Abstract
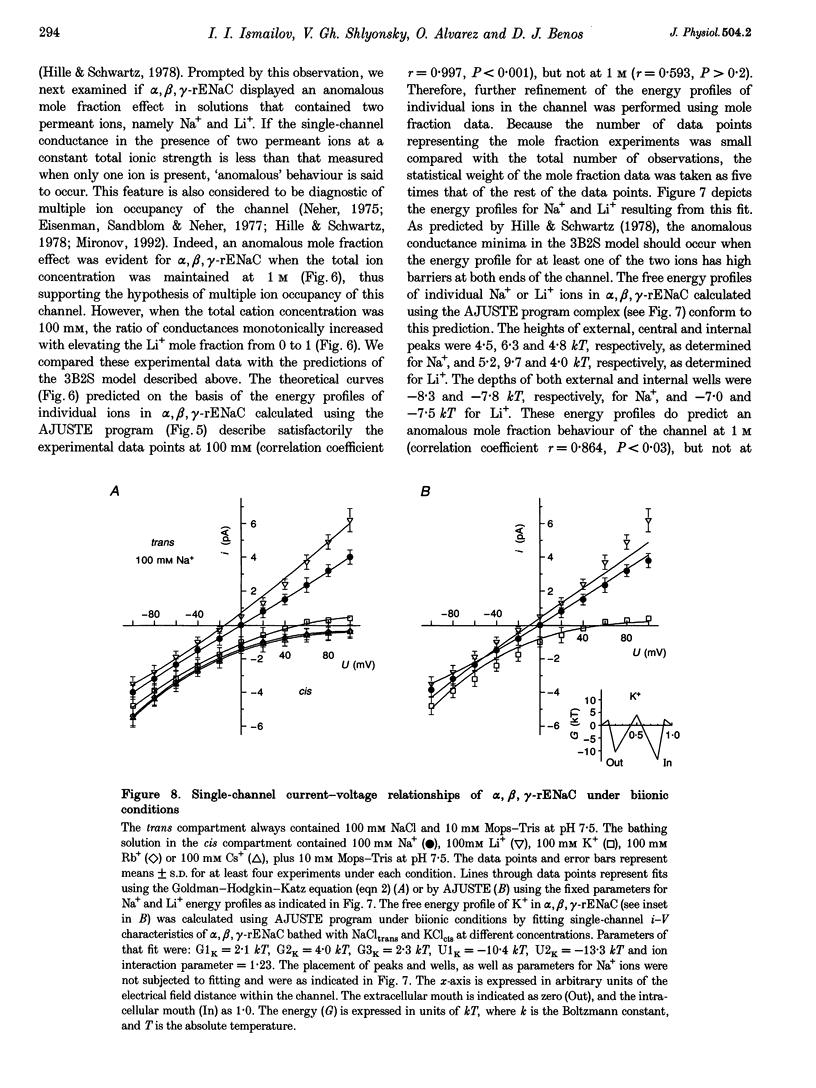
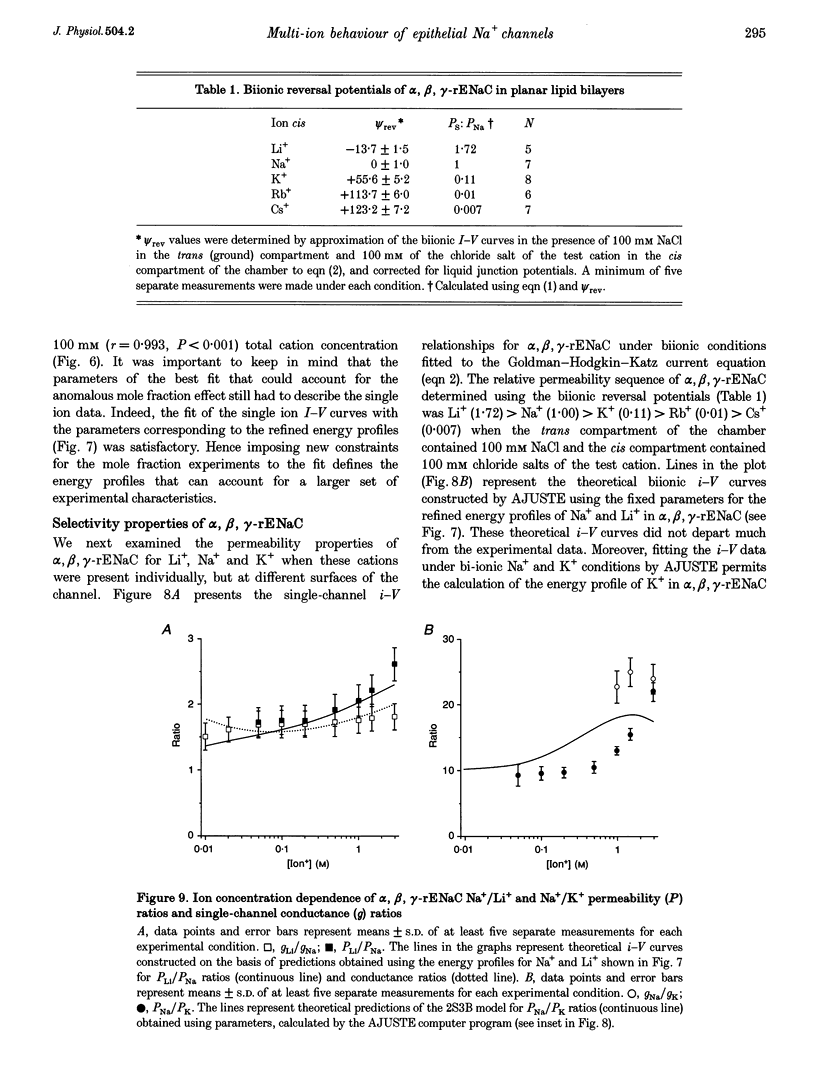
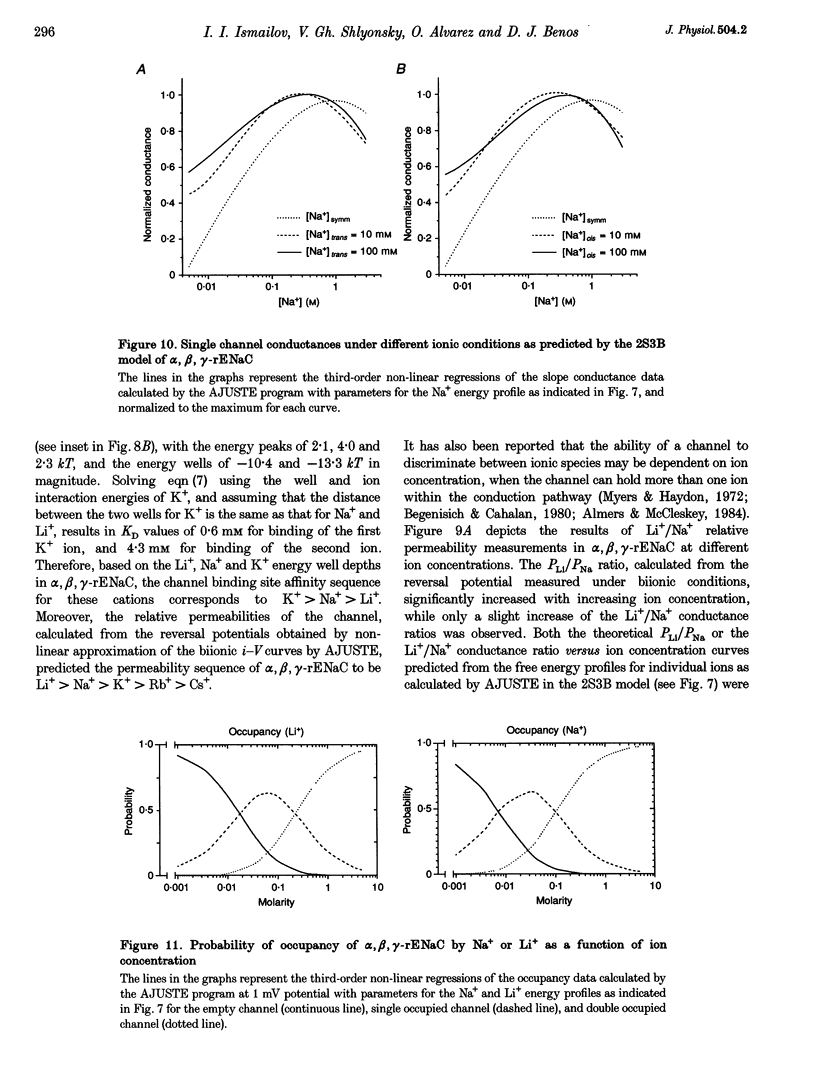
1. Conductance of heterotrimeric rat epithelial Na+ channels (alpha, beta, gamma-rENaCs) for Li+ and Na+ in planar lipid bilayers was a non-linear function of ion concentration, with a maximum of 30.4 +/- 2.9 pS and 18.5 +/- 1.9 pS at 1 M Li+ and Na+, respectively. 2. The alpha, beta, gamma-rENaC conductance measured in symmetrical mixtures of Na(+)-Li+ (1 M) exhibited an anomalous mole fraction dependence, with a minimum at 4:1 Li+ to Na+ molar ratio. 3. Permeability ratios PK/PNa and PLi/PNa of the channel calculated from the bionic reversal potentials were dependent on ion concentration: PK/PNa was 0.11 +/- 0.01, and PLi/PNa was 1.6 +/- 0.3 at 50 mM; PK/PNa was 0.04 +/- 0.01 and PLi/PNa was 2.5 +/- 0.4 at 3 M, but differed from the ratios of single-channel conductances in symmetrical Li+, Na+ or K+ solutions. The permeability sequence determined by either method was Li+ > Na+ > K+ >> Rb+ Cs+. 4. Predictions of a model featuring two binding sites and three energy barriers (2S3B), and allowing double occupancy, developed on the basis of single ion current-voltage relationships, are in agreement with the observed conductance maximum in single ion experiments, conductance minimum in the mole fraction experiments, non-linearity of the current-voltage curves in bionic experiments, and the concentration dependence of permeability ratios. 5. Computer simulations using the 2S3B model recreate the ion concentration dependencies of single-channel conductance observed for the immunopurified bovine renal amiloride-sensitive Na+ channel, and short-circuit current in frog skin, thus supporting the hypothesis that ENaCs form a core conduction unit of epithelial Na+ channels.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., McCleskey E. W. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984 Aug;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez O., Villarroel A., Eisenman G. Calculation of ion currents from energy profiles and energy profiles from ion currents in multibarrier, multisite, multioccupancy channel model. Methods Enzymol. 1992;207:816–854. doi: 10.1016/0076-6879(92)07058-v. [DOI] [PubMed] [Google Scholar]
- Awayda M. S., Ismailov I. I., Berdiev B. K., Benos D. J. A cloned renal epithelial Na+ channel protein displays stretch activation in planar lipid bilayers. Am J Physiol. 1995 Jun;268(6 Pt 1):C1450–C1459. doi: 10.1152/ajpcell.1995.268.6.C1450. [DOI] [PubMed] [Google Scholar]
