Abstract
Sitafloxacin is a 4th generation fluoroquinolone antibiotic with broad activity against a wide range of Gram-negative and Gram-positive bacteria. It is approved in Japan and used to treat pneumonia and urinary tract infections (UTIs) as well as other upper and lower respiratory infections, genitourinary infections, oral infections and otitis media. Compared to other fluoroquinolones, sitafloxacin displays a low minimal inhibitory concentration (MIC) for many bacterial species but also activity against anaerobes, intracellular bacteria, and persisters. Furthermore, it has also shown strong activity against biofilms of P. aeruginosa and S. aureus in vitro, which was recently validated in vivo with murine models of S. aureus implant-associated bone infection. Although limited in scale at present, the published literature supports the further evaluation of sitafloxacin in implant-related infections and other biofilm-related infections. The aim of this review is to summarize the chemical-positioning-based mechanisms, activity, resistance profile, and future clinical potential of sitafloxacin.
Keywords: sitafloxacin, fluoroquinolone, biofilm, persister, UTI
1. Introduction
Sitafloxacin (DU-6859a) is a broad spectrum, 4th generation fluoroquinolone antibiotic clinically approved in Japan in 2008 and produced under the name Gracevit by Daiichi Sankyo company [1]. Sitafloxacin is utilized against a wide range of Gram-positive and -negative bacterial infections [2]. Most commonly, sitafloxacin is prescribed for pneumonia and urinary tract infections (UTIs); however, it is also approved for other upper and lower respiratory infections (sinusitis, tonsillitis, laryngopharyngitis, and acute bronchitis), genitourinary infections (cervicitis and urethritis), oral infections (periodontitis, pericoronitis, and osteitis of the jaw), and otitis media [1]. It is also a third-line antibiotic for rescue therapy of resistant Helicobacter pylori and a second- or third-line treatment against non-gonococcal urethritis caused by both Mycoplasma genitalium and Chlamydia trachomatis [3,4]. As sitafloxacin is still a relatively new antibiotic, further indications may emerge in the future, particularly since it seems to have potential in infections that are recalcitrant to other treatments. This review explains the mode of action of sitafloxacin, starting with its chemical composition, and summarizes its activity in vitro, in vivo, and in clinical implementation. Finally, the review concludes with potential future applications where the clinical impact and indications for which sitafloxacin may be beneficial are discussed.
2. Chemical Composition and Properties
As a 4th generation fluoroquinolone, sitafloxacin has been substantially altered from the initial 1st generation quinolones. The early quinolones originated from the development of nalidixic acid in the 1960s, which is a by-product of the synthesis of the anti-malaria drug chloroquine [5]. These early quinolones evolved into the 2nd generation with the addition of a fluorine and a piperazine ring with norfloxacin and ciprofloxacin being two prominent examples [6,7,8,9,10,11,12,13,14,15]. Subsequently, 3rd and 4th generation fluoroquinolones were introduced in the following decades, which included various additional features such as a broader spectrum of activity (streptococci, anaerobes), longer half-life (enabling once daily dosing) or dual DNA gyrase and topoisomerase IV activity. Prominent examples of these latter generation fluoroquinolones are gemifloxacin, levofloxacin, sparfloxacin and moxifloxacin [16,17,18,19,20,21,22,23,24,25,26]. However, the distinction between the different generations is not clinically important [27].
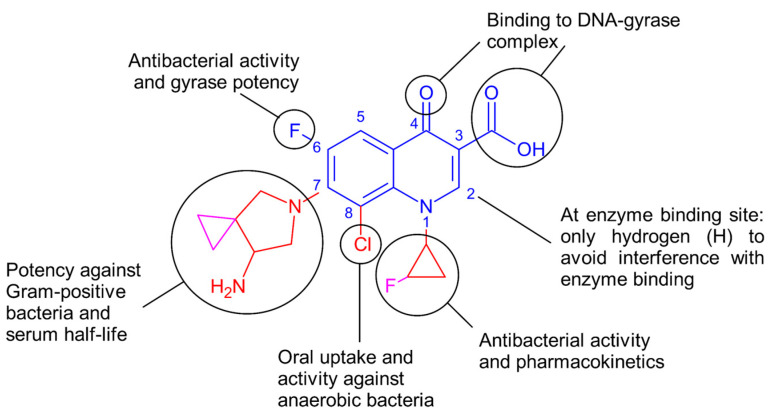
As is typical for all quinolones, sitafloxacin consists of the quinolone core (quinoline ring fused to pyridone ring) with a keto group (C=O) at position 4 and a carboxylic acid at position 3 (Figure 1). These two groups are essential for binding to the DNA–gyrase complex, which is the target of these antibiotics [27,28]. Small groups like hydrogen (H) are favored at position 2 so as not to interfere with enzyme binding. As a fluoroquinolone, it also has a fluorine at the 6th carbon on the quinoline ring, enhancing antibacterial activity and gyrase potency. In contrast to the 2nd generation fluoroquinolones, the piperazine ring at the 7th carbon in sitafloxacin is replaced with a piperazine derivative (pyrrolidine with an amine and integrated cyclopropane). The addition of this group has contributed toward increased potency against Gram-positive bacteria compared to the original piperazine ring, which enhances activity against Gram-negative bacteria. Similar to some other fluoroquinolones, sitafloxacin also contains an alkyl substitution that contributes to even higher activity against Gram-positive bacteria and increased serum half-life. At the N1 position, it further contains a non-polar cyclopropyl (three-membered carbon ring) group, which was shown to be the best group to improve overall potency and pharmacokinetics. In contrast to other fluoroquinolones, the cyclopropyl in sitafloxacin contains a fluoride substitution (fluorocyclopropyl). At the 8th carbon of the main quinolone ring, sitafloxacin has a chloride substitution, which improves oral uptake and activity against anaerobic bacteria [28]. The fluorine on the cyclopropyl and the chlorine collectively pull the electrons from the neighboring carbon atoms closer. This increases the hydrophobicity of sitafloxacin compared to other fluoroquinolones, which can influence the uptake of sitafloxacin through the bacterial envelope [29,30].
Figure 1.
Chemical composition of sitafloxacin with composition-based activity. In blue are the chemical groups typical for all fluoroquinolones. In red are the side-groups that are also present in other fluoroquinolones, and in pink are specific for sitafloxacin. This diagram was adapted from [27,28].
Fluoroquinolones have been shown to have reduced activity at low pH [31], which may be due to changes in charge of the functional groups at different pH values. For example, the carboxylic acid can lose a proton at high pH (-COO−), while the amine group can become protonated at low pH (-NH3+). However, whether sitafloxacin has reduced efficacy at low pH has not been determined to date to the best of our knowledge.
In addition to influencing pH-dependent activity, the carbonyl groups of the main quinolone composition are responsible for the interaction with the DNA gyrase complex within bacteria [32,33,34,35].
3. Mechanism of Action
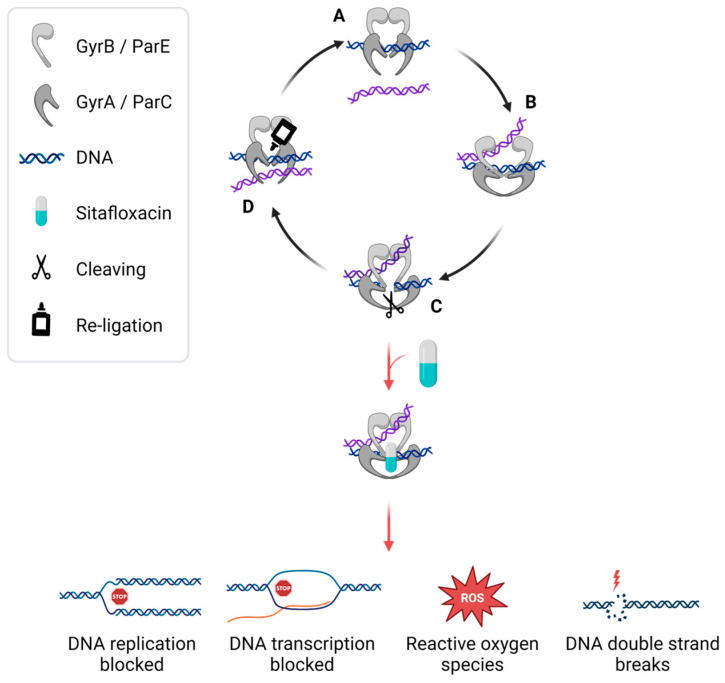
Like all fluoroquinolones, sitafloxacin targets prokaryotic DNA gyrase and topoisomerase IV enzymes. DNA gyrase is responsible for the catalytic relaxation of negatively supercoiled DNA and topoisomerase IV disentangles newly replicated DNA, both of which are essential for transcription and replication [36,37,38,39]. DNA gyrase is composed of two subunits, GyrA and GyrB. Topoisomerase IV is composed of the subunits ParC and ParE. Both protein complexes bind two double strands of DNA and cleave one of them. This allows the second DNA to pass through the gap, after which it is united again (Figure 2). The inhibition of these enzymes by fluoroquinolones is not fully understood, but binding of the fluoroquinolone to both DNA gyrase or topoisomerase IV and DNA is believed to be important [38,40]. All fluoroquinolones block the topology changes of DNA, and the whole complex leads to double-strand breaks, which results in cell death [41,42,43,44,45]. Only the latter generation fluoroquinolones, such as sitafloxacin, can target both enzymes with similar affinity, which is called dual targeting [46].
Figure 2.
Graphical representation of antibacterial mechanism proposed for sitafloxacin. The regular function of the enzymes DNA gyrase and topoisomerase IV is to first bind one double-stranded (ds) DNA strand (blue strand) (A), which is followed by binding to a second dsDNA (purple strand) (B). Together, these enzymes lead to a controlled break in one of the dsDNA strands (C). The resultant gap allows the second dsDNA to pass through and the re-ligation of the DNA (D). This serves to change DNA topology (relax or supercoil). Gyrase consists of the subunits GyrA and GyrB, topoisomerase IV consists of the subunits ParC and ParE. Fluoroquinolones such as sitafloxacin interfere in this process (at C) by binding to the DNA and both subunits of the enzyme, which stabilizes the cleavage complex. This blocks DNA replication and transcription, leading to the accumulation of reactive oxygen species (ROS) and DNA double-strand breaks, which results in bacterial cell death [27]. Made with BioRender.
Sitafloxacin has a balanced inhibition of both enzymes, as revealed by its IC50 ratio of 1.4. This compares with other fluoroquinolones, such as moxifloxacin (6.24) and ciprofloxacin (110), which have a greater activity against one of the enzymes. Furthermore, sitafloxacin showed the highest direct inhibitory activity against purified DNA gyrase and topoisomerase IV in vitro compared to levofloxacin, ciprofloxacin, gatifloxacin, tosufloxacin, and sparfloxacin [47,48,49].
