Abstract
1. The involvement of the cytoplasmic and core regions of K+ channel Kir3.1 and Kir3.2 subunits in determining the cell surface expression and G protein-gated activity of homomeric and heteromeric channel complexes was investigated by heterologous expression of chimeric and wild-type subunits together with the m2 muscarinic receptor in Xenopus oocytes. 2. Co-expression of Kir3.1 and Kir3.2 subunits yielded currents severalfold larger than those elicited by the individual expression of these subunits. Immunofluorescence labelling indicated that Kir3.2 homomeric channels and Kir3.1-Kir3.2 heteromeric channels were expressed at high levels at the cell surface whereas Kir3.1 homomeric complexes were not expressed at the cell surface. Chimeric subunits composed of Kir3.1 and Kir3.2 showed that the presence of either the cytoplasmic tails or the core region of Kir3.1 in all subunits inhibits expression of channels at the plasma membrane. 3. Substituting the cytoplasmic tails of Kir3.1 for the cytoplasmic tails of Kir3.2, generated a chimeric subunit (121) which displayed dramatically increased acetylcholine-induced channel activity compared with the wild-type Kir3.2 homomeric channel. Cell-attached, single-channel recordings revealed that chimera 121 channel openings were longer than Kir3.2 openings. 4. Individually substituting the N- and C-terminal tails of Kir3.1 for those of Kir3.2 showed that the C-terminal tail of Kir3.1 enhanced the activity of heteromeric channels independently of the N-terminal or core regions of this subunit. 5. The chimeric channel, 121, displayed a higher ratio of ACh-induced to basal activity than the Kir3.1-Kir3.2 or Kir3.2 channels. A smaller proportion of chimera 121 channels appear to be activated by the basal turnover of G proteins, implying that they have a lower affinity for G beta gamma. Our results suggest that substituting the Kir3.1 C-terminal tail for the Kir3.2 tail promotes the opening conformational change of the G beta gamma-bound channel. 6. The core and C-terminal regions of Kir3.1 independently conferred time dependence on voltage-dependent activation. The time constant (tau) was between 5 and 10 ms and varied little over the voltage range -60 to -120 mV.
