Abstract
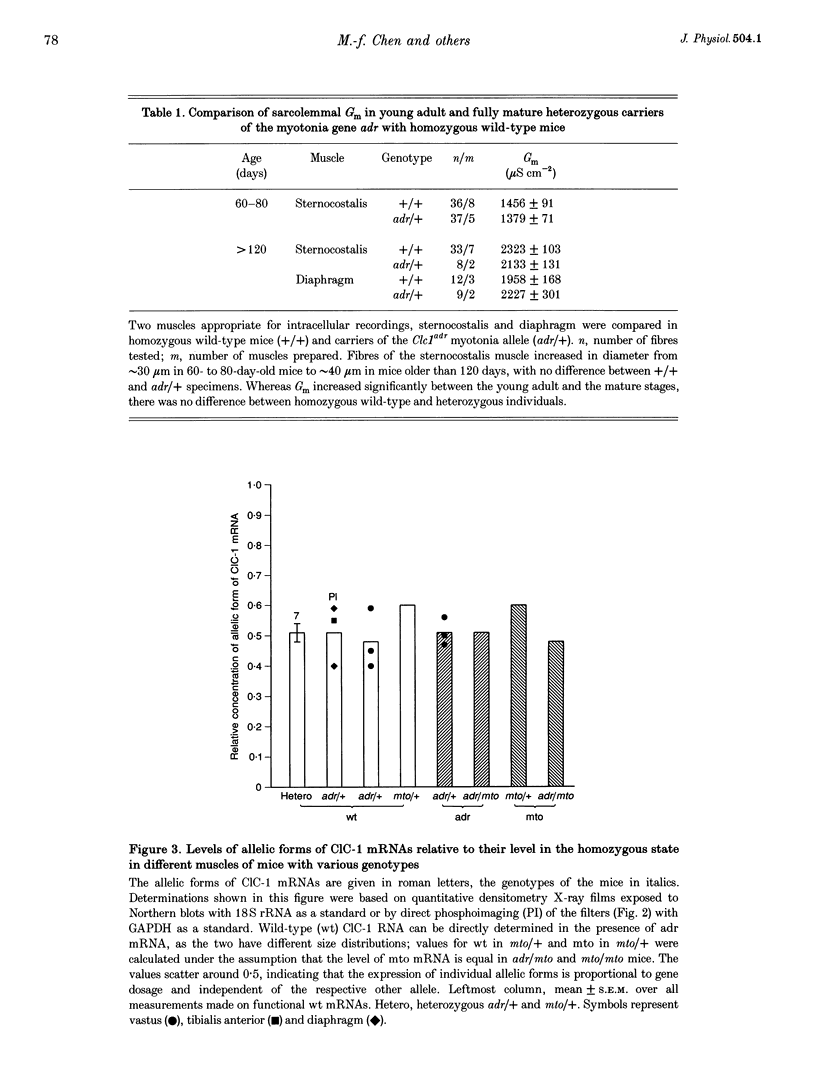
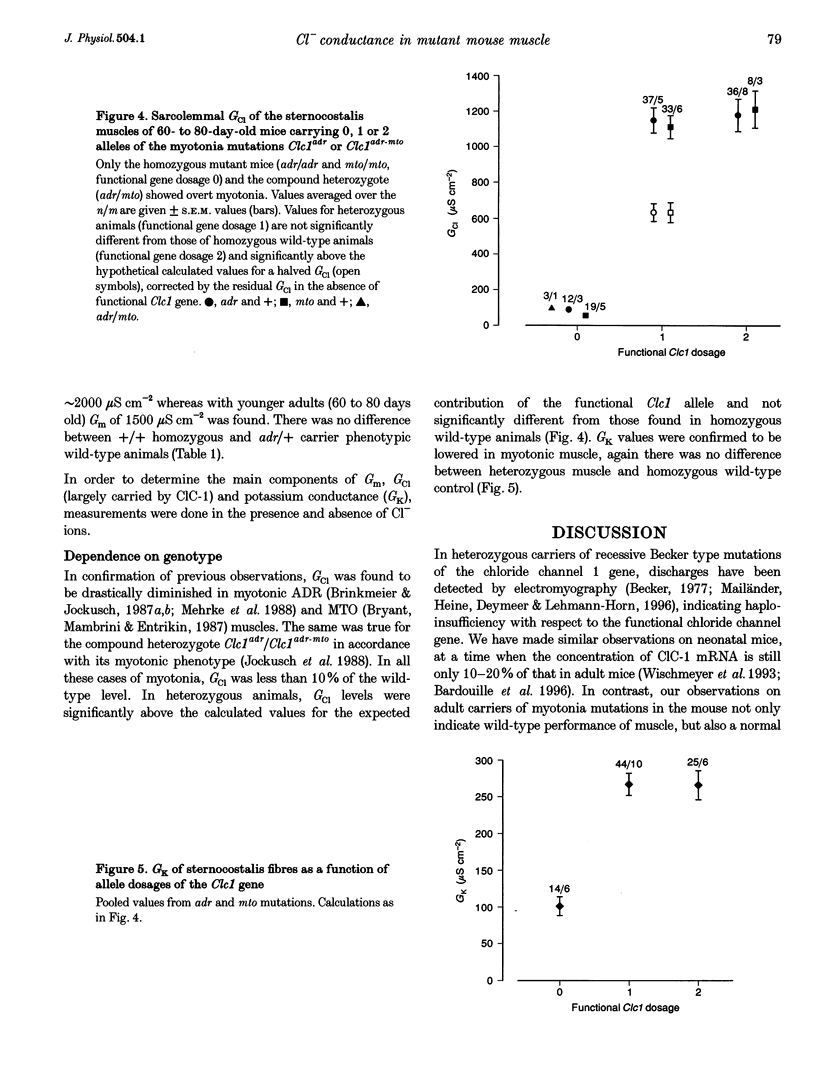
1. In mature mammalian muscle, the muscular chloride channel ClC-1 contributes about 75% of the sarcolemmal resting conductance (Gm). In mice carrying two defective alleles of the corresponding Clc1 gene, chloride conductance (GCl) is reduced to less than 10% of that of wild-type, and this causes hyperexcitability, the salient feature of the disease myotonia. Potassium conductance (GK) values in myotonic mouse muscle fibres are lowered by about 60% compared with wild-type. 2. The defective Clcadr allele causes loss of the 4.5 kb ClC-1 mRNA. Mice heterozygous for the defective Clc1adr allele contain about 50% functional mRNA in their muscles compared with homozygous wild-type mice. 3. Despite a halved functional gene dosage, heterozygous muscles display an average GCl which is not significantly different from that of homozygous wild-type animals. The GK values in heterozygotes are also indistinguishable from homozygous wild-type animals. 4. These results indicate that a regulatory mechanism acting at the post-transcriptional level limits the density of ClC-1 channels. GK is probably indirectly regulated by muscle activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barchi R. L. Myotonia. An evaluation of the chloride hypothesis. Arch Neurol. 1975 Mar;32(3):175–180. doi: 10.1001/archneur.1975.00490450055007. [DOI] [PubMed] [Google Scholar]
- Bardouille C., Vullhorst D., Jockusch H. Expression of chloride channel 1 mRNA in cultured myogenic cells: a marker of myotube maturation. FEBS Lett. 1996 Nov 4;396(2-3):177–180. doi: 10.1016/0014-5793(96)01098-8. [DOI] [PubMed] [Google Scholar]
- Brinkmeier H., Jockusch H. Activators of protein kinase C induce myotonia by lowering chloride conductance in muscle. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1383–1389. doi: 10.1016/s0006-291x(87)80285-1. [DOI] [PubMed] [Google Scholar]