4. Spectrum of Activity
Sitafloxacin is utilized against a wide range of Gram-positive and -negative bacterial infections, including Staphylococcus spp., Streptococcus pneumoniae, other Streptococcus spp., Enterococcus spp., Moraxella catarrhalis, Escherichia coli, Citrobacter spp., Klebsiella spp., Enterobacter spp., Serratia spp., Proteus spp., Morganella morganii, Haemophilus influenzae, Pseudomonas aeruginosa, Legionella pneumophila, Peptostreptococcus spp., Prevotella spp., Porphyromonas spp., Fusobacterium spp., C. trachomatis, Chlamydophila pneumoniae, and Mycobacterium pneumoniae [2]. Sitafloxacin has remarkably low minimal inhibitory concentrations (MICs) compared to other fluoroquinolones for a number of medically important bacterial species. For example, its MIC(90) was 0.06 µg/mL against clinical isolates of Streptococcus milleri, which is 8-fold lower than that of ciprofloxacin and 16-fold lower than that of levofloxacin [50,51,52,53,54,55,56,57,58]. Why this is the case is not quite understood. It is not related to the drug accumulation within the bacteria, as sitafloxacin showed very low accumulation compared to other quinolone antibiotics in S. pneumoniae [59]. Sitafloxacin also has a lower in vitro MIC against more anaerobic bacteria than older fluoroquinolones such as ciprofloxacin or ofloxacin [60,61,62].
A common factor limiting antibiotic efficacy is related to bacterial metabolic and respiratory activity, which is altered in small colony variants (SCVs) and so-called persisters [63,64,65].
SCVs can be found in patients with chronic Staphylococcal infections, which are in most cases linked to device or prosthetic joint-related infections or osteomyelitis [66,67,68,69]. Antibiotics, including the aminoglycoside gentamicin and the fluoroquinolone moxifloxacin, could not reduce the bacterial numbers of chronic SCVs in vitro and in vivo in an osteomyelitis mouse model. Moxifloxacin was even shown to induce increased SCV formation [70]. However, another study identified sitafloxacin as a potent bactericidal treatment for SCVs of Staphylococcus aureus [71]. The study was especially interesting, as sitafloxacin not only killed SCVs of methicillin-susceptible S. aureus (MSSA) but also of methicillin-resistant S. aureus (MRSA) [71].
Persisters are bacterial sub-populations, which are, amongst other things, more tolerant to antibiotics. They are often connected to recurrent infections and are especially abundant in biofilms [72,73,74]. Sitafloxacin has been proven to kill persisters of P. aeruginosa [75]. As S. aureus and P. aeruginosa can both lead to very problematic infections by forming biofilms [76,77,78,79,80,81], it is especially crucial to note that these studies also saw a potent anti-biofilm activity of sitafloxacin against these two pathogens. Sitafloxacin lead to a 4 log10 reduction in surviving bacteria within S. aureus biofilms, which is 100-fold more effective than gentamicin [71]. It also eliminated biofilms of P. aeruginosa in vitro by 7 logs and reduced the biofilm survival of an in vivo implant-related infection mouse model by at least 2 logs [75].
Sitafloxacin also displayed greater activity against intracellular S. aureus in infected murine macrophages in vitro than levofloxacin or moxifloxacin and showed good antibacterial activity in a mouse peritonitis in vivo model [82]. Fluoroquinolones generally seem to accumulate within monocytes [83]; however, this is not universal within this class of antibiotics, as moxifloxacin does accumulate, but levofloxacin does not [84]. With regard to cytotoxicity, sitafloxacin did not show adverse effects (AEs) against human embryonic kidney 293T cells up to a concentration of 64 µg/mL [71].
5. Antibiotic Resistance
As fluoroquinolones have been heavily prescribed in recent decades, resistance is rising [85,86,87,88,89,90,91]. However, few studies have been published so far concerning resistance to sitafloxacin. Resistance has been shown to develop during treatment with 1 out of 10 patients carrying sitafloxacin-resistant M. genitalium after treatment failure in one clinical report [4]. Still, resistance to sitafloxacin usually increases the MIC much less in comparison with other fluoroquinolones [46,53]; for example, a point mutation in gyrA led to a 16-fold increase in MIC for sparfloxacin or moxifloxacin but only a 4-fold increase in MIC for sitafloxacin [47].
The dual targeting of sitafloxacin against both DNA gyrase and topoisomerase IV might lead to reduced resistance formation, as suggested for the similar fluoroquinolone moxifloxacin [92,93,94,95]. This might be because the most common mechanism for antibiotic resistance in fluoroquinolones is point mutation in the quinolone-resistance-determining regions (QRDRs) of gyrA and/or parC. In Gram-negative bacteria, weaker resistance has been shown to usually only come from mutations in gyrA, while stronger resistance seems to originate from mutations in both gyrA and parC genes [96,97]. Mutations in parC are more likely to be found in Gram-positive bacteria [98,99]. In a combination treatment with doxycycline, sitafloxacin was shown to be more efficient in treating patients infected by M. genitalium having a mutation in parC. Still, this mutation was found in 50% of cases with failed doxycycline-sitafloxacin treatment, and treatment failure was even more likely if there was also a gyrA mutation [100]. Another clinical study checked for mutations prior to initiating sitafloxacin monotherapy against fluoroquinolone-resistant M. genitalium. They found a 100% cure rate for patients infected with M. genitalium without having any parC or gyrA mutations, while it was 92.9% for parC only mutations and 41.7% for parC and gyrA double mutants [101].
In S. pneumoniae, the mutant prevention concentration (MPC) was much lower for sitafloxacin (1 mg/L) compared to other fluoroquinolones such as ciprofloxacin (128 mg/L), levofloxacin (64 mg/L) and moxifloxacin (8 mg/L) and further showed less mutant frequencies [47]. Alteration of the drug transport mechanism, for example, the upregulation of efflux pump genes, can also lead to resistance in certain cases, specifically for the 2nd generation fluoroquinolones ciprofloxacin and norfloxacin [59,102]. In S. pneumoniae, sitafloxacin accumulated the least compared to all other fluoroquinolones tested, which might suggest it is pumped out by efflux systems. However, inhibiting potential efflux pumps did not change the intra-bacterial concentrations of sitafloxacin nor its activity measured in MIC [59]. Therefore, efflux pumps do not seem to be the primary resistance mechanism against sitafloxacin, at least not in S. pneumoniae.
Another mechanism for increased fluoroquinolone resistance is downregulation of the target enzyme, such as topoisomerase IV in S. aureus, which increases the MIC drastically [103]. Furthermore, the AAC(6′)-Ib-cr mutant protein has been found to be carried on a plasmid in several clinical isolates of Gram-negative bacteria, which makes some fluoroquinolones ineffective. Those enzymes inactivate the piperazine ring and therefore lead to resistance to fluoroquinolones with this chemical composition, such as ciprofloxacin [104]. Sitafloxacin has a piperazine derivative, which means it is not clear yet if the AAC(6′)-Ib-cr mutant protein would also lead to resistance against this antibiotic.
Sitafloxacin has been shown to be highly active against fluoroquinolone-resistant mutants. For example, sitafloxacin remains active against mutated DNA gyrase and topoisomerase IV in resistant M. genitalium [105]. Similarly, H. pylori with resistance to levofloxacin remained susceptible to sitafloxacin in vitro [106,107]. Furthermore, another study showed a high activity of sitafloxacin against levofloxacin-resistant E. coli [108]. Sitafloxacin was also shown to be more effective against gyrA mutants of M. tuberculosis tested on clinical isolates compared to moxifloxacin, levofloxacin, and ciprofloxacin in vitro [109], making it a valuable option against infections with resistance against commonly used fluoroquinolones.
However, sitafloxacin has been described to display reduced activity against ciprofloxacin-resistant strains [58]. This suggests there can be a shared resistance for more than one of these antibiotics from the same class. For M. tuberculosis, a cross-resistance between sitafloxacin and moxifloxacin was observed in 40.6% of the tested clinical isolates in vitro [110]. It seems as if the specific mutation location can either lead to resistance to sitafloxacin or not, so they are not universal between different fluoroquinolones.
Considering the increase in resistance, linked with increased mutation rates in bacterial DNA, the use of fluoroquinolones may warrant careful monitoring into the future. The mechanism of higher mutation rates is not completely clear, but the increased mutation rate is a consequence of DNA repair [27,111,112,113,114,115,116,117,118] and can lead to bacteria adapting to new drugs more quickly.
The resistance mechanism is not universal between classes, as sitafloxacin was shown to be highly effective against multiple bacteria resistant to an antibiotic of another class. One example is the antibacterial activity of multi-resistant enterococci in vitro, where sitafloxacin was effective against strains resistant to antibiotics of any tested class except to fluroquinolones [119]. Another example is the successful eradication of carbapenem-resistant E. coli or P. aeruginosa and vancomycin-resistant E. faecium [120].
6. Synergy with Other Drugs or Compounds
Combining sitafloxacin with another antibacterial may increase killing efficacy as well as reduce antibiotic resistance development. Therefore, several studies tested combinations between sitafloxacin and other antibiotics in vitro. For example, combining sitafloxacin with the protein synthesis targeting aminoglycoside antibiotic arbekacin led to a synergistic effect on the majority of the tested clinical isolates of Mycobacterium abscessus [121]. Combination with the aminoglycoside amikacin or the cell-wall inhibiting beta-lactam antibiotic imipenem was also beneficial for some of the strains [121]. Sitafloxacin further showed high in vitro activity for extensively drug-resistant Acinetobacter baumannii and showed possible synergy with the RNA synthesis inhibiting antibiotic rifampicin and the polymyxin antibiotic colistin [122]. The combination of sitafloxacin with colistin was even able to kill colistin-resistant A. baumannii. This could be due to the membrane-disruptive properties of colistin, which would increase uptake of the hydrophobic sitafloxacin [123]. Against Mycobacterium ulcerans, the combination of sitafloxacin and rifampicin also led to synergistic effects [124].
Some synergies observed in vitro could also be reproduced in vivo, such as the combination of sitafloxacin and rifampicin against M. ulcerans in a mouse footpad infection study [125]. However, synergy between sitafloxacin and colistin for the treatment of carbapenem-resistant A. baumannii that was observed in vitro was not found in a patient study compared to colistin monotherapy [126].
Antimicrobial activity can also be increased by partnering with other drugs that influence the drug-inhibiting pathways or antibiotic resistance mechanisms. Adding sitafloxacin together with the beta-lactamase inhibitor sulbactam increases the activity against multiple strains of A. baumannii [122,127]. Another approach is to change the environment the drug will be facing to make it more active or simply make the drug reach the target more efficiently. In vitro testing on H. pylori showed synergy with lansoprazole, which is a proton pump inhibitor (PPI) used to reduce stomach acid [53]. For both studies, the mechanisms behind these enhanced treatments have not been further investigated.
By conjugating sitafloxacin with a bisphosphonate, the bacterium S. aureus can be reached within the canaliculi of the bone shown in a murine model, as the bisphosphonate binds to bone and therefore transports the drug to the bone infection site [128,129,130].
7. Utility in Clinical Settings
The primary agent causing UTIs, E. coli, is becoming increasingly resistant to established therapeutics. Over 30% of UTI E. coli isolates in the Asian–Pacific region were resistant to third and fourth generation cephalosporins. Of these, half also had decreased susceptibility to commonly used fluoroquinolones such as levofloxacin and ciprofloxacin, which are the first-line treatments for complicated UTI [131,132,133]. Sitafloxacin exhibits similar efficacy compared to established levofloxacin therapy (88.6% vs. 94.9%) for uncomplicated UTIs, corroborating its utility as an option to overcome emerging resistance amongst uropathogens [134].
