Abstract
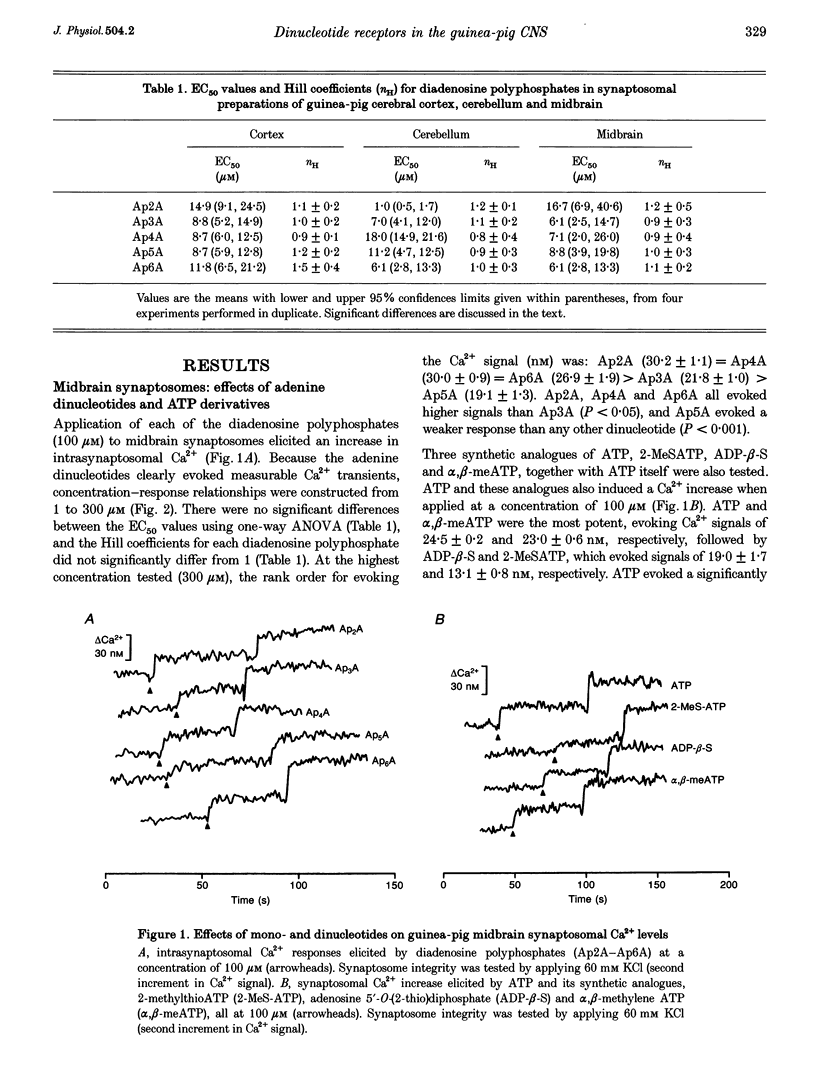
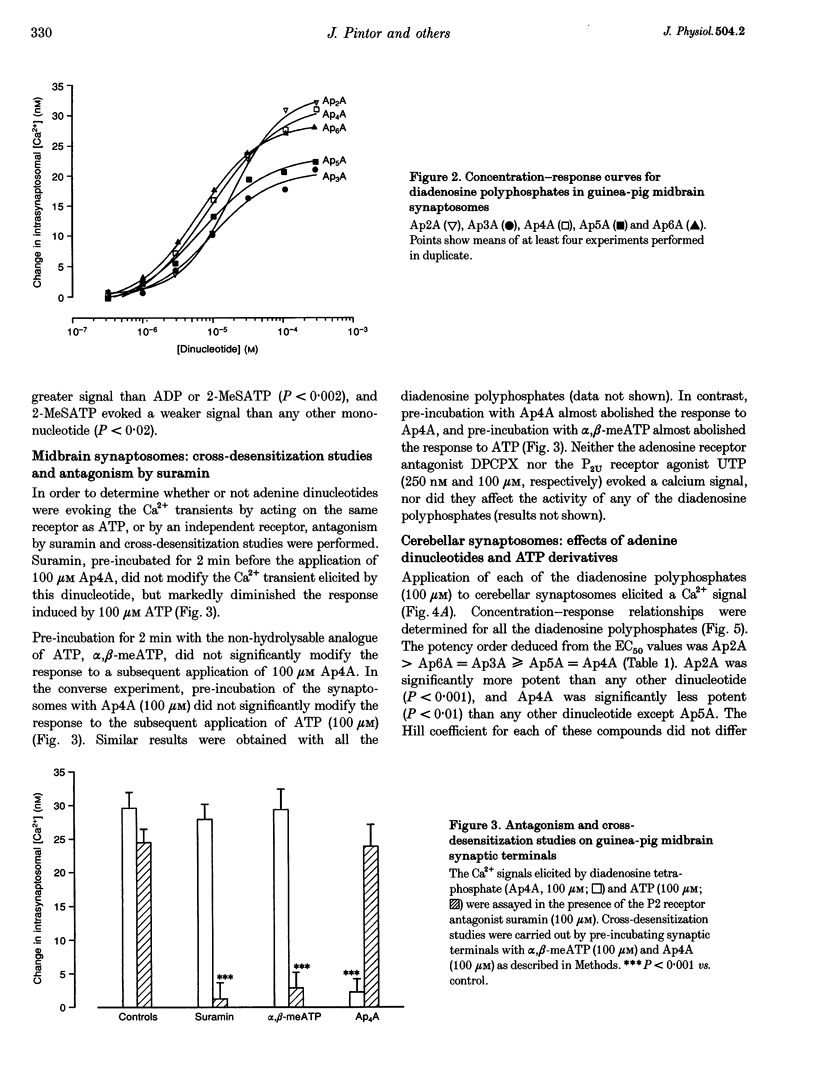
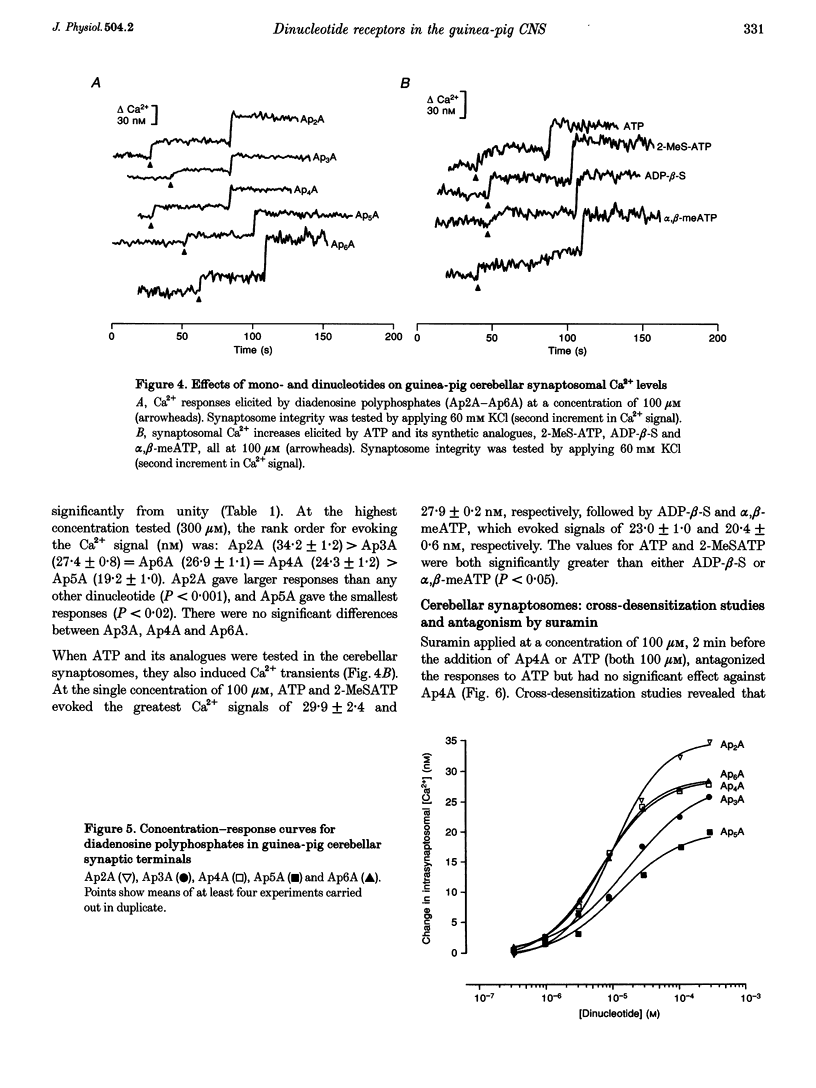
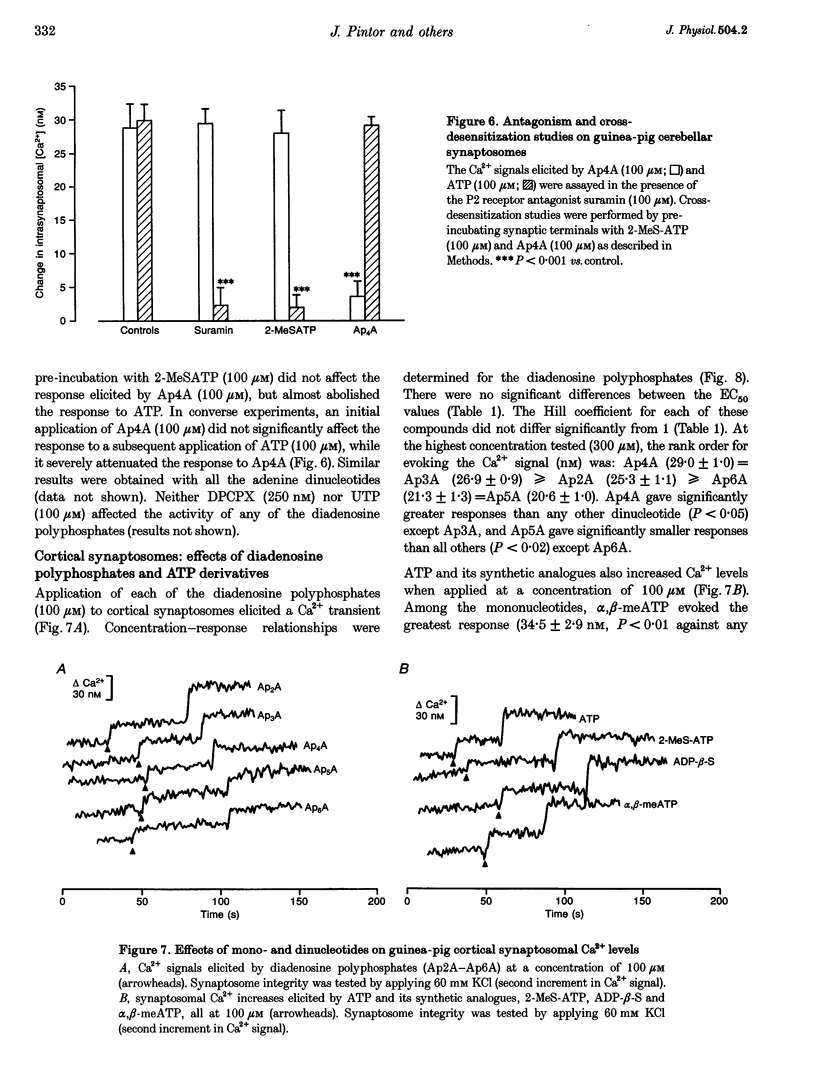
1. The ability of diadenosine polyphosphates, namely P1,P2-di(adenosine) pyrophosphate (Ap2A), P1,P3-di(adenosine) triphosphate (Ap3A), P1,P4-di(adenosine) tetraphosphate (Ap4A), P1,P5-di(adenosine) pentaphosphate (Ap5A) and P1,P6-di(adenosine) hexaphosphate (Ap6A) to evoke Ca2+ signals in synaptosomes prepared from three different regions of the guinea-pig brain was examined. 2. In synaptosomal preparations from the paleocortex (cortex), diencephalon/brainstem (midbrain) and cerebellum all the dinucleotides evoked Ca2+ signals that were concentration dependent over the range 1-300 microM. ATP and its synthetic analogues, alpha,beta-methylene ATP, 2-methylthio ATP and adenosine 5'-O-(2-thio)diphosphate (all 100 microM) also evoked Ca2+ signals in these preparations. 3. In the midbrain and cerebellum preparations, responses to ATP and its analogues were attenuated or abolished by the P2 receptor antagonist suramin (100 microM) but responses to the dinucleotides were not. Also, desensitization by a dinucleotide blocked responses to dinucleotides but not mononucleotides, and desensitization by a mononucleotide blocked responses to mononucleotides but not dinucleotides. 