- Bryant S. H. Cable properties of external intercostal muscle fibres from myotonic and nonmyotonic goats. J Physiol. 1969 Oct;204(3):539–550. doi: 10.1113/jphysiol.1969.sp008930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronemeier M., Condie A., Prosser J., Steinmeyer K., Jentsch T. J., Jockusch H. Nonsense and missense mutations in the muscular chloride channel gene Clc-1 of myotonic mice. J Biol Chem. 1994 Feb 25;269(8):5963–5967. [PubMed] [Google Scholar]
- Gurnett C. A., Kahl S. D., Anderson R. D., Campbell K. P. Absence of the skeletal muscle sarcolemma chloride channel ClC-1 in myotonic mice. J Biol Chem. 1995 Apr 21;270(16):9035–9038. doi: 10.1074/jbc.270.16.9035. [DOI] [PubMed] [Google Scholar]
- Heller A. H., Eicher E. M., Hallett M., Sidman R. L. Myotonia, a new inherited muscle disease in mice. J Neurosci. 1982 Jul;2(7):924–933. doi: 10.1523/JNEUROSCI.02-07-00924.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch H., Bertram K., Schenk S. The genes for two neuromuscular diseases of the mouse, 'arrested development of righting response', adr, and 'myotonia', mto, are allelic. Genet Res. 1988 Dec;52(3):203–205. doi: 10.1017/s001667230002766x. [DOI] [PubMed] [Google Scholar]
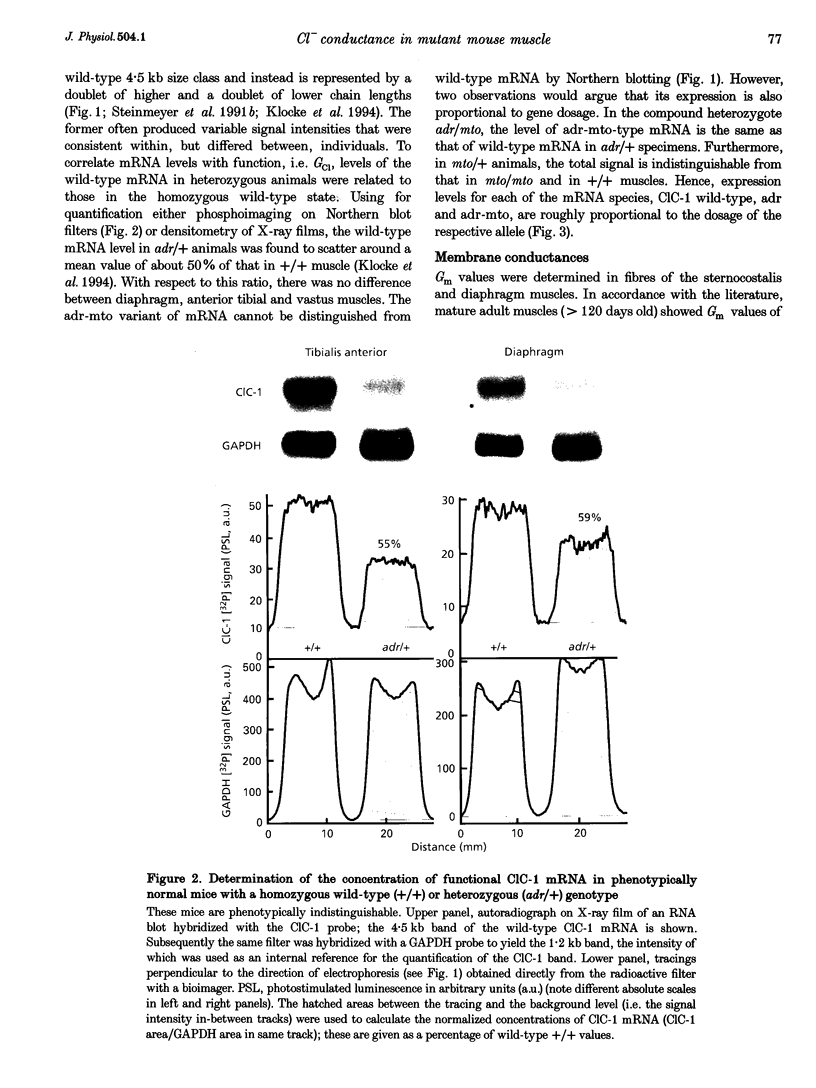
- Klocke R., Steinmeyer K., Jentsch T. J., Jockusch H. Role of innervation, excitability, and myogenic factors in the expression of the muscular chloride channel ClC-1. A study on normal and myotonic muscle. J Biol Chem. 1994 Nov 4;269(44):27635–27639. [PubMed] [Google Scholar]
- Koch M. C., Steinmeyer K., Lorenz C., Ricker K., Wolf F., Otto M., Zoll B., Lehmann-Horn F., Grzeschik K. H., Jentsch T. J. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992 Aug 7;257(5071):797–800. doi: 10.1126/science.1379744. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F., Küther G., Ricker K., Grafe P., Ballanyi K., Rüdel R. Adynamia episodica hereditaria with myotonia: a non-inactivating sodium current and the effect of extracellular pH. Muscle Nerve. 1987 May;10(4):363–374. doi: 10.1002/mus.880100414. [DOI] [PubMed] [Google Scholar]