Sitafloxacin also retains its efficacy when used in the treatment of acute pyelonephritis (kidney infection) and complicated UTI, which refers to an infection in specific patient populations (elderly, immunocompromised, pregnant, male) or patients with structural/functional abnormalities of the urinary tract (prostatic hypertrophy, indwelling catheter, neurogenic bladder) [135]. A study in Thailand of 289 patients with complicated UTI deemed sitafloxacin non-inferior to ceftriaxone with clinical success rates of 86.6% vs. 83.8% for intention-to-treat analysis and 97.2% vs. 99% for per-protocol analysis [136]. Another study in China of 59 patients found superior cure rates of sitafloxacin (80%) compared to levofloxacin (54%) for complicated UTI [134].
Regarding other genitourinary infections, sitafloxacin eradicated M. genitalium in 92.3% of patients with uterine cervicitis with analogous rates to azithromycin and moxifloxacin [137]. Another study demonstrated sitafloxacin could clear non-gonococcal urethritis in 95.7% of patients with C. trachomatis, 93.8% with M. genitalium, and 100% with Ureaplasma urealyticum [138]. In addition, sitafloxacin is being continuously evaluated for its safety and efficacy in other urologic infections, such as bacterial prostatitis and epididymitis [139]. With the incidence and degree of adverse events remaining similar between sitafloxacin and control groups, sitafloxacin is a valuable addition to the arsenal of antibiotics physicians can use to treat infections of the genitourinary tract.
Sitafloxacin has also been shown to be effective against respiratory infections such as community-acquired pneumonia (CAP). With the most common pathogens including S. pneumoniae, H. influenzae, and M. catarrhalis, CAP has also become more challenging to treat due to increased resistance to empiric therapy, consisting of penicillins, macrolides, and fluoroquinolones [140,141,142]. In a study comprising 300 S. pneumoniae clinical isolates in China, 44.7% were classified as resistant to oral penicillin, 60% were resistant to oral cefuroxime, and 96% were resistant to erythromycin; in contrast; all strains were found to be susceptible to levofloxacin [143]. Furthermore, the American Thoracic Society and Infectious Diseases Society of America provide a strong recommendation for fluoroquinolone monotherapy in CAP outpatients with comorbidities such as heart, lung, liver, renal disease, and diabetes mellitus given the class’s broad coverage of CAP-causing organisms, decreased resistance rates, high oral bioavailability, and increased likelihood of patient compliance with a single antimicrobial [142]. Amongst fluoroquinolones, oral sitafloxacin has been ascribed similar cure rates (>92%) for CAP when compared to oral levofloxacin and moxifloxacin with the highest cure rates presenting at a 100 mg bid dosing regimen [144]. Intravenous (IV) sitafloxacin also had comparable cure rates to the beta-lactam antibiotic imipenem (94% vs. 97%) in patients hospitalized with pneumonia [145]. Considering sitafloxacin’s high efficacy against inpatient and outpatient cases of pneumonia and decreased resistance of respiratory pathogens to fluoroquinolones, there is rationale for sitafloxacin use for respiratory infections, especially when first-line therapeutics fail or evoke AEs.
Sitafloxacin is also used as a third-line rescue therapy for H. pylori infection, which is highly associated with gastric adenocarcinoma, mucosa-associated lymphoid tissue lymphoma, and peptic ulcer disease [146,147,148]. Increased rates of antibiotic resistance have rendered first-line (PPI–clarithromycin–amoxicillin) and second-line (PPI–metronidazole–amoxicillin) triple therapy less efficacious [149,150]. In Japan, a randomized-control trial (RCT) established that sitafloxacin is superior to levofloxacin, and guidelines recommended its inclusion as part of the third-line regimen for H. pylori [150,151]. A systematic review of 12 clinical studies found 7-day treatment with the acid blocker vonoprazan or PPI–sitafloxacin–amoxicillin to have an eradication rate of 80.6% (95% CI, 75.2–85.0) [151,152,153,154,155,156,157,158,159,160,161,162,163]. Importantly, logistic regression analysis in another study demonstrated that decreased susceptibility to sitafloxacin, determined by increased MIC and association with gyrA mutation, independently influenced clearance by sitafloxacin-based triple therapy [159]. Therefore, given the duration of treatment and potential for fluoroquinolone-associated AEs, sensitivity to sitafloxacin should be confirmed prior to the initiation of therapy.
8. Safety Profile and Adverse Effects
As with most fluoroquinolones, the clinical usage of sitafloxacin requires careful consideration of both the benefits and potential life-threatening risks to the patient. Common AEs of the quinolone class that should be monitored in all patients include but are not limited to gastrointestinal (gastritis and hepatotoxicity), neurologic (altered mental status and peripheral neuropathy), cardiovascular (QT interval prolongation leading to torsades de pointes, a deadly arrhythmia), tendinopathy, dysglycemia, and phototoxicity [164,165,166,167,168,169,170,171,172]. In contrast, commonly reported side effects for sitafloxacin are more limited to the gastrointestinal tract [2]. Sitafloxacin elicits a dose-dependent mild phototoxic effect in Caucasian subjects, which was not observed in Asian subjects [173].
A meta-analysis of four RCTs that compared the safety of sitafloxacin in the treatment of a variety of bacterial infections, mainly UTI and pneumonia, demonstrated a similar risk of AEs between sitafloxacin and comparator antibiotics, including other fluoroquinolones (OR, 1.14; 95% CI, 0.64–2.01; I2 = 61%) [136,145,174,175,176]. Another study evaluating therapeutic efficacy and safety in CAP (n = 340) did not find a significant difference in the incidence of drug-related AEs (including clinical, lab, or electrocardiogram abnormalities) between sitafloxacin 100 mg once daily (qd) (29.8%), sitafloxacin 100 mg twice daily (bid) (29.8%), and moxifloxacin 400 mg qd (28.2%). The most common clinical AE in the sitafloxacin 100 mg qd group was dizziness, while that in the 100 mg bid group was nausea/diarrhea. Notably, an increased frequency of sitafloxacin dosing did increase rates of alanine transaminase/aspartate transaminase elevation from 2.6% in the qd group to 9.6% in the bid group, speaking to a potential dose-dependent deleterious effect of sitafloxacin on hepatic function [144]. The authors also performed a study for patients with UTI (n = 206) and found similar rates of sitafloxacin AEs for uncomplicated (27.5%) as well as complicated infections (26.5%). Levofloxacin had a lower incidence of AEs in uncomplicated UTIs (21.1%) but similar rates for complicated infections (28.1%) [134]. Numerous other studies further support that the range at which sitafloxacin evokes drug-related AEs varies between approximately 20–30% with hepatotoxicity being the most common that requires monitoring [1,145,174,177]. Nonetheless, considering sitafloxacin belongs to the fluoroquinolones, a class that can potentially elicit life-threatening side effects, its utilization must be warranted by the nature of the patient’s infection.
9. Dosing Regimens and Pharmacokinetics
Sitafloxacin has demonstrated efficacy over a range of doses. The appropriate dosage and frequency of administration are selected based on the type of infection, severity of clinical symptoms, and efforts to mitigate potential AE, and medical history. The standard oral tablet of sitafloxacin is 50 mg with the recommended dose for adults being 50 mg bid. Poor response and failure to clear the infection merit increasing dosage to 100 mg bid [1,2,134]. A dosage of 100 mg bid is also warranted for H. pylori infection and non-gonococcal urethritis [138,156]; 100 mg qd can be used in the treatment of CAP [178]. Guidelines regarding dosage adjustments for patients who are elderly or have decreased hepatic function remain unclear; nonetheless, drug toxicities may require careful monitoring in these specific patient populations [179]. Sitafloxacin safety in newborns, children, and pregnant individuals has not been established and is therefore not recommended for these patients [179]. In patients with impaired renal function, 50 mg qd is advised for individuals with creatinine clearance (CrCl) between 30–49 mL/min and 50 mg every 48 h for those with a CrCl less than 30 mL/min [1,2]. Critically ill patients who require higher serum concentrations of sitafloxacin may necessitate an IV infusion of 400 mg. However, this is an infrequent occurrence, as the oral form of the drug has good oral bioavailability [145,179].
Investigation of the pharmacokinetics of sitafloxacin has the potential to optimize dosage. For fluoroquinolones, bactericidal efficacy correlates with the 24 h area under the serum concentration–time curve (AUC) to MIC ratio [180]. Specifically, studies in both humans and animal models have demonstrated that an AUC:MIC ratio between 30 and 40 is required for fluoroquinolones (including sitafloxacin) to successfully eradicate bacterial infection [181,182]. Notably, the standard dosage of 50 mg bid for sitafloxacin achieves an AUC:MIC ratio > 100 against 90% of susceptible respiratory pathogens [183]. A different prospective study comparing oral sitafloxacin administration at 100 mg qd to 50 mg bid found higher peak serum concentrations for the 100 mg qd group, suggesting this would be the preferred regimen to treat infection with decreased susceptibility to the antimicrobial. Interestingly, AE rates were increased in the 50 mg bid group (40.4%) versus the 100 mg qd group (33.7%), although the difference was not found to be statistically significant. Nonetheless, the relationship between the dosing schedule of sitafloxacin and AEs deserves further exploration [184].
10. Conclusions
Sitafloxacin is able to kill a wide variety of Gram-negative and Gram-positive bacteria. It displays favorable properties such as low MIC for many clinically important pathogens and a good safety profile. Unlike many other fluoroquinolones or antibacterials, it also has potent activity against biofilms. This makes it an interesting option for biofilm-forming infections, such as implant-associated infections. Furthermore, it is able to treat persisters and SCVs, which makes it even more interesting in the fight against recurring infections. Although more studies are needed, sitafloxacin should not only be considered outside of Asia but especially for infections associated with high recurrence coming from persisters or biofilm formation. One such potential future niche is implant- and bone-related infection, as these infections are known to have high recurrence, and often biofilm and persister formation are involved.
Acknowledgments
We would like to thank Nicolas Devantay for his expert advice on the chemistry heavy section.
