ferrara P., De Gara L., Khazrai Y.M. Nutritional Approaches in Children with Overweight or Obesity and Hepatic Steatosis. Nutrients. 2023;15:2435. doi: 10.3390/nu15112435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore J.B. COVID-19, childhood obesity, and NAFLD: Colliding pandemics. Lancet Gastroenterol. Hepatol. 2022;7:499–501. doi: 10.1016/S2468-1253(22)00100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith S.K., Perito E.R. Nonalcoholic Liver Disease in Children and Adolescents. Clin. Liver Dis. 2018;22:723–733. doi: 10.1016/j.cld.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Adams L.A., Anstee Q.M., Tilg H., Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 6.Neuschwander-Tetri B.A. Non-alcoholic fatty liver disease. BMC Med. 2017;15:45. doi: 10.1186/s12916-017-0806-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan R.S., Bril F., Cusi K., Newsome P.N. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;70:711–724. doi: 10.1002/hep.30429. [DOI] [PubMed] [Google Scholar]
- 8.Chung G.E., Kim M.J., Yim J.Y., Kim J.S., Yoon J.W. Sarcopenia Is Significantly Associated with Presence and Severity of Nonalcoholic Fatty Liver Disease. J. Obes. Metab. Syndr. 2019;28:129–138. doi: 10.7570/jomes.2019.28.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zambon Azevedo V., Silaghi C.A., Maurel T., Silaghi H., Ratziu V., Pais R. Impact of Sarcopenia on the Severity of the Liver Damage in Patients With Non-alcoholic Fatty Liver Disease. Front. Nutr. 2022;8:774030. doi: 10.3389/fnut.2021.774030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim S., Kim J.H., Yoon J.W., Kang S.M., Choi S.H., Park Y.J., Kim K.W., Lim J.Y., Park K.S., Jang H.C. Sarcopenic Obesity: Prevalence and Association With Metabolic Syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA) Diabetes Care. 2010;33:1652–1654. doi: 10.2337/dc10-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stephen W.C., Janssen I. Sarcopenic-obesity and cardiovascular disease risk in the elderly. J. Nutr. Health Aging. 2009;13:460–466. doi: 10.1007/s12603-009-0084-z. [DOI] [PubMed] [Google Scholar]
- 12.Kim T.N., Park M.S., Yang S.J., Yoo H.J., Kang H.J., Song W., Seo J.A., Kim S.G., Kim N.H., Baik S.H., et al. Prevalence and Determinant Factors of Sarcopenia in Patients With Type 2 Diabetes: The Korean Sarcopenic Obesity Study (KSOS) Diabetes Care. 2010;33:1497–1499. doi: 10.2337/dc09-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee Y.H., Jung K.S., Kim S.U., Yoon H.J., Yun Y.J., Lee B.W., Kang E.S., Han K.H., Lee H.C., Cha B.S. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008-2011) J. Hepatol. 2015;63:486–493. doi: 10.1016/j.jhep.2015.02.051. [DOI] [PubMed] [Google Scholar]
- 14.Noh J. Sarcopenia as a Novel Risk Factor for Nonalcoholic Fatty Liver Disease. JOMES. 2020;29:1–3. doi: 10.7570/jomes20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zembura M., Matusik P. Sarcopenic Obesity in Children and Adolescents: A Systematic Review. Front. Endocrinol. 2022;13:914740. doi: 10.3389/fendo.2022.914740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon Y., Jeong S.J. Relative Skeletal Muscle Mass Is an Important Factor in Non-Alcoholic Fatty Liver Disease in Non-Obese Children and Adolescents. J. Clin. Med. 2020;9:3355. doi: 10.3390/jcm9103355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon Y., Kim J.H., Ha E.K., Jee H.M., Baek H.S., Han M.Y., Jeong S.J. Serum YKL-40 Levels Are Associated with the Atherogenic Index of Plasma in Children. Mediat. Inflamm. 2020;2020:8713908. doi: 10.1155/2020/8713908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobiásová M. AIP--atherogenic index of plasma as a significant predictor of cardiovascular risk: From research to practice. Vnitr. Lek. 2006;52:64–71. [PubMed] [Google Scholar]