- Begenisich T. B., Cahalan M. D. Sodium channel permeation in squid axons. I: Reversal potential experiments. J Physiol. 1980 Oct;307:217–242. doi: 10.1113/jphysiol.1980.sp013432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benos D. J., Hyde B. A., Latorre R. Sodium flux ratio through the amiloride-sensitive entry pathway in frog skin. J Gen Physiol. 1983 May;81(5):667–685. doi: 10.1085/jgp.81.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa C. M., Horisberger J. D., Rossier B. C. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993 Feb 4;361(6411):467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- Canessa C. M., Schild L., Buell G., Thorens B., Gautschi I., Horisberger J. D., Rossier B. C. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994 Feb 3;367(6462):463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Coronado R., Rosenberg R. L., Miller C. Ionic selectivity, saturation, and block in a K+-selective channel from sarcoplasmic reticulum. J Gen Physiol. 1980 Oct;76(4):425–446. doi: 10.1085/jgp.76.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D. C., Hamilton K. L. The amiloride-blockable sodium channel of epithelial tissue. Ion Channels. 1988;1:251–282. doi: 10.1007/978-1-4615-7302-9_7. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Latorre R., Miller C. Multi-ion conduction and selectivity in the high-conductance Ca++-activated K+ channel from skeletal muscle. Biophys J. 1986 Dec;50(6):1025–1034. doi: 10.1016/S0006-3495(86)83546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehland E., Hoshiko T., Machlup S. Competitive blocking of apical sodium channels in epithelia. Biochim Biophys Acta. 1983 Aug 10;732(3):636–646. doi: 10.1016/0005-2736(83)90241-9. [DOI] [PubMed] [Google Scholar]
- Fuller C. M., Awayda M. S., Arrate M. P., Bradford A. L., Morris R. G., Canessa C. M., Rossier B. C., Benos D. J. Cloning of a bovine renal epithelial Na+ channel subunit. Am J Physiol. 1995 Sep;269(3 Pt 1):C641–C654. doi: 10.1152/ajpcell.1995.269.3.C641. [DOI] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladky S. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. I. Studies of the unit conductance channel. Biochim Biophys Acta. 1972 Aug 9;274(2):294–312. doi: 10.1016/0005-2736(72)90178-2. [DOI] [PubMed] [Google Scholar]