Author Contributions
E.M.A.K.; writing—original draft preparation, L.A.S.; writing—original draft preparation, M.C.; writing—review and editing, T.F.M.; writing—review and editing, E.M.S.; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by grants from AO Trauma, grant number AR2022_01, and National Institute of Health grants P50 AR072000, T32 GM007356, and T32 GM152318.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Daiichi Sankyo Co., Ltd. Fine Granules 10%: Prescribing Information, Japan. Gracevit® Tablets 50 mg, Fine Granules 10%: Prescribing Information. Daiichi Sankyo Co., Ltd.; Tokyo, Japan: 2009. [Google Scholar]
- 2.Keating G.M. Sitafloxacin: In bacterial infections. Drugs. 2011;71:731–744. doi: 10.2165/11207380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Ito S., Yasuda M., Seike K., Sugawara T., Tsuchiya T., Yokoi S., Nakano M., Deguchi T. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J. Infect. Chemother. 2012;18:414–418. doi: 10.1007/s10156-012-0392-9. [DOI] [PubMed] [Google Scholar]
- 4.Durukan D., Doyle M., Murray G., Bodiyabadu K., Vodstrcil L., Chow E.P.F., Jensen J.S., Fairley C.K., Aguirre I., Bradshaw C.S. Doxycycline and Sitafloxacin Combination Therapy for Treating Highly Resistant Mycoplasma genitalium. Emerg. Infect. Dis. 2020;26:1870–1874. doi: 10.3201/eid2608.191806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lesher G.Y., Froelich E.J., Gruett M.D., Bailey J.H., Brundage R.P. 1,8-Naphthyridine Derivatives. A New Class of Chemotherapeutic Agents. J. Med. Pharm. Chem. 1962;5:1063–1065. doi: 10.1021/jm01240a021. [DOI] [PubMed] [Google Scholar]
- 6.Rohlfing S.R., Gerster J.R., Kvam D.C. Bioevaluation of the antibacterial flumequine for urinary tract use. Antimicrob. Agents Chemother. 1976;10:20–24. doi: 10.1128/AAC.10.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito A., Hirai K., Inoue M., Koga H., Suzue S., Irikura T., Mitsuhashi S. In vitro antibacterial activity of AM-715, a new nalidixic acid analog. Antimicrob. Agents Chemother. 1980;17:103–108. doi: 10.1128/AAC.17.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfson J.S., Hooper D.C. Norfloxacin: A new targeted fluoroquinolone antimicrobial agent. Ann. Intern. Med. 1988;108:238–251. doi: 10.7326/0003-4819-108-2-238. [DOI] [PubMed] [Google Scholar]
- 9.Wolfson J.S., Hooper D.C. Fluoroquinolone antimicrobial Agents. Clin. Microbiol. Rev. 1989;2:378–424. doi: 10.1128/CMR.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Bambeke F., Michot J.M., Van Eldere J., Tulkens P.M. Quinolones in 2005: An update. Clin. Microbiol. Infect. 2005;11:256–280. doi: 10.1111/j.1469-0691.2005.01131.x. [DOI] [PubMed] [Google Scholar]
- 11.Emmerson A.M., Jones A.M. The quinolones: Decades of development and use. J. Antimicrob. Chemother. 2003;51((Suppl. 1)):13–20. doi: 10.1093/jac/dkg208. [DOI] [PubMed] [Google Scholar]
- 12.Adhami Z.N., Wise R., Weston D., Crump B. The pharmacokinetics and tissue penetration of norfloxacin. J. Antimicrob. Chemother. 1984;13:87–92. doi: 10.1093/jac/13.1.87. [DOI] [PubMed] [Google Scholar]
- 13.Aznar J., Caballero M.C., Lozano M.C., de Miguel C., Palomares J.C., Perea E.J. Activities of new quinoline derivatives against genital pathogens. Antimicrob. Agents Chemother. 1985;27:76–78. doi: 10.1128/AAC.27.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schacht P., Arcieri G., Branolte J., Bruck H., Chyský V., Griffith E., Gruenwaldt G., Hullmann R., Konopka C.A., O’Brien B., et al. Worldwide clinical data on efficacy and safety of ciprofloxacin. Infection. 1988;16((Suppl. 1)):S29–S43. doi: 10.1007/BF01650504. [DOI] [PubMed] [Google Scholar]
- 15.Segev S., Yaniv I., Haverstock D., Reinhart H. Safety of long-term therapy with ciprofloxacin: Data analysis of controlled clinical trials and review. Clin. Infect. Dis. 1999;28:299–308. doi: 10.1086/515132. [DOI] [PubMed] [Google Scholar]
- 16.Ball P. Quinolone generations: Natural history or natural selection? J. Antimicrob. Chemother. 2000;46((Suppl. 3)):17–24. doi: 10.1093/oxfordjournals.jac.a020889. [DOI] [PubMed] [Google Scholar]
- 17.Andriole V.T. The quinolones: Past, present, and future. Clin. Infect. Dis. 2005;41((Suppl. 2)):S113–S119. doi: 10.1086/428051. [DOI] [PubMed] [Google Scholar]
- 18.Mitscher L.A. Bacterial topoisomerase inhibitors: Quinolone and pyridone antibacterial Agents. Chem. Rev. 2005;105:559–592. doi: 10.1021/cr030101q. [DOI] [PubMed] [Google Scholar]
- 19.Wimer S.M., Schoonover L., Garrison M.W. Levofloxacin: A therapeutic review. Clin. Ther. 1998;20:1049–1070. doi: 10.1016/S0149-2918(98)80104-5. [DOI] [PubMed] [Google Scholar]
- 20.North D.S., Fish D.N., Redington J.J. Levofloxacin, a second-generation fluoroquinolone. Pharmacotherapy. 1998;18:915–935. doi: 10.1002/j.1875-9114.1998.tb03925.x. [DOI] [PubMed] [Google Scholar]
- 21.Davis R., Bryson H.M. Levofloxacin. A review of its antibacterial activity, pharmacokinetics and therapeutic efficacy. Drugs. 1994;47:677–700. doi: 10.2165/00003495-199447040-00008. [DOI] [PubMed] [Google Scholar]
- 22.Culley C.M., Lacy M.K., Klutman N., Edwards B. Moxifloxacin: Clinical efficacy and safety. Am. J. Health Syst. Pharm. 2001;58:379–388. doi: 10.1093/ajhp/58.5.379. [DOI] [PubMed] [Google Scholar]
- 23.Balfour J.A., Wiseman L.R. Moxifloxacin. Drugs. 1999;57:363–373; discussion 374. doi: 10.2165/00003495-199957030-00007. [DOI] [PubMed] [Google Scholar]
- 24.Yoo B.K., Triller D.M., Yong C.S., Lodise T.P. Gemifloxacin: A new fluoroquinolone approved for treatment of respiratory infections. Ann. Pharmacother. 2004;38:1226–1235. doi: 10.1345/aph.1E003. [DOI] [PubMed] [Google Scholar]
- 25.File T.M., Jr., Tillotson G.S. Gemifloxacin: A new, potent fluoroquinolone for the therapy of lower respiratory tract infections. Expert Rev. Ant. Infect. Ther. 2004;2:831–843. doi: 10.1586/14789072.2.6.831. [DOI] [PubMed] [Google Scholar]
- 26.Schentag J.J. Sparfloxacin: A review. Clin. Ther. 2000;22:372–387; discussion 371. doi: 10.1016/S0149-2918(00)89007-4. [DOI] [PubMed] [Google Scholar]
- 27.Bush N.G., Diez-Santos I., Abbott L.R., Maxwell A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules. 2020;25:5662. doi: 10.3390/molecules25235662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domagala J.M. Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J. Antimicrob. Chemother. 1994;33:685–706. doi: 10.1093/jac/33.4.685. [DOI] [PubMed] [Google Scholar]
- 29.Bean R.C., Shepherd W.C., Chan H. Permeability of lipid bilayer membranes to organic solutes. J. Gen. Physiol. 1968;52:495–508. doi: 10.1085/jgp.52.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikaido H., Thanassi D.G. Penetration of lipophilic agents with multiple protonation sites into bacterial cells: Tetracyclines and fluoroquinolones as examples. Antimicrob. Agents Chemother. 1993;37:1393–1399. doi: 10.1128/AAC.37.7.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lode H. Drug interactions with quinolones. Rev. Infect. Dis. 1988;10((Suppl. 1)):S132–S136. doi: 10.1093/clinids/10.Supplement_1.S132. [DOI] [PubMed] [Google Scholar]
- 32.Mustaev A., Malik M., Zhao X., Kurepina N., Luan G., Oppegard L.M., Hiasa H., Marks K.R., Kerns R.J., Berger J.M., et al. Fluoroquinolone-gyrase-DNA complexes: Two modes of drug binding. J. Biol. Chem. 2014;289:12300–12312. doi: 10.1074/jbc.M113.529164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aldred K.J., Breland E.J., Vlčková V., Strub M.P., Neuman K.C., Kerns R.J., Osheroff N. Role of the water-metal ion bridge in mediating interactions between quinolones and Escherichia coli topoisomerase IV. Biochemistry. 2014;53:5558–5567. doi: 10.1021/bi500682e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aldred K.J., McPherson S.A., Turnbough C.L., Jr., Kerns R.J., Osheroff N. Topoisomerase IV-quinolone interactions are mediated through a water-metal ion bridge: Mechanistic basis of quinolone resistance. Nucleic Acids Res. 2013;41:4628–4639. doi: 10.1093/nar/gkt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter H.E., Wildman B., Schwanz H.A., Kerns R.J., Aldred K.J. Role of the Water-Metal Ion Bridge in Quinolone Interactions with Escherichia coli Gyrase. Int. J. Mol. Sci. 2023;24:2879. doi: 10.3390/ijms24032879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crumplin G.C., Smith J.T. Nalidixic acid and bacterial chromosome replication. Nature. 1976;260:643–645. doi: 10.1038/260643a0. [DOI] [PubMed] [Google Scholar]
- 37.Gellert M., Mizuuchi K., O’Dea M.H., Nash H.A. DNA gyrase: An enzyme that introduces superhelical turns into DNA. Proc. Natl. Acad. Sci. USA. 1976;73:3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gellert M., Mizuuchi K., O’Dea M.H., Itoh T., Tomizawa J.I. Nalidixic acid resistance: A second genetic character involved in DNA gyrase activity. Proc. Natl. Acad. Sci. USA. 1977;74:4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bush N.G., Evans-Roberts K., Maxwell A. DNA Topoisomerases. EcoSal Plus. 2015;6:10–1128. doi: 10.1128/ecosalplus.esp-0010-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugino A., Peebles C.L., Kreuzer K.N., Cozzarelli N.R. Mechanism of action of nalidixic acid: Purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc. Natl. Acad. Sci. USA. 1977;74:4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willmott C.J., Critchlow S.E., Eperon I.C., Maxwell A. The complex of DNA gyrase and quinolone drugs with DNA forms a barrier to transcription by RNA polymerase. J. Mol. Biol. 1994;242:351–363. doi: 10.1006/jmbi.1994.1586. [DOI] [PubMed] [Google Scholar]
- 42.Chen C.R., Malik M., Snyder M., Drlica K. DNA gyrase and topoisomerase IV on the bacterial chromosome: Quinolone-induced DNA cleavage. J. Mol. Biol. 1996;258:627–637. doi: 10.1006/jmbi.1996.0274. [DOI] [PubMed] [Google Scholar]
- 43.Drlica K., Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malik M., Zhao X., Drlica K. Lethal fragmentation of bacterial chromosomes mediated by DNA gyrase and quinolones. Mol. Microbiol. 2006;61:810–825. doi: 10.1111/j.1365-2958.2006.05275.x. [DOI] [PubMed] [Google Scholar]