Full text
PDF

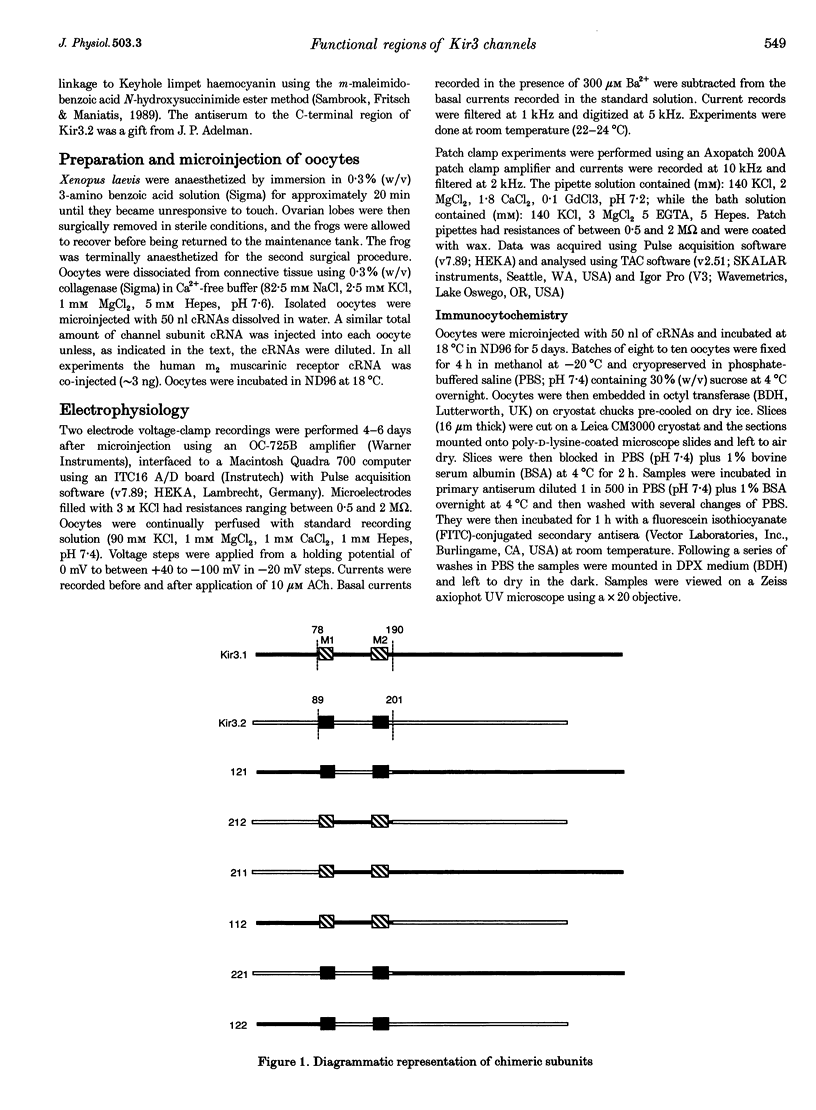
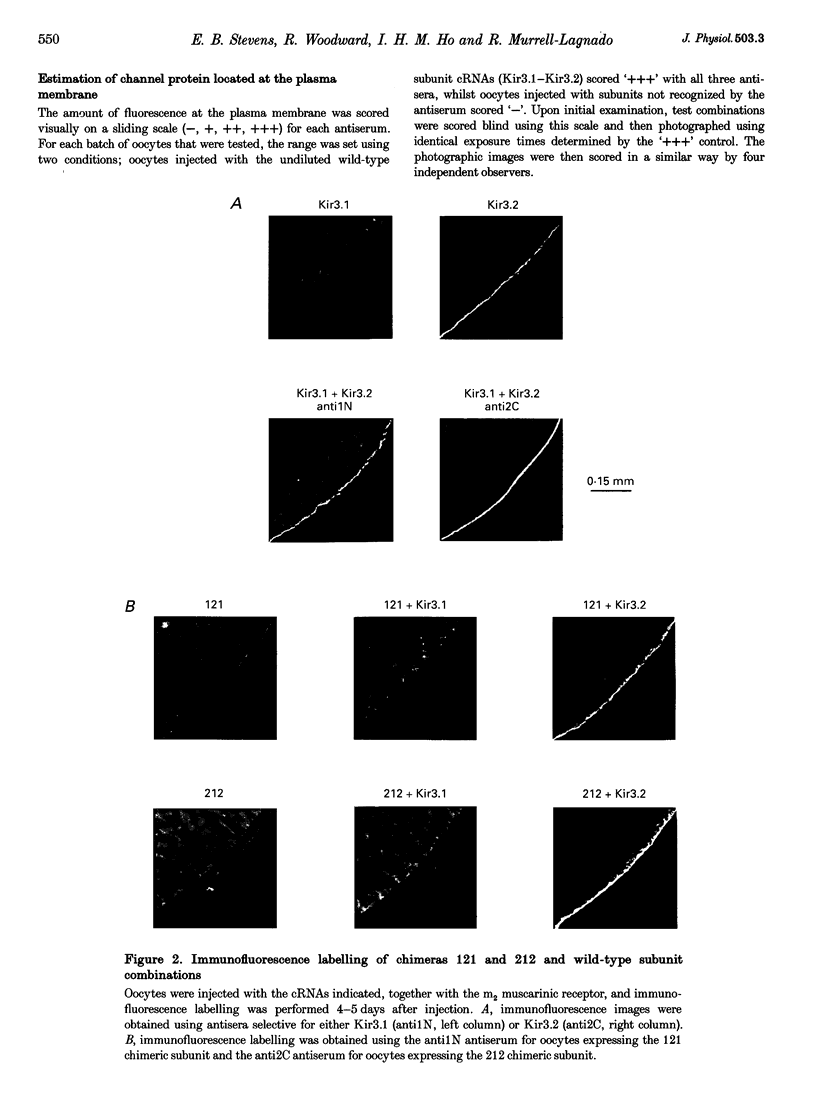



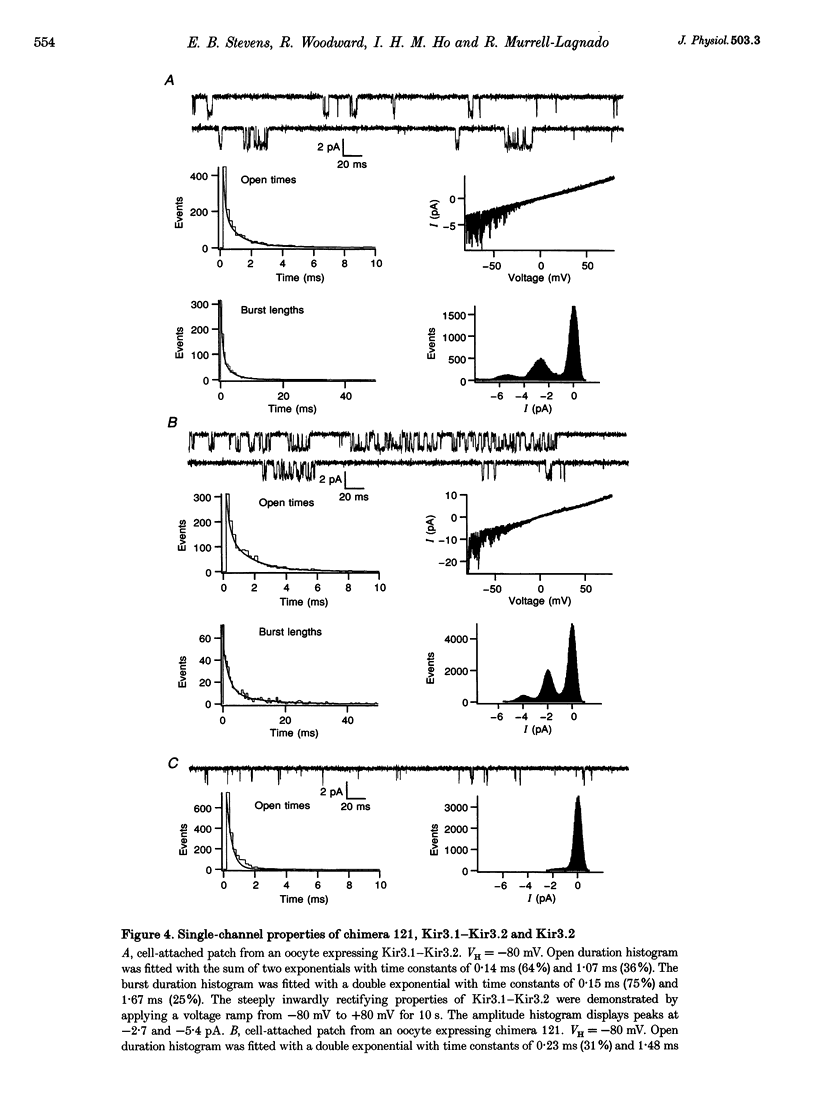



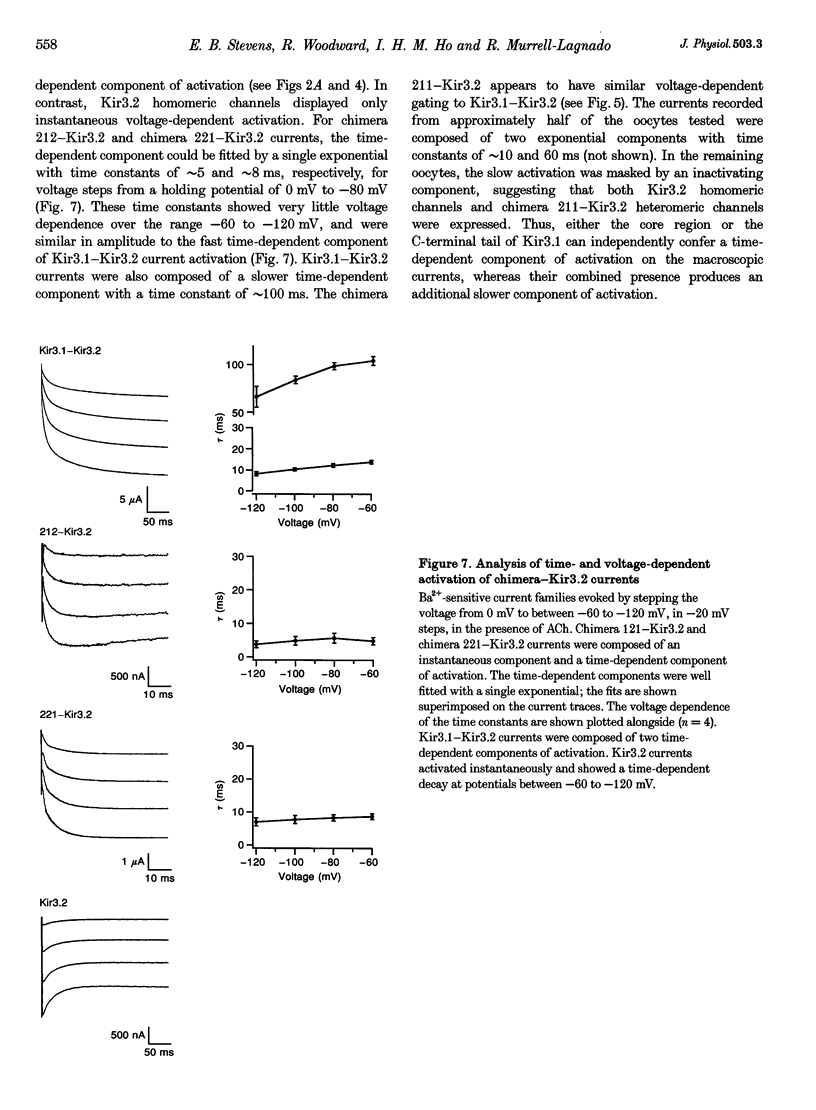




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond C. T., Ammälä C., Ashfield R., Blair T. A., Gribble F., Khan R. N., Lee K., Proks P., Rowe I. C., Sakura H. Cloning and functional expression of the cDNA encoding an inwardly-rectifying potassium channel expressed in pancreatic beta-cells and in the brain. FEBS Lett. 1995 Jun 19;367(1):61–66. doi: 10.1016/0014-5793(95)00497-w. [DOI] [PubMed] [Google Scholar]
- Chan K. W., Langan M. N., Sui J. L., Kozak J. A., Pabon A., Ladias J. A., Logothetis D. E. A recombinant inwardly rectifying potassium channel coupled to GTP-binding proteins. J Gen Physiol. 1996 Mar;107(3):381–397. doi: 10.1085/jgp.107.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K. W., Sui J. L., Vivaudou M., Logothetis D. E. Control of channel activity through a unique amino acid residue of a G protein-gated inwardly rectifying K+ channel subunit. Proc Natl Acad Sci U S A. 1996 Nov 26;93(24):14193–14198. doi: 10.1073/pnas.93.24.14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K. W., Sui J. L., Vivaudou M., Logothetis D. E. Specific regions of heteromeric subunits involved in enhancement of G protein-gated K+ channel activity. J Biol Chem. 1997 Mar 7;272(10):6548–6555. doi: 10.1074/jbc.272.10.6548. [DOI] [PubMed] [Google Scholar]
- Dascal N., Schreibmayer W., Lim N. F., Wang W., Chavkin C., DiMagno L., Labarca C., Kieffer B. L., Gaveriaux-Ruff C., Trollinger D. Atrial G protein-activated K+ channel: expression cloning and molecular properties. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10235–10239. doi: 10.1073/pnas.90.21.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F., Lesage F., Guillemare E., Fink M., Hugnot J. P., Bigay J., Lazdunski M., Romey G., Barhanin J. Heterologous multimeric assembly is essential for K+ channel activity of neuronal and cardiac G-protein-activated inward rectifiers. Biochem Biophys Res Commun. 1995 Jul 17;212(2):657–663. doi: 10.1006/bbrc.1995.2019. [DOI] [PubMed] [Google Scholar]