- Ismailov I. I., Awayda M. S., Berdiev B. K., Bubien J. K., Lucas J. E., Fuller C. M., Benos D. J. Triple-barrel organization of ENaC, a cloned epithelial Na+ channel. J Biol Chem. 1996 Jan 12;271(2):807–816. doi: 10.1074/jbc.271.2.807. [DOI] [PubMed] [Google Scholar]
- Ismailov I. I., Berdiev B. K., Benos D. J. Regulation by Na+ and Ca2+ of renal epithelial Na+ channels reconstituted into planar lipid bilayers. J Gen Physiol. 1995 Sep;106(3):445–466. doi: 10.1085/jgp.106.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismailov I. I., Berdiev B. K., Bradford A. L., Awayda M. S., Fuller C. M., Benos D. J. Associated proteins and renal epithelial Na+ channel function. J Membr Biol. 1996 Jan;149(2):123–132. doi: 10.1007/s002329900013. [DOI] [PubMed] [Google Scholar]
- Ismailov I. I., Berdiev B. K., Shlyonsky V. G., Benos D. J. Mechanosensitivity of an epithelial Na+ channel in planar lipid bilayers: release from Ca2+ block. Biophys J. 1997 Mar;72(3):1182–1192. doi: 10.1016/S0006-3495(97)78766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismailov I. I., Shlyonsky V. G., Benos D. J. Streaming potential measurements in alphabetagamma-rat epithelial Na+ channel in planar lipid bilayers. Proc Natl Acad Sci U S A. 1997 Jul 8;94(14):7651–7654. doi: 10.1073/pnas.94.14.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D. G. Electrostatic calculations for an ion channel. I. Energy and potential profiles and interactions between ions. Biophys J. 1978 May;22(2):209–219. doi: 10.1016/S0006-3495(78)85485-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingueglia E., Voilley N., Waldmann R., Lazdunski M., Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel. A new channel type with homologies to Caenorhabditis elegans degenerins. FEBS Lett. 1993 Feb 22;318(1):95–99. doi: 10.1016/0014-5793(93)81336-x. [DOI] [PubMed] [Google Scholar]
- Lu Z., MacKinnon R. A conductance maximum observed in an inward-rectifier potassium channel. J Gen Physiol. 1994 Sep;104(3):477–486. doi: 10.1085/jgp.104.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P. Ion transport through pores: a rate-theory analysis. Biochim Biophys Acta. 1973 Jul 6;311(3):423–441. doi: 10.1016/0005-2736(73)90323-4. [DOI] [PubMed] [Google Scholar]
- Mironov S. L. Conformational model for ion permeation in membrane channels: a comparison with multi-ion models and applications to calcium channel permeability. Biophys J. 1992 Aug;63(2):485–496. doi: 10.1016/S0006-3495(92)81628-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers V. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta. 1972 Aug 9;274(2):313–322. doi: 10.1016/0005-2736(72)90179-4. [DOI] [PubMed] [Google Scholar]