- 45.Hooper D.C., Jacoby G.A. Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016;6:a025320. doi: 10.1101/cshperspect.a025320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Onodera Y., Okuda J., Tanaka M., Sato K. Inhibitory activities of quinolones against DNA gyrase and topoisomerase IV of Enterococcus faecalis. Antimicrob. Agents Chemother. 2002;46:1800–1804. doi: 10.1128/AAC.46.6.1800-1804.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okumura R., Hirata T., Onodera Y., Hoshino K., Otani T., Yamamoto T. Dual-targeting properties of the 3-aminopyrrolidyl quinolones, DC-159a and sitafloxacin, against DNA gyrase and topoisomerase IV: Contribution to reducing in vitro emergence of quinolone-resistant Streptococcus pneumoniae. J. Antimicrob. Chemother. 2008;62:98–104. doi: 10.1093/jac/dkn136. [DOI] [PubMed] [Google Scholar]
- 48.Onodera Y., Uchida Y., Tanaka M., Sato K. Dual inhibitory activity of sitafloxacin (DU-6859a) against DNA gyrase and topoisomerase IV of Streptococcus pneumoniae. J. Antimicrob. Chemother. 1999;44:533–536. doi: 10.1093/jac/44.4.533. [DOI] [PubMed] [Google Scholar]
- 49.Morrissey I., George J.T. Inhibition of Pneumococcal Topoisomerases by Sitafloxacin. Drugs. 1999;58:354–355. doi: 10.2165/00003495-199958002-00120. [DOI] [Google Scholar]
- 50.Zhou X., Wang L., Wu S., Yang Y., Guo Y., Shen S., Hu F., Yang F. Antimicrobial activities of sitafloxacin and comparators against the clinical isolates of less common nonfermenting Gram-negative bacteria. J. Glob. Antimicrob. Resist. 2022;30:123–126. doi: 10.1016/j.jgar.2022.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Tiengrim S., Phiboonbanakit D., Thunyaharn S., Tantisiriwat W., Santiwatanakul S., Susaengrat W., Srisurat N., Malithong A., Srisangchan P., Thamlikitkul V. Comparative in vitro activity of sitafloxacin against bacteria isolated from Thai patients with urinary tract infections and lower respiratory tract infections. J. Med. Assoc. Thai. 2012;95((Suppl. 2)):S6–S17. [PubMed] [Google Scholar]
- 52.Huang Y.S., Wang J.T., Sheng W.H., Chuang Y.C., Chang S.C. Comparative in vitro activity of sitafloxacin against bacteremic isolates of carbapenem resistant Acinetobacter baumannii complex. J. Microbiol. Immunol. Infect. 2015;48:545–551. doi: 10.1016/j.jmii.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto T., Takano T., Higuchi W., Nishiyama A., Taneike I., Yoshida K., Kanda H., Imamura Y. Helicobacter pylori eradication by sitafloxacin-lansoprazole combination and sitafloxacin pharmacokinetics in Mongolian gerbils and its in vitro activity and resistance development. Antimicrob. Agents Chemother. 2011;55:4261–4266. doi: 10.1128/AAC.01105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi Y.I., Lee S.M., Chung J.W., Kim K.O., Kwon K.A., Kim Y.J., Kim J.H., Lee S.M., Jeong J.Y., Park D.K. Therapeutic Potential of Sitafloxacin as a New Drug Candidate for Helicobacter Eradication in Korea: An In Vitro Culture-Based Study. Antibiotics. 2021;10:1242. doi: 10.3390/antibiotics10101242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto N., Fujita J., Shinzato T., Higa F., Tateyama M., Tohyama M., Nakasone I., Yamane N. In vitro activity of sitafloxacin compared with several fluoroquinolones against Streptococcus anginosus and Streptococcus constellatus. Int. J. Antimicrob. Agents. 2006;27:171–173. doi: 10.1016/j.ijantimicag.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Jönsson A., Foerster S., Golparian D., Hamasuna R., Jacobsson S., Lindberg M., Jensen J.S., Ohnishi M., Unemo M. In vitro activity and time-kill curve analysis of sitafloxacin against a global panel of antimicrobial-resistant and multidrug-resistant Neisseria gonorrhoeae isolates. Apmis. 2018;126:29–37. doi: 10.1111/apm.12777. [DOI] [PubMed] [Google Scholar]
- 57.Schmitz F.J., Fluit A.C., Milatovic D., Verhoef J., Heinz H.P., Brisse S. In vitro potency of moxifloxacin, clinafloxacin and sitafloxacin against 248 genetically defined clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 2000;46:109–113. doi: 10.1093/jac/46.1.109. [DOI] [PubMed] [Google Scholar]
- 58.Wu S., Yang Y., Guo Y., Yin D., Zheng Y., Han R., Ding L., Zhu D., Hu F. Comparative activities of sitafloxacin against recent clinical isolates in hospitals across China. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:2271–2283. doi: 10.1007/s10096-021-04278-3. [DOI] [PubMed] [Google Scholar]
- 59.Piddock L.J., Johnson M.M. Accumulation of 10 fluoroquinolones by wild-type or efflux mutant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2002;46:813–820. doi: 10.1128/AAC.46.3.813-820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldstein E.J. Possible role for the new fluoroquinolones (levofloxacin, grepafloxacin, trovafloxacin, clinafloxacin, sparfloxacin, and DU-6859a) in the treatment of anaerobic infections: Review of current information on efficacy and safety. Clin. Infect. Dis. 1996;23((Suppl. 1)):S25–S30. doi: 10.1093/clinids/23.Supplement_1.S25. [DOI] [PubMed] [Google Scholar]
- 61.Wexler H.M., Molitoris E., Reeves D., Finegold S.M. In vitro activity of DU-6859a against anaerobic bacteria. Antimicrob. Agents Chemother. 1994;38:2504–2509. doi: 10.1128/AAC.38.10.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kato N., Kato H., Tanaka-Bando K., Watanabe K., Ueno K. Comparison of in vitro activities of DU-6859a and other fluoroquinolones against Japanese isolates of anaerobic bacteria. Clin. Infect. Dis. 1996;23((Suppl. 1)):S31–S35. doi: 10.1093/clinids/23.Supplement_1.S31. [DOI] [PubMed] [Google Scholar]
- 63.Proctor R.A., von Eiff C., Kahl B.C., Becker K., McNamara P., Herrmann M., Peters G. Small colony variants: A pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 2006;4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 64.Lewis K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007;5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 65.Vulin C., Leimer N., Huemer M., Ackermann M., Zinkernagel A.S. Prolonged bacterial lag time results in small colony variants that represent a sub-population of persisters. Nat. Commun. 2018;9:4074. doi: 10.1038/s41467-018-06527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kahl B.C., Becker K., Loffler B. Clinical Significance and Pathogenesis of Staphylococcal Small Colony Variants in Persistent Infections. Clin. Microbiol. Rev. 2016;29:401–427. doi: 10.1128/CMR.00069-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johns B.E., Purdy K.J., Tucker N.P., Maddocks S.E. Phenotypic and Genotypic Characteristics of Small Colony Variants and Their Role in Chronic Infection. Microbiol. Insights. 2015;8:15–23. doi: 10.4137/MBI.S25800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia L.G., Lemaire S., Kahl B.C., Becker K., Proctor R.A., Denis O., Tulkens P.M., Van Bambeke F. Antibiotic activity against small-colony variants of Staphylococcus aureus: Review of in vitro, animal and clinical data. J. Antimicrob. Chemother. 2013;68:1455–1464. doi: 10.1093/jac/dkt072. [DOI] [PubMed] [Google Scholar]
- 69.von Eiff C., Peters G., Becker K. The small colony variant (SCV) concept—The role of staphylococcal SCVs in persistent infections. Injury. 2006;37((Suppl. 2)):S26–S33. doi: 10.1016/j.injury.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 70.Tuchscherr L., Kreis C.A., Hoerr V., Flint L., Hachmeister M., Geraci J., Bremer-Streck S., Kiehntopf M., Medina E., Kribus M., et al. Staphylococcus aureus develops increased resistance to antibiotics by forming dynamic small colony variants during chronic osteomyelitis. J. Antimicrob. Chemother. 2015;71:438–448. doi: 10.1093/jac/dkv371. [DOI] [PubMed] [Google Scholar]
- 71.Trombetta R.P., Dunman P.M., Schwarz E.M., Kates S.L., Awad H.A. A High-Throughput Screening Approach To Repurpose FDA-Approved Drugs for Bactericidal Applications against Staphylococcus aureus Small-Colony Variants. mSphere. 2018;3:10–1128. doi: 10.1128/mSphere.00422-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huemer M., Mairpady Shambat S., Bergada-Pijuan J., Söderholm S., Boumasmoud M., Vulin C., Gómez-Mejia A., Antelo Varela M., Tripathi V., Götschi S., et al. Molecular reprogramming and phenotype switching in Staphylococcus aureus lead to high antibiotic persistence and affect therapy success. Proc. Natl. Acad. Sci. USA. 2021;118:2014920118. doi: 10.1073/pnas.2014920118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kamble E., Pardesi K. Antibiotic Tolerance in Biofilm and Stationary-Phase Planktonic Cells of Staphylococcus aureus. Microb. Drug Resist. 2021;27:3–12. doi: 10.1089/mdr.2019.0425. [DOI] [PubMed] [Google Scholar]
- 74.Lechner S., Lewis K., Bertram R. Staphylococcus aureus persisters tolerant to bactericidal antibiotics. J. Mol. Microbiol. Biotechnol. 2012;22:235–244. doi: 10.1159/000342449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.She P., Li S., Liu Y., Xu L., Zhou L., Zeng X., Li Y., Liu S., Li Z., Hussain Z., et al. Repurposing Sitafloxacin, Prulifloxacin, Tosufloxacin, and Sisomicin as Antimicrobials Against Biofilm and Persister Cells of Pseudomonas aeruginosa. Curr. Microbiol. 2021;79:12. doi: 10.1007/s00284-021-02729-w. [DOI] [PubMed] [Google Scholar]
- 76.Otto M. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 2008;322:207–228. doi: 10.1007/978-3-540-75418-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paharik A.E., Horswill A.R. The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response. Microbiol. Spectr. 2016;4:529–566. doi: 10.1128/microbiolspec.VMBF-0022-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thi M.T.T., Wibowo D., Rehm B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020;21:8671. doi: 10.3390/ijms21228671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee K., Yoon S.S. Pseudomonas aeruginosa Biofilm, a Programmed Bacterial Life for Fitness. J. Microbiol. Biotechnol. 2017;27:1053–1064. doi: 10.4014/jmb.1611.11056. [DOI] [PubMed] [Google Scholar]
- 80.Gilbert P., Das J., Foley I. Biofilm susceptibility to antimicrobials. Adv. Dent. Res. 1997;11:160–167. doi: 10.1177/08959374970110010701. [DOI] [PubMed] [Google Scholar]