- Fink M., Duprat F., Lesage F., Heurteaux C., Romey G., Barhanin J., Lazdunski M. A new K+ channel beta subunit to specifically enhance Kv2.2 (CDRK) expression. J Biol Chem. 1996 Oct 18;271(42):26341–26348. doi: 10.1074/jbc.271.42.26341. [DOI] [PubMed] [Google Scholar]
- Glowatzki E., Fakler G., Brändle U., Rexhausen U., Zenner H. P., Ruppersberg J. P., Fakler B. Subunit-dependent assembly of inward-rectifier K+ channels. Proc Biol Sci. 1995 Aug 22;261(1361):251–261. doi: 10.1098/rspb.1995.0145. [DOI] [PubMed] [Google Scholar]
- Hedin K. E., Lim N. F., Clapham D. E. Cloning of a Xenopus laevis inwardly rectifying K+ channel subunit that permits GIRK1 expression of IKACh currents in oocytes. Neuron. 1996 Feb;16(2):423–429. doi: 10.1016/s0896-6273(00)80060-4. [DOI] [PubMed] [Google Scholar]
- Huang C. L., Jan Y. N., Jan L. Y. Binding of the G protein betagamma subunit to multiple regions of G protein-gated inward-rectifying K+ channels. FEBS Lett. 1997 Apr 1;405(3):291–298. doi: 10.1016/s0014-5793(97)00197-x. [DOI] [PubMed] [Google Scholar]
- Huang C. L., Slesinger P. A., Casey P. J., Jan Y. N., Jan L. Y. Evidence that direct binding of G beta gamma to the GIRK1 G protein-gated inwardly rectifying K+ channel is important for channel activation. Neuron. 1995 Nov;15(5):1133–1143. doi: 10.1016/0896-6273(95)90101-9. [DOI] [PubMed] [Google Scholar]
- Inanobe A., Morishige K. I., Takahashi N., Ito H., Yamada M., Takumi T., Nishina H., Takahashi K., Kanaho Y., Katada T. G beta gamma directly binds to the carboxyl terminus of the G protein-gated muscarinic K+ channel, GIRK1. Biochem Biophys Res Commun. 1995 Jul 26;212(3):1022–1028. doi: 10.1006/bbrc.1995.2072. [DOI] [PubMed] [Google Scholar]
- Keller S. H., Lindstrom J., Taylor P. Involvement of the chaperone protein calnexin and the acetylcholine receptor beta-subunit in the assembly and cell surface expression of the receptor. J Biol Chem. 1996 Sep 13;271(37):22871–22877. doi: 10.1074/jbc.271.37.22871. [DOI] [PubMed] [Google Scholar]