- 19.Wu T.T., Gao Y., Zheng Y.Y., Ma Y.T., Xie X. Atherogenic index of plasma (AIP): A novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018;17:197. doi: 10.1186/s12944-018-0828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon J.S., Shim Y.S., Lee H.S., Hwang I.T., Hwang J.S. A population-based study of TyG index distribution and its relationship to cardiometabolic risk factors in children and adolescents. Sci. Rep. 2021;11:23660. doi: 10.1038/s41598-021-03138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brito A.D.M., Hermsdorff H.H.M., Filgueiras M.S., Vieira-Ribeiro S.A., Franceschini S., Novaes J.F. TAG-glucose (TyG) index in childhood: An estimate of cut-off points and the relation to cardiometabolic risk in 4- to 9-year-old children. Public Health Nutr. 2021;24:2603–2610. doi: 10.1017/S1368980020000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim G., Lee S.E., Lee Y.B., Jun J.E., Ahn J., Bae J.C., Jin S.M., Hur K.Y., Jee J.H., Lee M.K., et al. Relationship Between Relative Skeletal Muscle Mass and Nonalcoholic Fatty Liver Disease: A 7-Year Longitudinal Study. Hepatology. 2018;68:1755–1768. doi: 10.1002/hep.30049. [DOI] [PubMed] [Google Scholar]
- 23.Kim K., Hong S., Kim E.Y. Reference Values of Skeletal Muscle Mass for Korean Children and Adolescents Using Data from the Korean National Health and Nutrition Examination Survey 2009–2011. PLoS ONE. 2016;11:e0153383. doi: 10.1371/journal.pone.0153383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y.N., Fowler K.J., Hamilton G., Cui J.Y., Sy E.Z., Balanay M., Hooker J.C., Szeverenyi N., Sirlin C.B. Liver fat imaging-a clinical overview of ultrasound, CT, and MR imaging. Br. J. Radiol. 2018;91:20170959. doi: 10.1259/bjr.20170959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zwiebel W.J. Sonographic diagnosis of diffuse liver disease. Semin. Ultrasound CT MR. 1995;16:8–15. doi: 10.1016/0887-2171(95)90011-X. [DOI] [PubMed] [Google Scholar]
- 26.Ferraioli G., Soares Monteiro L.B. Ultrasound-based techniques for the diagnosis of liver steatosis. World J. Gastroenterol. 2019;25:6053–6062. doi: 10.3748/wjg.v25.i40.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saadeh S., Younossi Z.M., Remer E.M., Gramlich T., Ong J.P., Hurley M., Mullen K.D., Cooper J.N., Sheridan M.J. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 28.Simental-Mendía L.E., Rodríguez-Morán M., Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008;6:299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 29.Song K., Park G., Lee H.S., Choi Y., Oh J.S., Choi H.S., Suh J., Kwon A., Kim H.S., Chae H.W. Prediction of Insulin Resistance by Modified Triglyceride Glucose Indices in Youth. Life. 2021;11:286. doi: 10.3390/life11040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernández-Macías J.C., Ochoa-Martínez A.C., Varela-Silva J.A., Pérez-Maldonado I.N. Atherogenic Index of Plasma: Novel Predictive Biomarker for Cardiovascular Illnesses. Arch. Med. Res. 2019;50:285–294. doi: 10.1016/j.arcmed.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Lioy B., Webb R.J., Amirabdollahian F. The Association between the Atherogenic Index of Plasma and Cardiometabolic Risk Factors: A Review. Healthcare. 2023;11:966. doi: 10.3390/healthcare11070966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J.W., Park S.H., Kim Y., Im M., Han H.-S. The cutoff values of indirect indices for measuring insulin resistance for metabolic syndrome in Korean children and adolescents. Ann. Pediatr. Endocrinol. Metab. 2016;21:143. doi: 10.6065/apem.2016.21.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook S., Weitzman M., Auinger P., Nguyen M., Dietz W.H. Prevalence of a Metabolic Syndrome Phenotype in Adolescents: Findings From the Third National Health and Nutrition Examination Survey, 1988–1994. Arch. Pediatr. Adolesc. Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 34.Ferranti S.D.d., Gauvreau K., Ludwig D.S., Neufeld E.J., Newburger J.W., Rifai N. Prevalence of the Metabolic Syndrome in American Adolescents. Circulation. 2004;110:2494–2497. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 35.Zimmet P., Alberti G., Kaufman F., Tajima N., Silink M., Arslanian S., Wong G., Bennett P., Shaw J., Caprio S. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–2061. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 36.Ahn S.H., Lee J.H., Lee J.W. Inverse association between triglyceride glucose index and muscle mass in Korean adults: 2008–2011 KNHANES. Lipids Health Dis. 2020;19:243. doi: 10.1186/s12944-020-01414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A.A., et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malmstrom T.K., Miller D.K., Simonsick E.M., Ferrucci L., Morley J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle. 2016;7:28–36. doi: 10.1002/jcsm.