- 81.Ciofu O., Rojo-Molinero E., Macià M.D., Oliver A. Antibiotic treatment of biofilm infections. Apmis. 2017;125:304–319. doi: 10.1111/apm.12673. [DOI] [PubMed] [Google Scholar]
- 82.Shi G., Chen X., Wang H., Wang S., Guo X., Zhang X. Activity of sitafloxacin against extracellular and intracellular Staphylococcus aureus in vitro and in vivo: Comparison with levofloxacin and moxifloxacin. J. Antibiot. 2012;65:229–236. doi: 10.1038/ja.2012.7. [DOI] [PubMed] [Google Scholar]
- 83.Dorian M., Grellet J., Saux M.C. Uptake of fluoroquinolones in human monocytes isolated from peripheral blood. J. Pharm. Pharmacol. 1998;50:783–788. doi: 10.1111/j.2042-7158.1998.tb07140.x. [DOI] [PubMed] [Google Scholar]
- 84.Van de Velde S., Nguyen H.A., Van Bambeke F., Tulkens P.M., Grellet J., Dubois V., Quentin C., Saux M.C. Contrasting effects of human THP-1 cell differentiation on levofloxacin and moxifloxacin intracellular accumulation and activity against Staphylococcus aureus and Listeria monocytogenes. J. Antimicrob. Chemother. 2008;62:518–521. doi: 10.1093/jac/dkn232. [DOI] [PubMed] [Google Scholar]
- 85.Phillips I., Casewell M., Cox T., De Groot B., Friis C., Jones R., Nightingale C., Preston R., Waddell J. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J. Antimicrob. Chemother. 2004;53:28–52. doi: 10.1093/jac/dkg483. [DOI] [PubMed] [Google Scholar]
- 86.Davies J., Davies D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010;74:417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dalhoff A. Global fluoroquinolone resistance epidemiology and implictions for clinical use. Interdiscip. Perspect. Infect. Dis. 2012;2012:976273. doi: 10.1155/2012/976273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Livermore D.M., Hope R., Reynolds R., Blackburn R., Johnson A.P., Woodford N. Declining cephalosporin and fluoroquinolone non-susceptibility among bloodstream Enterobacteriaceae from the UK: Links to prescribing change? J. Antimicrob. Chemother. 2013;68:2667–2674. doi: 10.1093/jac/dkt212. [DOI] [PubMed] [Google Scholar]
- 89.Fair R.J., Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Medicin. Chem. 2014;6:25–64. doi: 10.4137/PMC.S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim E.S., Hooper D.C. Clinical importance and epidemiology of quinolone resistance. Infect. Chemother. 2014;46:226–238. doi: 10.3947/ic.2014.46.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spellberg B., Doi Y. The Rise of Fluoroquinolone-Resistant Escherichia coli in the Community: Scarier Than We Thought. J. Infect. Dis. 2015;212:1853–1855. doi: 10.1093/infdis/jiv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Varon E., Janoir C., Kitzis M.D., Gutmann L. ParC and GyrA may be interchangeable initial targets of some fluoroquinolones in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 1999;43:302–306. doi: 10.1128/AAC.43.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takei M., Fukuda H., Kishii R., Hosaka M. Target preference of 15 quinolones against Staphylococcus aureus, based on antibacterial activities and target inhibition. Antimicrob. Agents Chemother. 2001;45:3544–3547. doi: 10.1128/AAC.45.12.3544-3547.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Houssaye S., Gutmann L., Varon E. Topoisomerase mutations associated with in vitro selection of resistance to moxifloxacin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2002;46:2712–2715. doi: 10.1128/AAC.46.8.2712-2715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li X., Zhao X., Drlica K. Selection of Streptococcus pneumoniae mutants having reduced susceptibility to moxifloxacin and levofloxacin. Antimicrob. Agents Chemother. 2002;46:522–524. doi: 10.1128/AAC.46.2.522-524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakano M., Deguchi T., Kawamura T., Yasuda M., Kimura M., Okano Y., Kawada Y. Mutations in the gyrA and parC genes in fluoroquinolone-resistant clinical isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1997;41:2289–2291. doi: 10.1128/AAC.41.10.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kumagai Y., Kato J.I., Hoshino K., Akasaka T., Sato K., Ikeda H. Quinolone-resistant mutants of escherichia coli DNA topoisomerase IV parC gene. Antimicrob. Agents Chemother. 1996;40:710–714. doi: 10.1128/AAC.40.3.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferrero L., Cameron B., Manse B., Lagneaux D., Crouzet J., Famechon A., Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: A primary target of fluoroquinolones. Mol. Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 99.Tanaka M., Onodera Y., Uchida Y., Sato K., Hayakawa I. Inhibitory activities of quinolones against DNA gyrase and topoisomerase IV purified from Staphylococcus aureus. Antimicrob. Agents Chemother. 1997;41:2362–2366. doi: 10.1128/AAC.41.11.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Murray G.L., Bodiyabadu K., Danielewski J., Garland S.M., Machalek D.A., Fairley C.K., Jensen J.S., Williamson D.A., Tan L.Y., Mokany E., et al. Moxifloxacin and Sitafloxacin Treatment Failure in Mycoplasma genitalium Infection: Association with parC Mutation G248T (S83I) and Concurrent gyrA Mutations. J. Infect. Dis. 2020;221:1017–1024. doi: 10.1093/infdis/jiz550. [DOI] [PubMed] [Google Scholar]
- 101.Ando N., Mizushima D., Takano M., Mitobe M., Kobayashi K., Kubota H., Miyake H., Suzuki J., Sadamasu K., Aoki T., et al. Effectiveness of sitafloxacin monotherapy for quinolone-resistant rectal and urogenital Mycoplasma genitalium infections: A prospective cohort study. J. Antimicrob. Chemother. 2023;78:2070–2079. doi: 10.1093/jac/dkad208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhanel G.G., Walkty A., Nichol K., Smith H., Noreddin A., Hoban D.J. Molecular characterization of fluoroquinolone resistant Streptococcus pneumoniae clinical isolates obtained from across Canada. Diagn. Microbiol. Infect. Dis. 2003;45:63–67. doi: 10.1016/S0732-8893(02)00498-4. [DOI] [PubMed] [Google Scholar]
- 103.Ince D., Hooper D.C. Quinolone resistance due to reduced target enzyme expression. J. Bacteriol. 2003;185:6883–6892. doi: 10.1128/JB.185.23.6883-6892.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Robicsek A., Strahilevitz J., Jacoby G.A., Macielag M., Abbanat D., Park C.H., Bush K., Hooper D.C. Fluoroquinolone-modifying enzyme: A new adaptation of a common aminoglycoside acetyltransferase. Nat. Med. 2006;12:83–88. doi: 10.1038/nm1347. [DOI] [PubMed] [Google Scholar]
- 105.Deguchi T., Kikuchi M., Yasuda M., Ito S. Sitafloxacin: Antimicrobial activity against ciprofloxacin-selected laboratory mutants of Mycoplasma genitalium and inhibitory activity against its DNA gyrase and topoisomerase IV. J. Infect. Chemother. 2015;21:74–75. doi: 10.1016/j.jiac.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 106.Murakami K., Okimoto T., Kodama M., Tanahashi J., Fujioka T., Ikeda F., Muraoka H., Takigawa M., Saika T., Hasegawa M., et al. Sitafloxacin activity against Helicobacter pylori isolates, including those with gyrA mutations. Antimicrob. Agents Chemother. 2009;53:3097–3099. doi: 10.1128/AAC.01552-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suzuki H., Nishizawa T., Muraoka H., Hibi T. Sitafloxacin and garenoxacin may overcome the antibiotic resistance of Helicobacter pylori with gyrA mutation. Antimicrob. Agents Chemother. 2009;53:1720–1721. doi: 10.1128/AAC.00049-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hiyama Y., Sato T., Takahashi S., Yamamoto S., Fukushima Y., Nakajima C., Suzuki Y., Yokota S.I., Masumori N. Sitafloxacin has a potent activity for eradication of extended spectrum β-lactamase-producing fluoroquinolone-resistant Escherichia coli forming intracellular bacterial communities in uroepithelial cells. J. Infect. Chemother. 2020;26:1272–1277. doi: 10.1016/j.jiac.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 109.Yi L., Aono A., Chikamatsu K., Igarashi Y., Yamada H., Takaki A., Mitarai S. In vitro activity of sitafloxacin against Mycobacterium tuberculosis with gyrA/B mutations isolated in Japan. J. Med. Microbiol. 2017;66:770–776. doi: 10.1099/jmm.0.000493. [DOI] [PubMed] [Google Scholar]
- 110.Kong Y., Geng Z., Jiang G., Jia J., Wang F., Jiang X., Gu Y., Qi Z., Chu N., Huang H., et al. Comparison of the in vitro antibacterial activity of ofloxacin, levofloxacin, moxifloxacin, sitafloxacin, finafloxacin, and delafloxacin against Mycobacterium tuberculosis strains isolated in China. Heliyon. 2023;9:e21216. doi: 10.1016/j.heliyon.2023.e21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gillespie S.H., Basu S., Dickens A.L., O’Sullivan D.M., McHugh T.D. Effect of subinhibitory concentrations of ciprofloxacin on Mycobacterium fortuitum mutation rates. J. Antimicrob. Chemother. 2005;56:344–348. doi: 10.1093/jac/dki191. [DOI] [PubMed] [Google Scholar]
- 112.Cirz R.T., O’Neill B.M., Hammond J.A., Head S.R., Romesberg F.E. Defining the Pseudomonas aeruginosa SOS response and its role in the global response to the antibiotic ciprofloxacin. J. Bacteriol. 2006;188:7101–7110. doi: 10.1128/JB.00807-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cirz R.T., Jones M.B., Gingles N.A., Minogue T.D., Jarrahi B., Peterson S.N., Romesberg F.E. Complete and SOS-mediated response of Staphylococcus aureus to the antibiotic ciprofloxacin. J. Bacteriol. 2007;189:531–539. doi: 10.1128/JB.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.López E., Elez M., Matic I., Blázquez J. Antibiotic-mediated recombination: Ciprofloxacin stimulates SOS-independent recombination of divergent sequences in Escherichia coli. Mol. Microbiol. 2007;64:83–93. doi: 10.1111/j.1365-2958.2007.05642.x. [DOI] [PubMed] [Google Scholar]