- Kennedy M. E., Nemec J., Clapham D. E. Localization and interaction of epitope-tagged GIRK1 and CIR inward rectifier K+ channel subunits. Neuropharmacology. 1996;35(7):831–839. doi: 10.1016/0028-3908(96)00132-3. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Ikeda K., Ichikawa T., Abe S., Togashi S., Kumanishi T. Molecular cloning of a mouse G-protein-activated K+ channel (mGIRK1) and distinct distributions of three GIRK (GIRK1, 2 and 3) mRNAs in mouse brain. Biochem Biophys Res Commun. 1995 Mar 28;208(3):1166–1173. doi: 10.1006/bbrc.1995.1456. [DOI] [PubMed] [Google Scholar]
- Kofuji P., Davidson N., Lester H. A. Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by G beta gamma subunits and function as heteromultimers. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6542–6546. doi: 10.1073/pnas.92.14.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P., Doupnik C. A., Davidson N., Lester H. A. A unique P-region residue is required for slow voltage-dependent gating of a G protein-activated inward rectifier K+ channel expressed in Xenopus oocytes. J Physiol. 1996 Feb 1;490(Pt 3):633–645. doi: 10.1113/jphysiol.1996.sp021173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P., Hofer M., Millen K. J., Millonig J. H., Davidson N., Lester H. A., Hatten M. E. Functional analysis of the weaver mutant GIRK2 K+ channel and rescue of weaver granule cells. Neuron. 1996 May;16(5):941–952. doi: 10.1016/s0896-6273(00)80117-8. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G., Gordon E. A., Wickman K., Velimirović B., Krapivinsky L., Clapham D. E. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature. 1995 Mar 9;374(6518):135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G., Krapivinsky L., Wickman K., Clapham D. E. G beta gamma binds directly to the G protein-gated K+ channel, IKACh. J Biol Chem. 1995 Dec 8;270(49):29059–29062. doi: 10.1074/jbc.270.49.29059. [DOI] [PubMed] [Google Scholar]
- Kubo Y., Reuveny E., Slesinger P. A., Jan Y. N., Jan L. Y. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 1993 Aug 26;364(6440):802–806. doi: 10.1038/364802a0. [DOI] [PubMed] [Google Scholar]