4. In cortical preparations, suramin (100 microM) blocked responses to both classes of nucleotides. Furthermore, there was mutual cross-desensitization between the mono- and dinucleotides. 5. The adenosine A1 receptor antagonist, 8-cyclopentyl-1,3-dipropylxanthine, did not affect responses evoked by the dinucleotides, nor did the pyrimidine UTP. 6. It is concluded that there are specific dinucleotide receptors, activated by diadenosine polyphosphates, but not ATP or UTP, on synaptic terminals in guinea-pig diencephalon/ brainstem and cerebellum. These receptors bear a similarity to the dinucleotide receptor (P4 receptor) in rat brain. In guinea-pig cerebral cortex synaptosomes, diadenosine polyphosphates appear to act via the same receptor as ATP.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxi M. D., Vishwanatha J. K. Diadenosine polyphosphates: their biological and pharmacological significance. J Pharmacol Toxicol Methods. 1995 Jun;33(3):121–128. doi: 10.1016/1056-8719(94)00127-p. [DOI] [PubMed] [Google Scholar]
- Castro E., Torres M., Miras-Portugal M. T., Gonzalez M. P. Effect of diadenosine polyphosphates on catecholamine secretion from isolated chromaffin cells. Br J Pharmacol. 1990 Jun;100(2):360–364. doi: 10.1111/j.1476-5381.1990.tb15809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. P., Levy A., Lightman S. L. Nucleotides as extracellular signalling molecules. J Neuroendocrinol. 1995 Feb;7(2):83–96. doi: 10.1111/j.1365-2826.1995.tb00671.x. [DOI] [PubMed] [Google Scholar]