- 115.López E., Blázquez J. Effect of subinhibitory concentrations of antibiotics on intrachromosomal homologous recombination in Escherichia coli. Antimicrob. Agents Chemother. 2009;53:3411–3415. doi: 10.1128/AAC.00358-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tattevin P., Basuino L., Chambers H.F. Subinhibitory fluoroquinolone exposure selects for reduced beta-lactam susceptibility in methicillin-resistant Staphylococcus aureus and alterations in the SOS-mediated response. Res. Microbiol. 2009;160:187–192. doi: 10.1016/j.resmic.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 117.O’Sullivan D.M., Hinds J., Butcher P.D., Gillespie S.H., McHugh T.D. Mycobacterium tuberculosis DNA repair in response to subinhibitory concentrations of ciprofloxacin. J. Antimicrob. Chemother. 2008;62:1199–1202. doi: 10.1093/jac/dkn387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Torres-Barceló C., Kojadinovic M., Moxon R., MacLean R.C. The SOS response increases bacterial fitness, but not evolvability, under a sublethal dose of antibiotic. Proc. Biol. Sci. 2015;282:20150885. doi: 10.1098/rspb.2015.0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Korten V., Erdem I., Murray B.E. Bactericidal activity of the fluoroquinolone DU-6859a alone and in combination with other antimicrobial agents against multiresistant enterococci. Diagn. Microbiol. Infect. Dis. 1996;26:79–85. doi: 10.1016/S0732-8893(96)00197-6. [DOI] [PubMed] [Google Scholar]
- 120.Guo S., Li X., Li Y., Tong H., Wei M., Yan B., Tian M., Xu B., Shao J. Sitafloxacin pharmacokinetics/pharmacodynamics against multidrug-resistant bacteria in a dynamic urinary tract infection in vitro model. J. Antimicrob. Chemother. 2022;78:141–149. doi: 10.1093/jac/dkac365. [DOI] [PubMed] [Google Scholar]
- 121.Watanabe J., Ihara H., Takei S., Nakamura A., Fujimoto Y., Handoh T., Kurokawa K., Arai Y., Shibayama K., Sumiyoshi I., et al. The synergetic effect of sitafloxacin-arbekacin combination in the Mycobacterium abscessus species. Sci. Rep. 2023;13:2027. doi: 10.1038/s41598-023-29021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dong X., Chen F., Zhang Y., Liu H., Liu Y., Ma L. In vitro activities of sitafloxacin tested alone and in combination with rifampin, colistin, sulbactam, and tigecycline against extensively drug-resistant Acinetobacter baumannii. Int. J. Clin. Exp. Med. 2015;8:8135–8140. [PMC free article] [PubMed] [Google Scholar]
- 123.Rodjun V., Houngsaitong J., Montakantikul P., Paiboonvong T., Khuntayaporn P., Yanyongchaikit P., Sriyant P. In Vitro Activities of Colistin and Sitafloxacin Combinations against Multidrug-, Carbapenem-, and Colistin-Resistant Acinetobacter baumannii Using the Broth Microdilution Checkerboard and Time-Kill Methods. Antibiotics. 2020;9:516. doi: 10.3390/antibiotics9080516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dhople A.M., Namba K. In vitro activity of sitafloxacin (DU-6859a) alone, or in combination with rifampicin, against Mycobacterium ulcerans. J. Antimicrob. Chemother. 2002;50:727–729. doi: 10.1093/jac/dkf218. [DOI] [PubMed] [Google Scholar]
- 125.Dhople A.M., Namba K. Activities of sitafloxacin (DU-6859a), either singly or in combination with rifampin, against Mycobacterium ulcerans infection in mice. J. Chemother. 2003;15:47–52. doi: 10.1179/joc.2003.15.1.47. [DOI] [PubMed] [Google Scholar]
- 126.Sirijatuphat R., Thawornkaew S., Ruangkriengsin D., Thamlikitkul V. Colistin Monotherapy versus Colistin plus Sitafloxacin for Therapy of Carbapenem-Resistant Acinetobacter baumannii Infections: A Preliminary Study. Antibiotics. 2022;11:1707. doi: 10.3390/antibiotics11121707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu N., Wang G., Leng Y., Dong X., Chen F., Xing Q. Sulbactam enhances the in vitro activity of sitafloxacin against extensively-drug resistant Acinetobacter baumannii. Exp. Ther. Med. 2018;16:3485–3491. doi: 10.3892/etm.2018.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Adjei-Sowah E., Peng Y., Weeks J., Jonason J.H., de Mesy Bentley K.L., Masters E., Morita Y., Muthukrishnan G., Cherian P., Hu X.E., et al. Development of Bisphosphonate-Conjugated Antibiotics to Overcome Pharmacodynamic Limitations of Local Therapy: Initial Results with Carbamate Linked Sitafloxacin and Tedizolid. Antibiotics. 2021;10:732. doi: 10.3390/antibiotics10060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ren Y., Xue T., Rainbolt J., Bentley K.L.M., Galloway C.A., Liu Y., Cherian P., Neighbors J., Hofstee M.I., Ebetino F.H., et al. Efficacy of Bisphosphonate-Conjugated Sitafloxacin in a Murine Model of S. aureus Osteomyelitis: Evidence of “Target & Release” Kinetics and Killing of Bacteria Within Canaliculi. Front. Cell Infect. Microbiol. 2022;12:910970. doi: 10.3389/fcimb.2022.910970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ren Y., Weeks J., Xue T., Rainbolt J., de Mesy Bentley K.L., Shu Y., Liu Y., Masters E., Cherian P., McKenna C.E., et al. Evidence of bisphosphonate-conjugated sitafloxacin eradication of established methicillin-resistant S. aureus infection with osseointegration in murine models of implant-associated osteomyelitis. Bone Res. 2023;11:51. doi: 10.1038/s41413-023-00287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kang C.I., Kim J., Park D.W., Kim B.N., Ha U.S., Lee S.J., Yeo J.K., Min S.K., Lee H., Wie S.H. Clinical Practice Guidelines for the Antibiotic Treatment of Community-Acquired Urinary Tract Infections. Infect. Chemother. 2018;50:67–100. doi: 10.3947/ic.2018.50.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Carson C., Naber K.G. Role of fluoroquinolones in the treatment of serious bacterial urinary tract infections. Drugs. 2004;64:1359–1373. doi: 10.2165/00003495-200464120-00007. [DOI] [PubMed] [Google Scholar]
- 133.Hsueh P.R., Hoban D.J., Carmeli Y., Chen S.Y., Desikan S., Alejandria M., Ko W.C., Binh T.Q. Consensus review of the epidemiology and appropriate antimicrobial therapy of complicated urinary tract infections in Asia-Pacific region. J. Infect. 2011;63:114–123. doi: 10.1016/j.jinf.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 134.Li Y., Yin Y., Peng X., Zheng H., Fu F., Liu Z., Wu X., Wu X., Zheng S., Chen N., et al. A randomized, active-controlled, multicentre clinical trial to evaluate the efficacy and safety of oral sitafloxacin versus levofloxacin in Chinese adults with acute uncomplicated or complicated urinary tract infection. Ann. Med. 2021;53:217–226. doi: 10.1080/07853890.2020.1861322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nicolle L.E., Committee* A.C.G. Complicated urinary tract infection in adults. Can. J. Infect. Dis. Med. Microbiol. 2005;16:349–360. doi: 10.1155/2005/385768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lojanapiwat B., Nimitvilai S., Bamroongya M., Jirajariyavej S., Tiradechavat C., Malithong A., Predanon C., Tanphaichitra D., Lertsupphakul B. Oral sitafloxacin vs intravenous ceftriaxone followed by oral cefdinir for acute pyelonephritis and complicated urinary tract infection: A randomized controlled trial. Infect. Drug Resist. 2019;12:173–181. doi: 10.2147/IDR.S178183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Terada M., Izumi K., Ohki E., Yamagishi Y., Mikamo H. Antimicrobial efficacies of several antibiotics against uterine cervicitis caused by Mycoplasma genitalium. J. Infect. Chemother. 2012;18:313–317. doi: 10.1007/s10156-011-0329-8. [DOI] [PubMed] [Google Scholar]
- 138.Takahashi S., Hamasuna R., Yasuda M., Ito S., Ito K., Kawai S., Yamaguchi T., Satoh T., Sunaoshi K., Takeda K., et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J. Infect. Chemother. 2013;19:941–945. doi: 10.1007/s10156-013-0620-y. [DOI] [PubMed] [Google Scholar]
- 139.Iwata T., Sadahira T., Maruyama Y., Sekito T., Yoshinaga K., Watari S., Nagao K., Kawada T., Tominaga Y., Nishimura S., et al. Clinical Efficacy and Safety of Sitafloxacin 200 mg Once Daily for Refractory Genitourinary Tract Infections. Acta Med. Okayama. 2021;75:763–766. doi: 10.18926/amo/62820. [DOI] [PubMed] [Google Scholar]
- 140.Welte T., Kohnlein T. Global and local epidemiology of community-acquired pneumonia: The experience of the CAPNETZ Network. Semin. Respir. Crit. Care Med. 2009;30:127–135. doi: 10.1055/s-0029-1202941. [DOI] [PubMed] [Google Scholar]
- 141.Lee J.S., Giesler D.L., Gellad W.F., Fine M.J. Antibiotic Therapy for Adults Hospitalized with Community-Acquired Pneumonia: A Systematic Review. JAMA. 2016;315:593–602. doi: 10.1001/jama.2016.0115. [DOI] [PubMed] [Google Scholar]
- 142.Metlay J.P., Waterer G.W., Long A.C., Anzueto A., Brozek J., Crothers K., Cooley L.A., Dean N.C., Fine M.J., Flanders S.A., et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhou M., Wang L., Wang Z., Kudinha T., Wang Y., Xu Y., Liu Z. Molecular Characterization of Penicillin-Binding Protein2x, 2b and 1a of Streptococcus pneumoniae Causing Invasive Pneumococcal Diseases in China: A Multicenter Study. Front. Microbiol. 2022;13:838790. doi: 10.3389/fmicb.2022.838790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li Y., Zhu D., Peng Y., Tong Z., Ma Z., Xu J., Sun S., Tang H., Xiu Q., Liang Y., et al. A randomized, controlled, multicenter clinical trial to evaluate the efficacy and safety of oral sitafloxacin versus moxifloxacin in adult patients with community-acquired pneumonia. Curr. Med. Res. Opin. 2021;37:693–701. doi: 10.1080/03007995.2021.1885362. [DOI] [PubMed] [Google Scholar]
- 145.Feldman C., White H., O’Grady J., Flitcroft A., Briggs A., Richards G. An open, randomised, multi-centre study comparing the safety and efficacy of sitafloxacin and imipenem/cilastatin in the intravenous treatment of hospitalised patients with pneumonia. Int. J. Antimicrob. Agents. 2001;17:177–188. doi: 10.1016/S0924-8579(00)00344-7. [DOI] [PubMed] [Google Scholar]
- 146.Peek R.M., Jr., Blaser M.J. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 147.Farinha P., Gascoyne R.D. Helicobacter pylori and MALT lymphoma. Gastroenterology. 2005;128:1579–1605. doi: 10.1053/j.gastro.2005.03.083. [DOI] [PubMed] [Google Scholar]
- 148.Peterson W.L. Helicobacter pylori and peptic ulcer disease. N. Engl. J. Med. 1991;324:1043–1048. doi: 10.1056/NEJM199104113241507. [DOI] [PubMed] [Google Scholar]