- Kunkel M. T., Peralta E. G. Identification of domains conferring G protein regulation on inward rectifier potassium channels. Cell. 1995 Nov 3;83(3):443–449. doi: 10.1016/0092-8674(95)90122-1. [DOI] [PubMed] [Google Scholar]
- Lesage F., Duprat F., Fink M., Guillemare E., Coppola T., Lazdunski M., Hugnot J. P. Cloning provides evidence for a family of inward rectifier and G-protein coupled K+ channels in the brain. FEBS Lett. 1994 Oct 10;353(1):37–42. doi: 10.1016/0014-5793(94)01007-2. [DOI] [PubMed] [Google Scholar]
- Lesage F., Guillemare E., Fink M., Duprat F., Heurteaux C., Fosset M., Romey G., Barhanin J., Lazdunski M. Molecular properties of neuronal G-protein-activated inwardly rectifying K+ channels. J Biol Chem. 1995 Dec 1;270(48):28660–28667. doi: 10.1074/jbc.270.48.28660. [DOI] [PubMed] [Google Scholar]
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987 Jan 22;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Lopatin A. N., Makhina E. N., Nichols C. G. The mechanism of inward rectification of potassium channels: "long-pore plugging" by cytoplasmic polyamines. J Gen Physiol. 1995 Nov;106(5):923–955. doi: 10.1085/jgp.106.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro B., Kennedy M. E., Velimirovíc B., Bhat D., Peterson A. S., Clapham D. E. Nonselective and G betagamma-insensitive weaver K+ channels. Science. 1996 Jun 28;272(5270):1950–1953. doi: 10.1126/science.272.5270.1950. [DOI] [PubMed] [Google Scholar]
- Patil N., Cox D. R., Bhat D., Faham M., Myers R. M., Peterson A. S. A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nat Genet. 1995 Oct;11(2):126–129. doi: 10.1038/ng1095-126. [DOI] [PubMed] [Google Scholar]
- Pessia M., Bond C. T., Kavanaugh M. P., Adelman J. P. Contributions of the C-terminal domain to gating properties of inward rectifier potassium channels. Neuron. 1995 May;14(5):1039–1045. doi: 10.1016/0896-6273(95)90342-9. [DOI] [PubMed] [Google Scholar]