- Dunn P. M., Blakeley A. G. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br J Pharmacol. 1988 Feb;93(2):243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hilderman R. H., Martin M., Zimmerman J. K., Pivorun E. B. Identification of a unique membrane receptor for adenosine 5',5"'-P1,P4-tetraphosphate. J Biol Chem. 1991 Apr 15;266(11):6915–6918. [PubMed] [Google Scholar]
- Hoyle C. H., Knight G. E., Burnstock G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol. 1990 Mar;99(3):617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle C. H. Pharmacological activity of adenine dinucleotides in the periphery: possible receptor classes and transmitter function. Gen Pharmacol. 1990;21(6):827–831. doi: 10.1016/0306-3623(90)90440-w. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Postorino A., Burnstock G. Pre- and postjunctional effects of diadenosine polyphosphates in the guinea-pig vas deferens. J Pharm Pharmacol. 1995 Nov;47(11):926–931. doi: 10.1111/j.2042-7158.1995.tb03272.x. [DOI] [PubMed] [Google Scholar]
- Klishin A., Lozovaya N., Pintor J., Miras-Portugal M. T., Krishtal O. Possible functional role of diadenosine polyphosphates: negative feedback for excitation in hippocampus. Neuroscience. 1994 Jan;58(2):235–236. doi: 10.1016/0306-4522(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Lazarowski E. R., Watt W. C., Stutts M. J., Boucher R. C., Harden T. K. Pharmacological selectivity of the cloned human P2U-purinoceptor: potent activation by diadenosine tetraphosphate. Br J Pharmacol. 