- 149.Fallone C.A., Chiba N., van Zanten S.V., Fischbach L., Gisbert J.P., Hunt R.H., Jones N.L., Render C., Leontiadis G.I., Moayyedi P., et al. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology. 2016;151:51–69.e14. doi: 10.1053/j.gastro.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 150.Kato M., Ota H., Okuda M., Kikuchi S., Satoh K., Shimoyama T., Suzuki H., Handa O., Furuta T., Mabe K., et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 Revised Edition. Helicobacter. 2019;24:e12597. doi: 10.1111/hel.12597. [DOI] [PubMed] [Google Scholar]
- 151.Murakami K., Furuta T., Ando T., Nakajima T., Inui Y., Oshima T., Tomita T., Mabe K., Sasaki M., Suganuma T., et al. Multi-center randomized controlled study to establish the standard third-line regimen for Helicobacter pylori eradication in Japan. J. Gastroenterol. 2013;48:1128–1135. doi: 10.1007/s00535-012-0731-8. [DOI] [PubMed] [Google Scholar]
- 152.Nishizawa T., Munkjargal M., Ebinuma H., Toyoshima O., Suzuki H. Sitafloxacin for Third-Line Helicobacter pylori Eradication: A Systematic Review. J. Clin. Med. 2021;10:2722. doi: 10.3390/jcm10122722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hirata Y., Ohmae T., Yanai A., Sakitani K., Hayakawa Y., Yoshida S., Sugimoto T., Mitsuno Y., Akanuma M., Yamaji Y., et al. Sitafloxacin resistance in Helicobacter pylori isolates and sitafloxacin-based triple therapy as a third-line regimen in Japan. Int. J. Antimicrob. Agents. 2012;39:352–355. doi: 10.1016/j.ijantimicag.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 154.Hirata Y., Serizawa T., Shichijo S., Suzuki N., Sakitani K., Hayakawa Y., Yamada A., Koike K. Efficacy of triple therapy with esomeprazole, amoxicillin, and sitafloxacin as a third-line Helicobacter pylori eradication regimen. Int. J. Infect. Dis. 2016;51:66–69. doi: 10.1016/j.ijid.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 155.Sue S., Shibata W., Sasaki T., Kaneko H., Irie K., Kondo M., Maeda S. Randomized trial of vonoprazan-based versus proton-pump inhibitor-based third-line triple therapy with sitafloxacin for Helicobacter pylori. J. Gastroenterol. Hepatol. 2019;34:686–692. doi: 10.1111/jgh.14456. [DOI] [PubMed] [Google Scholar]
- 156.Matsuzaki J., Suzuki H., Nishizawa T., Hirata K., Tsugawa H., Saito Y., Okada S., Fukuhara S., Hibi T. Efficacy of sitafloxacin-based rescue therapy for Helicobacter pylori after failures of first- and second-line therapies. Antimicrob. Agents Chemother. 2012;56:1643–1645. doi: 10.1128/AAC.05941-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Saito Y., Konno K., Sato M., Nakano M., Kato Y., Saito H., Serizawa H. Vonoprazan-Based Third-Line Therapy Has a Higher Eradication Rate against Sitafloxacin-Resistant Helicobacter pylori. Cancers. 2019;11:116. doi: 10.3390/cancers11010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Furuta T., Sugimoto M., Kodaira C., Nishino M., Yamade M., Uotani T., Sahara S., Ichikawa H., Yamada T., Osawa S., et al. Sitafloxacin-based third-line rescue regimens for Helicobacter pylori infection in Japan. J. Gastroenterol. Hepatol. 2014;29:487–493. doi: 10.1111/jgh.12442. [DOI] [PubMed] [Google Scholar]
- 159.Mori H., Suzuki H., Matsuzaki J., Tsugawa H., Fukuhara S., Miyoshi S., Hirata K., Seino T., Matsushita M., Masaoka T., et al. Efficacy of 10-day Sitafloxacin-Containing Third-Line Rescue Therapies for Helicobacter pylori Strains Containing the gyrA Mutation. Helicobacter. 2016;21:286–294. doi: 10.1111/hel.12286. [DOI] [PubMed] [Google Scholar]
- 160.Mori H., Suzuki H., Matsuzaki J., Masaoka T., Kanai T. 10-Year Trends in Helicobacter pylori Eradication Rates by Sitafloxacin-Based Third-Line Rescue Therapy. Digestion. 2020;101:644–650. doi: 10.1159/000501610. [DOI] [PubMed] [Google Scholar]
- 161.Sugimoto M., Sahara S., Ichikawa H., Kagami T., Uotani T., Furuta T. High Helicobacter pylori cure rate with sitafloxacin-based triple therapy. Aliment. Pharmacol. Ther. 2015;42:477–483. doi: 10.1111/apt.13280. [DOI] [PubMed] [Google Scholar]
- 162.Sugimoto M., Hira D., Murata M., Kawai T., Terada T. Effect of Antibiotic Susceptibility and CYP3A4/5 and CYP2C19 Genotype on the Outcome of Vonoprazan-Containing Helicobacter pylori Eradication Therapy. Antibiotics. 2020;9:645. doi: 10.3390/antibiotics9100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tokunaga K., Tanaka A., Takahashi S. Clinical problems of H. pylori eradication therapy after application expansion of the health insurance. J. Germfree Life Gnotobiol. 2014;44:38–40. [Google Scholar]
- 164.Orman E.S., Conjeevaram H.S., Vuppalanchi R., Freston J.W., Rochon J., Kleiner D.E., Hayashi P.H., Group D.R. Clinical and histopathologic features of fluoroquinolone-induced liver injury. Clin. Gastroenterol. Hepatol. 2011;9:517–523.e513. doi: 10.1016/j.cgh.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.US Foot & Drug FDA Updates Warnings for Fluoroquinolone Antibiotics on Risks of Mental Health and Low Blood Sugar Adverse Reactions. [(accessed on 8 August 2024)]; Available online: https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics-risks-mental-health-and-low-blood-sugar-adverse.
- 166.Etminan M., Brophy J.M., Samii A. Oral fluoroquinolone use and risk of peripheral neuropathy: A pharmacoepidemiologic study. Neurology. 2014;83:1261–1263. doi: 10.1212/WNL.0000000000000846. [DOI] [PubMed] [Google Scholar]
- 167.Kang J., Wang L., Chen X.L., Triggle D.J., Rampe D. Interactions of a series of fluoroquinolone antibacterial drugs with the human cardiac K+ channel HERG. Mol. Pharmacol. 2001;59:122–126. doi: 10.1124/mol.59.1.122. [DOI] [PubMed] [Google Scholar]
- 168.Khaliq Y., Zhanel G.G. Fluoroquinolone-associated tendinopathy: A critical review of the literature. Clin. Infect. Dis. 2003;36:1404–1410. doi: 10.1086/375078. [DOI] [PubMed] [Google Scholar]
- 169.van der Linden P.D., Sturkenboom M.C., Herings R.M., Leufkens H.M., Rowlands S., Stricker B.H. Increased risk of achilles tendon rupture with quinolone antibacterial use, especially in elderly patients taking oral corticosteroids. Arch. Intern. Med. 2003;163:1801–1807. doi: 10.1001/archinte.163.15.1801. [DOI] [PubMed] [Google Scholar]
- 170.Aspinall S.L., Good C.B., Jiang R., McCarren M., Dong D., Cunningham F.E. Severe dysglycemia with the fluoroquinolones: A class effect? Clin. Infect. Dis. 2009;49:402–408. doi: 10.1086/600294. [DOI] [PubMed] [Google Scholar]
- 171.Van Bambeke F., Tulkens P.M. Safety profile of the respiratory fluoroquinolone moxifloxacin: Comparison with other fluoroquinolones and other antibacterial classes. Drug Saf. 2009;32:359–378. doi: 10.2165/00002018-200932050-00001. [DOI] [PubMed] [Google Scholar]
- 172.Hayashi N., Nakata Y., Yazaki A. New findings on the structure-phototoxicity relationship and photostability of fluoroquinolones with various substituents at position 1. Antimicrob. Agents Chemother. 2004;48:799–803. doi: 10.1128/AAC.48.3.799-803.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dawe R.S., Ibbotson S.H., Sanderson J.B., Thomson E.M., Ferguson J. A randomized controlled trial (volunteer study) of sitafloxacin, enoxacin, levofloxacin and sparfloxacin phototoxicity. Br. J. Dermatol. 2003;149:1232–1241. doi: 10.1111/j.1365-2133.2003.05582.x. [DOI] [PubMed] [Google Scholar]
- 174.Miyazaki T., Nakamura S., Hashiguchi K., Kobayashi T., Fukushima K., Fukuda Y., Kondo A., Inoue Y., Koga H., Sasaki E., et al. The efficacy and safety of sitafloxacin and garenoxacin for the treatment of pneumonia in elderly patients: A randomized, multicenter, open-label trial. J. Infect. Chemother. 2019;25:886–893. doi: 10.1016/j.jiac.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 175.Kawada Y., Yasuda M., Tanaka K., Moden K., Akasaka S., Egashira T., Kaku M., Hori S. Dose-comparative study of sitafloxacin in complicated urinary tract infections. Jpn J. Chemother. 2008;56:81–91. [Google Scholar]
- 176.Chen C.K., Cheng I.L., Chen Y.H., Lai C.C. Efficacy and Safety of Sitafloxacin in the Treatment of Acute Bacterial Infection: A Meta-analysis of Randomized Controlled Trials. Antibiotics. 2020;9:106. doi: 10.3390/antibiotics9030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shetty N., Wilson A.P. Sitafloxacin in the treatment of patients with infections caused by vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2000;46:633–638. doi: 10.1093/jac/46.4.633. [DOI] [PubMed] [Google Scholar]
- 178.Grayson M.L., Cosgrove S.E., Crowe S., Hope W., McCarthy J.S., Mills J., Mouton J.W., Paterson D.L. Kucers’ The Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs, Seventh Edition—Three Volume Set. 7th ed. Taylor and Francis; London, UK: 2017. [Google Scholar]
- 179.O’Grady J., Briggs A., Atarashi S., Kobayashi H., Smith R.L., Ward J., Ward C., Milatovic D. Pharmacokinetics and absolute bioavailability of sitafloxacin, a new fluoroquinolone antibiotic, in healthy male and female Caucasian subjects. Xenobiotica. 2001;31:811–822. doi: 10.1080/0049825011. [DOI] [PubMed] [Google Scholar]
- 180.Craig W.A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998;26:1–10; quiz 11–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 181.Leggett J.E., Fantin B., Ebert S., Totsuka K., Vogelman B., Calame W., Mattie H., Craig W.A. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J. Infect. Dis. 1989;159:281–292. doi: 10.1093/infdis/159.2.281. [DOI] [PubMed] [Google Scholar]
- 182.Yokota S., Ohkoshi Y., Fujii N. Susceptibility and bactericidal activity of 8 oral quinolones against conventional-fluoroquinolone-resistant Streptococcus pneumoniae clinical isolates. Diagn. Microbiol. Infect. Dis. 2009;65:76–80. doi: 10.1016/j.diagmicrobio.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 183.Saito A., Tanigawara Y., Watanabe A., Aoki N., Niki Y., Kohno S., Kaku M., Hori S., Totsuka K. Open study of sitafloxacin in patients with respiratory tract infections: PK/PD study. Jpn J. Chemother. 2008;56((Suppl. 1)):63–80. [Google Scholar]
- 184.Kohno S., Niki Y., Kadota J., Yanagihara K., Kaku M., Watanabe A., Aoki N., Hori S., Fujita J., Tanigawara Y. Clinical dose findings of sitafloxacin treatment: Pharmacokinetic-pharmacodynamic analysis of two clinical trial results for community-acquired respiratory tract infections. J. Infect. Chemother. 2013;19:486–494. doi: 10.1007/s10156-012-0543-z. [DOI] [PubMed] [Google Scholar]