- Signorini S., Liao Y. J., Duncan S. A., Jan L. Y., Stoffel M. Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc Natl Acad Sci U S A. 1997 Feb 4;94(3):923–927. doi: 10.1073/pnas.94.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slesinger P. A., Patil N., Liao Y. J., Jan Y. N., Jan L. Y., Cox D. R. Functional effects of the mouse weaver mutation on G protein-gated inwardly rectifying K+ channels. Neuron. 1996 Feb;16(2):321–331. doi: 10.1016/s0896-6273(00)80050-1. [DOI] [PubMed] [Google Scholar]
- Slesinger P. A., Reuveny E., Jan Y. N., Jan L. Y. Identification of structural elements involved in G protein gating of the GIRK1 potassium channel. Neuron. 1995 Nov;15(5):1145–1156. doi: 10.1016/0896-6273(95)90102-7. [DOI] [PubMed] [Google Scholar]
- Tucker S. J., Pessia M., Adelman J. P. Muscarine-gated K+ channel: subunit stoichiometry and structural domains essential for G protein stimulation. Am J Physiol. 1996 Jul;271(1 Pt 2):H379–H385. doi: 10.1152/ajpheart.1996.271.1.H379. [DOI] [PubMed] [Google Scholar]
- Velimirovic B. M., Gordon E. A., Lim N. F., Navarro B., Clapham D. E. The K+ channel inward rectifier subunits form a channel similar to neuronal G protein-gated K+ channel. FEBS Lett. 1996 Jan 22;379(1):31–37. doi: 10.1016/0014-5793(95)01465-9. [DOI] [PubMed] [Google Scholar]
- Wickman K. D., Iñiguez-Lluhl J. A., Davenport P. A., Taussig R., Krapivinsky G. B., Linder M. E., Gilman A. G., Clapham D. E. Recombinant G-protein beta gamma-subunits activate the muscarinic-gated atrial potassium channel. Nature. 1994 Mar 17;368(6468):255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- Woodward R., Stevens E. B., Murrell-Lagnado R. D. Molecular determinants for assembly of G-protein-activated inwardly rectifying K+ channels. J Biol Chem. 1997 Apr 18;272(16):10823–10830. doi: 10.1074/jbc.272.16.10823. [DOI] [PubMed] [Google Scholar]
- Yang J., Jan Y. N., Jan L. Y. Control of rectification and permeation by residues in two distinct domains in an inward rectifier K+ channel. Neuron. 1995 May;14(5):1047–1054. doi: 10.1016/0896-6273(95)90343-7. [DOI] [PubMed] [Google Scholar]
- Yang J., Jan Y. N., Jan L. Y. Determination of the subunit stoichiometry of an inwardly rectifying potassium channel. Neuron. 1995 Dec;15(6):1441–1447. doi: 10.1016/0896-6273(95)90021-7. [DOI] [PubMed] [Google Scholar]