1995 Sep;116(1):1619–1627. doi: 10.1111/j.1476-5381.1995.tb16382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintor J., Díaz-Rey M. A., Miras-Portugal M. T. Ap4A and ADP-beta-S binding to P2 purinoceptors present on rat brain synaptic terminals. Br J Pharmacol. 1993 Apr;108(4):1094–1099. doi: 10.1111/j.1476-5381.1993.tb13510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintor J., Díaz-Rey M. A., Torres M., Miras-Portugal M. T. Presence of diadenosine polyphosphates--Ap4A and Ap5A--in rat brain synaptic terminals. Ca2+ dependent release evoked by 4-aminopyridine and veratridine. Neurosci Lett. 1992 Mar 2;136(2):141–144. doi: 10.1016/0304-3940(92)90034-5. [DOI] [PubMed] [Google Scholar]
- Pintor J., Miras-Portugal M. T. A novel receptor for diadenosine polyphosphates coupled to calcium increase in rat midbrain synaptosomes. Br J Pharmacol. 1995 Jul;115(6):895–902. doi: 10.1111/j.1476-5381.1995.tb15894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintor J., Miras-Portugal M. T. P2 purinergic receptors for diadenosine polyphosphates in the nervous system. Gen Pharmacol. 1995 Mar;26(2):229–235. doi: 10.1016/0306-3623(94)00182-m. [DOI] [PubMed] [Google Scholar]