- Mailänder V., Heine R., Deymeer F., Lehmann-Horn F. Novel muscle chloride channel mutations and their effects on heterozygous carriers. Am J Hum Genet. 1996 Feb;58(2):317–324. [PMC free article] [PubMed] [Google Scholar]
- Mehrke G., Brinkmeier H., Jockusch H. The myotonic mouse mutant ADR: electrophysiology of the muscle fiber. Muscle Nerve. 1988 May;11(5):440–446. doi: 10.1002/mus.880110505. [DOI] [PubMed] [Google Scholar]
- Pusch M., Steinmeyer K., Jentsch T. J. Low single channel conductance of the major skeletal muscle chloride channel, ClC-1. Biophys J. 1994 Jan;66(1):149–152. doi: 10.1016/S0006-3495(94)80753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmeyer K., Klocke R., Ortland C., Gronemeier M., Jockusch H., Gründer S., Jentsch T. J. Inactivation of muscle chloride channel by transposon insertion in myotonic mice. Nature. 1991 Nov 28;354(6351):304–308. doi: 10.1038/354304a0. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K., Ortland C., Jentsch T. J. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature. 1991 Nov 28;354(6351):301–304. doi: 10.1038/354301a0. [DOI] [PubMed] [Google Scholar]
- Watkins W. J., Watts D. C. Biological features of the new A2G--adr mouse mutant with abnormal muscle function. Lab Anim. 1984 Jan;18(1):1–6. doi: 10.1258/002367784780865036. [DOI] [PubMed] [Google Scholar]
- Wischmeyer E., Nolte E., Klocke R., Jockusch H., Brinkmeier H. Development of electrical myotonia in the ADR mouse: role of chloride conductance in myotubes and neonatal animals. Neuromuscul Disord. 1993 Jul;3(4):267–274. doi: 10.1016/0960-8966(93)90019-g. [DOI] [PubMed] [Google Scholar]