Abstract
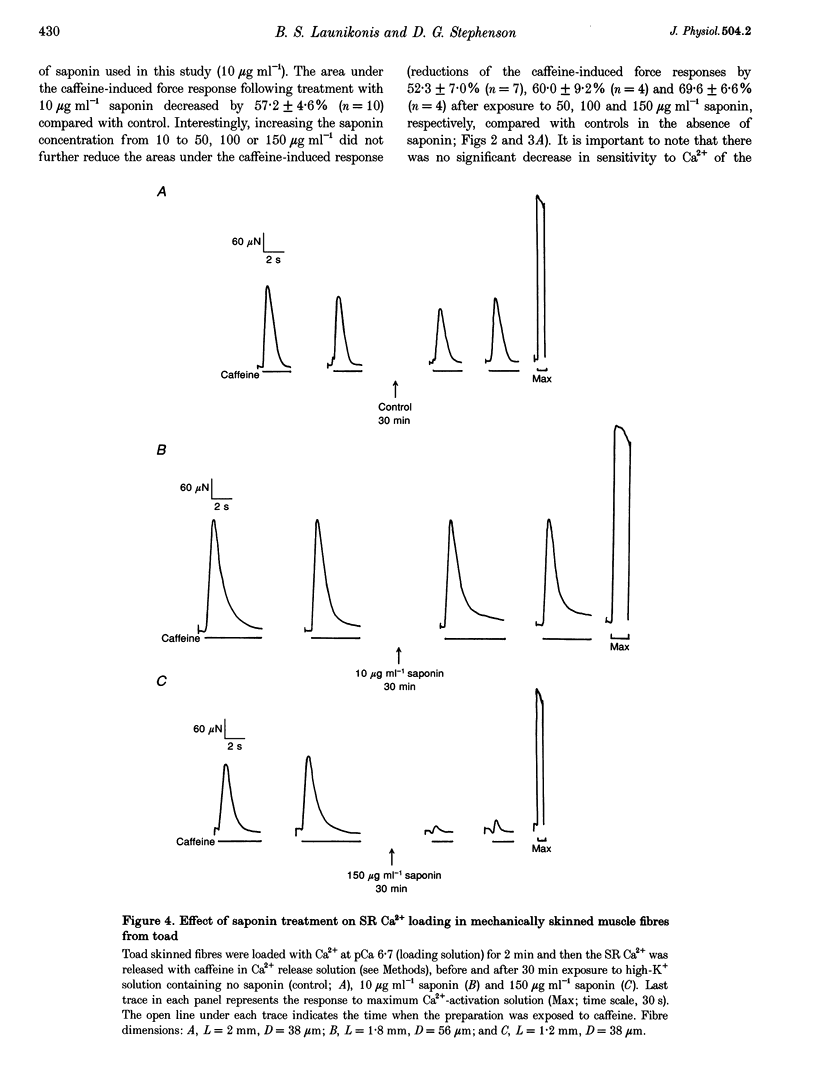
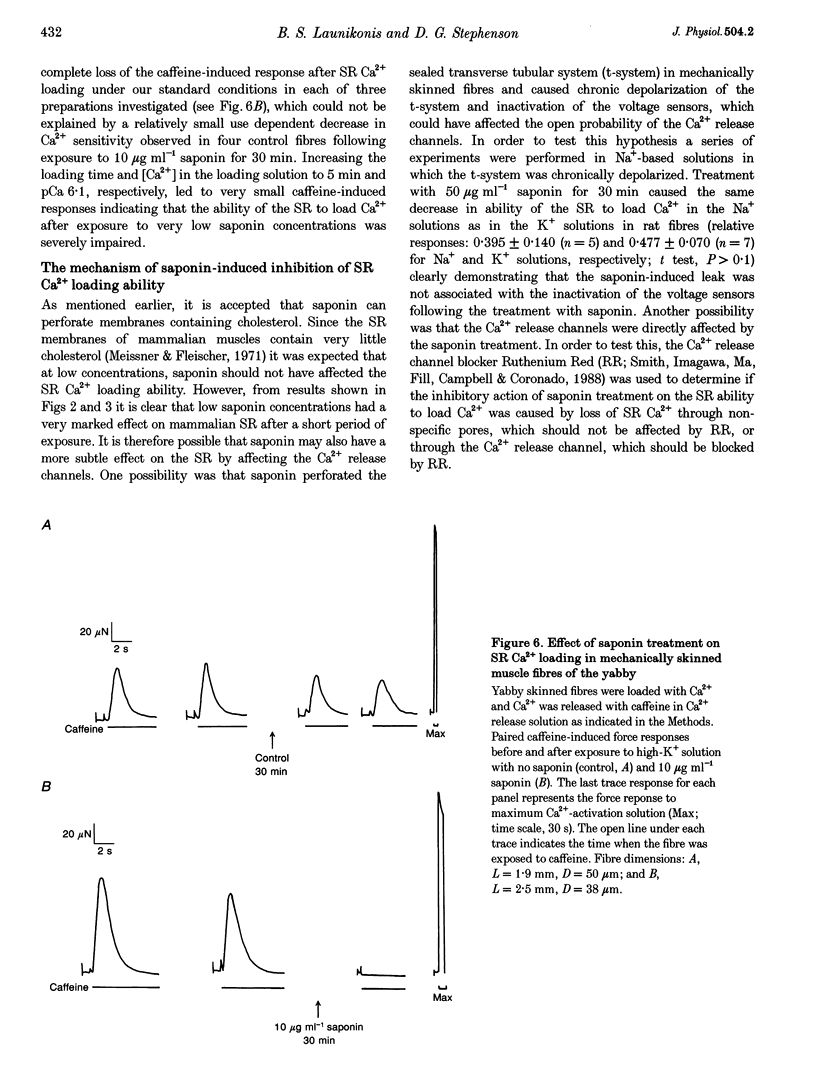
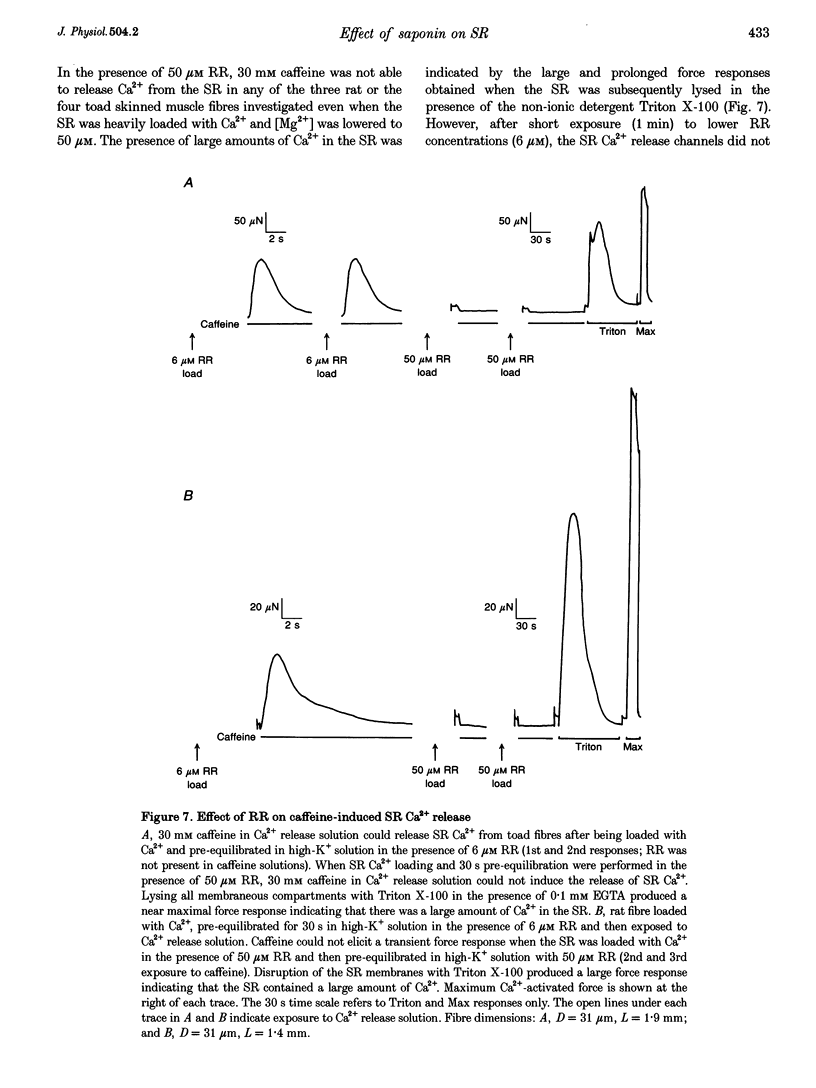
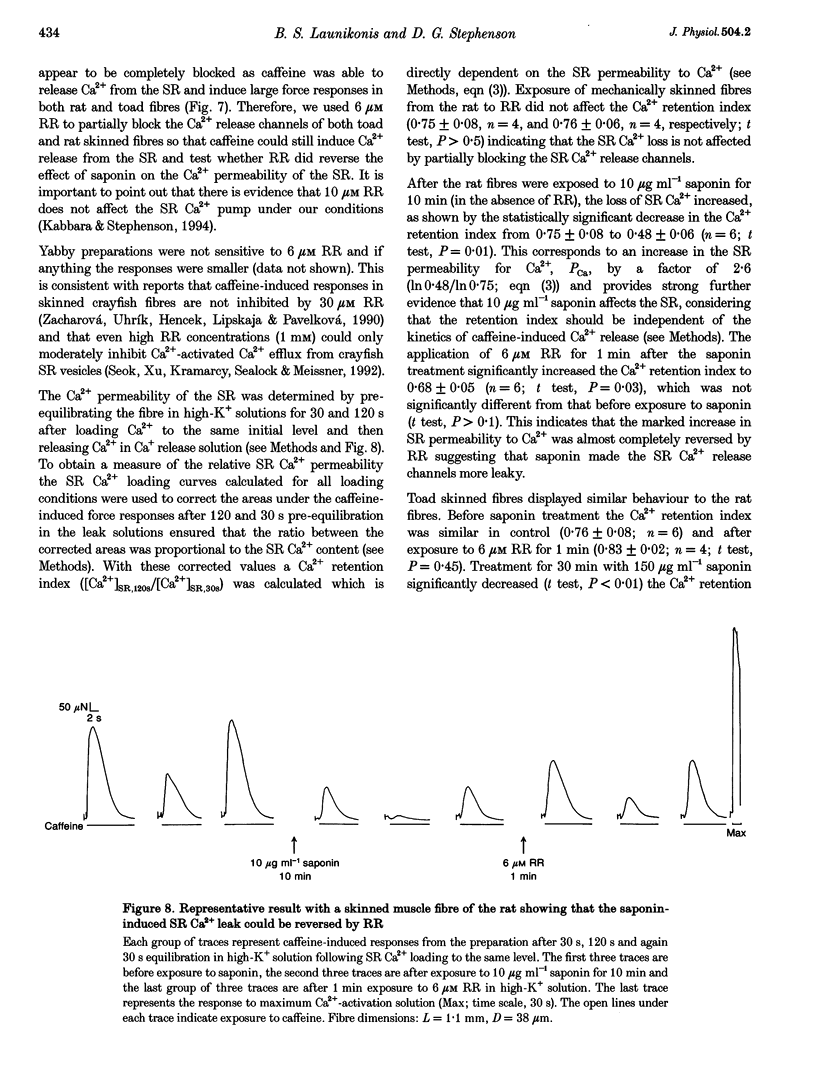
1. Mechanically skinned fibres from skeletal muscles of the rat, toad and yabby were used to investigate the effect of saponin treatment on sarcoplasmic reticulum (SR) Ca2+ loading properties. The SR was loaded submaximally under control conditions before and after treatment with saponin and SR Ca2+ was released with caffeine. 2. Treatment with 10 micrograms ml-1 saponin greatly reduced the SR Ca2+ loading ability of skinned fibres from the extensor digitorum longus muscle of the rat with a rate constant of 0.24 min-1. Saponin concentrations up to 150 micrograms ml-1 and increased exposure time up to 30 min did not further reduce the SR Ca2+ loading ability of the SR, which indicates that the inhibitory action of 10-150 micrograms ml-1 saponin is not dose dependent. The effect of saponin was also not dependent on the state of polarization of the transverse-tubular system. 3. Treatment with saponin at concentrations up to 100 micrograms ml-1 for 30 min did not affect the Ca2+ loading ability of SR in skinned skeletal muscle fibres from the twitch portion of the toad iliofibularis muscle but SR Ca2+ loading ability decreased markedly with a time constant of 0.22 min-1 in the presence of 150 micrograms ml-1 saponin. 4. The saponin dependent increase in permeability could be reversed in both rat and toad fibres by short treatment with 6 microM Ruthenium Red, a potent SR Ca2+ channel blocker, suggesting that saponin does affect the SR Ca2+ channel properties in mammalian and anuran skeletal muscle. 