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beaudart C., Biver E., Reginster J.Y., Rizzoli R., Rolland Y., Bautmans I., Petermans J., Gillain S., Buckinx F., Dardenne N., et al. Validation of the SarQoL®, a specific health-related quality of life questionnaire for Sarcopenia. J. Cachexia Sarcopenia Muscle. 2017;8:238–244. doi: 10.1002/jcsm.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dos Santos L., Cyrino E.S., Antunes M., Santos D.A., Sardinha L.B. Sarcopenia and physical independence in older adults: The independent and synergic role of muscle mass and muscle function. J. Cachexia Sarcopenia Muscle. 2017;8:245–250. doi: 10.1002/jcsm.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bone A.E., Hepgul N., Kon S., Maddocks M. Sarcopenia and frailty in chronic respiratory disease. Chronic Respir. Dis. 2017;14:85–99. doi: 10.1177/1479972316679664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang K.V., Hsu T.H., Wu W.T., Huang K.C., Han D.S. Association Between Sarcopenia and Cognitive Impairment: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2016;17:1164.e7–1164.e15. doi: 10.1016/j.jamda.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Bahat G., Ilhan B. Sarcopenia and the cardiometabolic syndrome: A narrative review. Eur. Geriatr. Med. 2016;6:220–223. doi: 10.1016/j.eurger.2015.12.012. [DOI] [Google Scholar]
- 44.Ooi P.H., Thompson-Hodgetts S., Pritchard-Wiart L., Gilmour S.M., Mager D.R. Pediatric Sarcopenia: A Paradigm in the Overall Definition of Malnutrition in Children? JPEN J. Parenter. Enter. Nutr. 2020;44:407–418. doi: 10.1002/jpen.1681. [DOI] [PubMed] [Google Scholar]
- 45.Prado C.M., Wells J.C., Smith S.R., Stephan B.C., Siervo M. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin. Nutr. 2012;31:583–601. doi: 10.1016/j.clnu.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Kalinkovich A., Livshits G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017;35:200–221. doi: 10.1016/j.arr.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Bhanji R.A., Narayanan P., Allen A.M., Malhi H., Watt K.D. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology. 2017;66:2055–2065. doi: 10.1002/hep.29420. [DOI] [PubMed] [Google Scholar]
- 48.Zhai Y., Xiao Q. The Common Mechanisms of Sarcopenia and NAFLD. Biomed. Res. Int. 2017;2017:6297651. doi: 10.1155/2017/6297651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beyer I., Mets T., Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care. 2012;15:12–22. doi: 10.1097/MCO.0b013e32834dd297. [DOI] [PubMed] [Google Scholar]
- 50.Dhillon R.J., Hasni S. Pathogenesis and Management of Sarcopenia. Clin. Geriatr. Med. 2017;33:17–26. doi: 10.1016/j.cger.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo T., Jou W., Chanturiya T., Portas J., Gavrilova O., McPherron A.C. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS ONE. 2009;4:e4937. doi: 10.1371/journal.pone.0004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elkina Y., von Haehling S., Anker S.D., Springer J. The role of myostatin in muscle wasting: An overview. J. Cachexia Sarcopenia Muscle. 2011;2:143–151. doi: 10.1007/s13539-011-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chakravarthy M.V., Siddiqui M.S., Forsgren M.F., Sanyal A.J. Harnessing Muscle-Liver Crosstalk to Treat Nonalcoholic Steatohepatitis. Front. Endocrinol. 2020;11:592373. doi: 10.3389/fendo.2020.592373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCarthy H.D., Samani-Radia D., Jebb S.A., Prentice A.M. Skeletal muscle mass reference curves for children and adolescents. Pediatr. Obes. 2014;9:249–259. doi: 10.1111/j.2047-6310.2013.00168.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data shown in the publication are openly available on GitHub at https://github.com/YOOWON-KWON/Sarcopenia_NAFLD_Rawdata.git (accessed on 16 October 2024). This dataset includes raw experimental data, and further inquiries are available from the corresponding author upon reasonable request.