- Oh Y., Benos D. J. Single-channel characteristics of a purified bovine renal amiloride-sensitive Na+ channel in planar lipid bilayers. Am J Physiol. 1993 Jun;264(6 Pt 1):C1489–C1499. doi: 10.1152/ajpcell.1993.264.6.C1489. [DOI] [PubMed] [Google Scholar]
- Olans L., Sariban-Sohraby S., Benos D. J. Saturation behavior of single, amiloride-sensitive Na+ channels in planar lipid bilayers. Biophys J. 1984 Dec;46(6):831–835. doi: 10.1016/S0006-3495(84)84082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. G., Frindt G. Amiloride-sensitive Na channels from the apical membrane of the rat cortical collecting tubule. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2767–2770. doi: 10.1073/pnas.83.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. G., Frindt G. Conductance and gating of epithelial Na channels from rat cortical collecting tubule. Effects of luminal Na and Li. J Gen Physiol. 1988 Jul;92(1):121–138. doi: 10.1085/jgp.92.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. G. Na+ transport and flux ratio through apical Na+ channels in toad bladder. Nature. 1982 Jun 24;297(5868):688–690. doi: 10.1038/297688a0. [DOI] [PubMed] [Google Scholar]
- Puoti A., May A., Canessa C. M., Horisberger J. D., Schild L., Rossier B. C. The highly selective low-conductance epithelial Na channel of Xenopus laevis A6 kidney cells. Am J Physiol. 1995 Jul;269(1 Pt 1):C188–C197. doi: 10.1152/ajpcell.1995.269.1.C188. [DOI] [PubMed] [Google Scholar]
- Schumaker M. F., MacKinnon R. A simple model for multi-ion permeation. Single-vacancy conduction in a simple pore model. Biophys J. 1990 Oct;58(4):975–984. doi: 10.1016/S0006-3495(90)82442-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien H. T., Diana A. L. Some physical properties of bimolecular lipid membranes produced from new lipid solutions. Nature. 1967 Sep 9;215(5106):1199–1200. doi: 10.1038/2151199a0. [DOI] [PubMed] [Google Scholar]
- Urban B. W., Hladky S. B., Haydon D. A. The kinetics of ion movements in the gramicidin channel. Fed Proc. 1978 Oct;37(12):2628–2632. [PubMed] [Google Scholar]
- Urban B. W., Hladky S. B. Ion transport in the simplest single file pore. Biochim Biophys Acta. 1979 Jul 5;554(2):410–429. doi: 10.1016/0005-2736(79)90381-x. [DOI] [PubMed] [Google Scholar]