5. Treatment of skinned fibres of long sarcomere length (> 6 microns) from the claw muscle of the yabby (a freshwater decapod crustacean) with 10 micrograms ml-1 saponin for 30 min abolished the ability of the SR to load Ca2+, indicating that saponin affects differently the SR from skeletal muscles of mammals, anurans and crustaceans. 6. It is concluded that at relatively low concentrations, saponin causes inhibition of the skeletal SR Ca2+ loading ability in a species dependent manner, probably by increasing the Ca2+ loss through SR Ca2+ release channels.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Donaldson S. K. Peeled mammalian skeletal muscle fibers. Possible stimulation of Ca2+ release via a transverse tubule-sarcoplasmic reticulum mechanism. J Gen Physiol. 1985 Oct;86(4):501–525. doi: 10.1085/jgp.86.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Endo M., Iino M. Specific perforation of muscle cell membranes with preserved SR functions by saponin treatment. J Muscle Res Cell Motil. 1980 Mar;1(1):89–100. doi: 10.1007/BF00711927. [DOI] [PubMed] [Google Scholar]
- Fanó G., Belia S., Fulle S., Angelella P., Panara F., Marsili V., Pascolini R. Functional aspects of calcium transport in sarcoplasmic reticulum vesicles derived from frog skeletal muscle treated with saponin. J Muscle Res Cell Motil. 1989 Aug;10(4):326–330. doi: 10.1007/BF01758428. [DOI] [PubMed] [Google Scholar]