- Pintor J., Porras A., Mora F., Miras-Portugal M. T. Dopamine receptor blockade inhibits the amphetamine-induced release of diadenosine polyphosphates, diadenosine tetraphosphate and diadenosine pentaphosphate, from neostriatum of the conscious rat. J Neurochem. 1995 Feb;64(2):670–676. doi: 10.1046/j.1471-4159.1995.64020670.x. [DOI] [PubMed] [Google Scholar]
- Pintor J., Torres M., Castro E., Miras-Portugal M. T. Characterization of diadenosine tetraphosphate (Ap4A) binding sites in cultured chromaffin cells: evidence for a P2y site. Br J Pharmacol. 1991 Aug;103(4):1980–1984. doi: 10.1111/j.1476-5381.1991.tb12363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino A., Burnstock G. Possible role of diadenosine polyphosphates as modulators of cardiac sensory-motor neurotransmission in guinea-pigs. J Physiol. 1996 Sep 1;495(Pt 2):515–523. doi: 10.1113/jphysiol.1996.sp021611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T. W. Actions of adenine dinucleotides on the vas deferens, guinea-pig taenia caeci and bladder. Eur J Pharmacol. 1981 Oct 22;75(2-3):93–102. doi: 10.1016/0014-2999(81)90066-2. [DOI] [PubMed] [Google Scholar]
- Stone T. W., Perkins M. N. Adenine dinucleotide effects on rat cortical neurones. Brain Res. 1981 Dec 14;229(1):241–245. doi: 10.1016/0006-8993(81)90764-2. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Späth L., Starke K. Evidence for P2-purinoceptor-mediated inhibition of noradrenaline release in rat brain cortex. Br J Pharmacol. 1994 Nov;113(3):815–822. doi: 10.1111/j.1476-5381.1994.tb17066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