- Fink R. H., Stephenson D. G. Ca2+-movements in muscle modulated by the state of K+-channels in the sarcoplasmic reticulum membranes. Pflugers Arch. 1987 Aug;409(4-5):374–380. doi: 10.1007/BF00583791. [DOI] [PubMed] [Google Scholar]
- Fryer M. W., Stephenson D. G. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J Physiol. 1996 Jun 1;493(Pt 2):357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herland J. S., Julian F. J., Stephenson D. G. Halothane increases Ca2+ efflux via Ca2+ channels of sarcoplasmic reticulum in chemically skinned rat myocardium. J Physiol. 1990 Jul;426:1–18. doi: 10.1113/jphysiol.1990.sp018124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth S., Zhao M., Baylor S. M. The amplitude and time course of the myoplasmic free [Ca2+] transient in fast-twitch fibers of mouse muscle. J Gen Physiol. 1996 Nov;108(5):455–469. doi: 10.1085/jgp.108.5.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Control of calcium release and the effect of ryanodine in skinned muscle fibres of the toad. J Physiol. 1990 Apr;423:519–542. doi: 10.1113/jphysiol.1990.sp018037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Effect of Mg2+ on the control of Ca2+ release in skeletal muscle fibres of the toad. J Physiol. 1991 Mar;434:507–528. doi: 10.1113/jphysiol.1991.sp018483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Effects of intracellular pH and [Mg2+] on excitation-contraction coupling in skeletal muscle fibres of the rat. J Physiol. 1994 Jul 15;478(Pt 2):331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Excitation-contraction coupling in skeletal muscle fibres of rat and toad in the presence of GTP gamma S. J Physiol. 1991 Dec;444:65–84. doi: 10.1113/jphysiol.1991.sp018866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Adenine nucleotide stimulation of Ca2+-induced Ca2+ release in sarcoplasmic reticulum. J Biol Chem. 1984 Feb 25;259(4):2365–2374. [PubMed] [Google Scholar]
- Meissner G., Fleischer S. Characterization of sarcoplasmic reticulum from skeletal muscle. Biochim Biophys Acta. 1971 Aug 13;241(2):356–378. doi: 10.1016/0005-2736(71)90036-8. [DOI] [PubMed] [Google Scholar]
- Mobley B. A., Eisenberg B. R. Sizes of components in frog skeletal muscle measured by methods of stereology. J Gen Physiol. 1975 Jul;66(1):31–45. doi: 10.1085/jgp.66.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Seok J. H., Xu L., Kramarcy N. R., Sealock R., Meissner G. The 30 S lobster skeletal muscle Ca2+ release channel (ryanodine receptor) has functional properties distinct from the mammalian channel proteins. J Biol Chem. 1992 Aug 5;267(22):15893–15901. [PubMed] [Google Scholar]
- Smith J. S., Imagawa T., Ma J., Fill M., Campbell K. P., Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol. 1988 Jul;92(1):1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981 Aug;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharová D., Uhrík B., Hencek M., Lipskaja E., Pavelková J. Effects of ruthenium red on excitation and contraction in muscle fibres with Ca2+ electrogenesis. Gen Physiol Biophys. 1990 Dec;9(6):545–568. [PubMed] [Google Scholar]


