Abstract
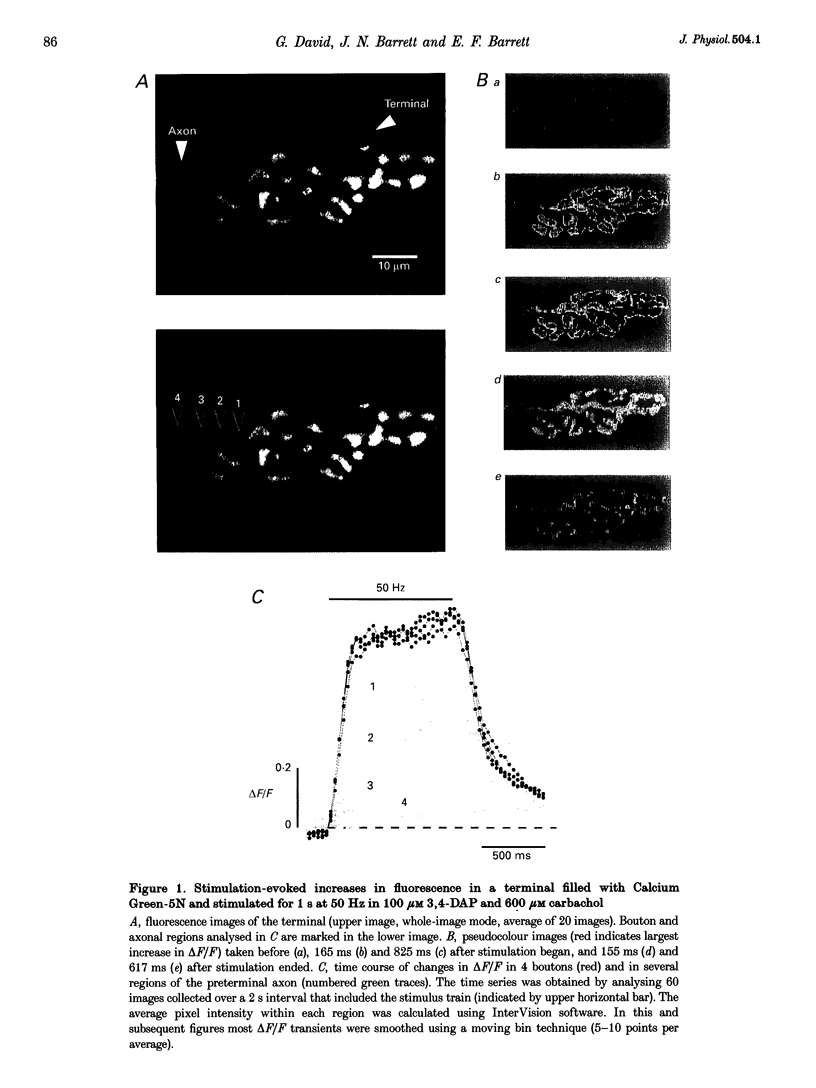
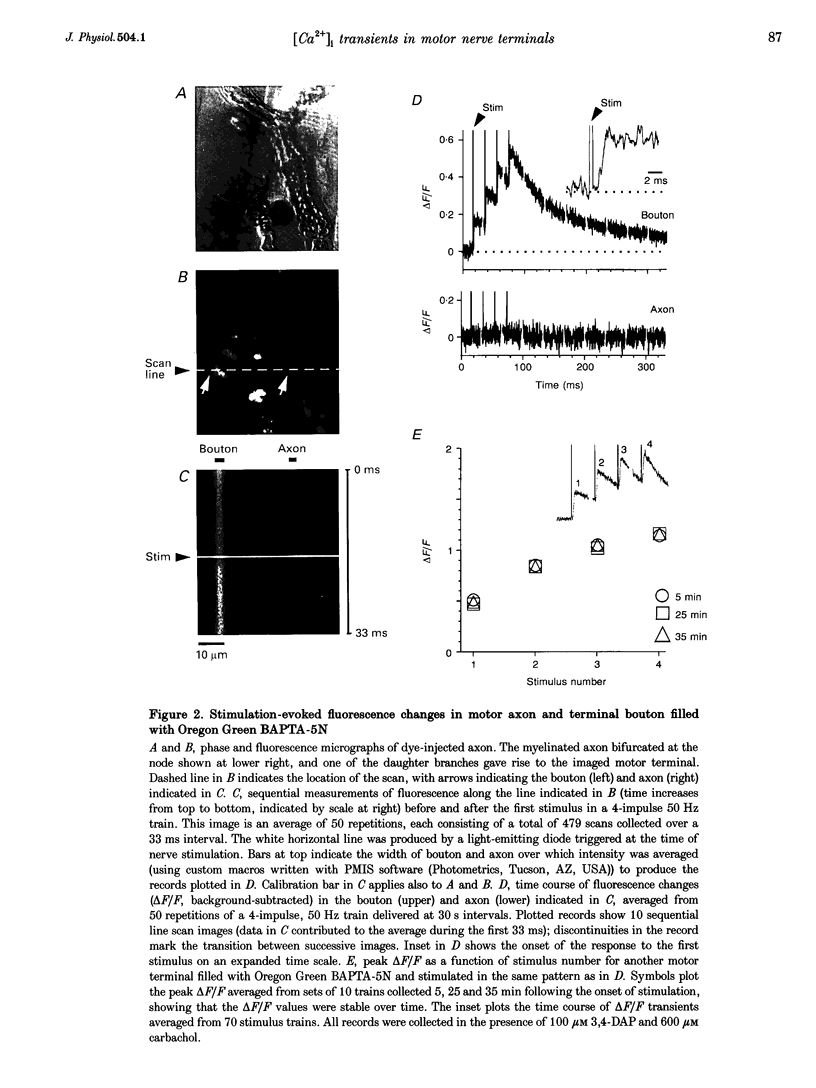
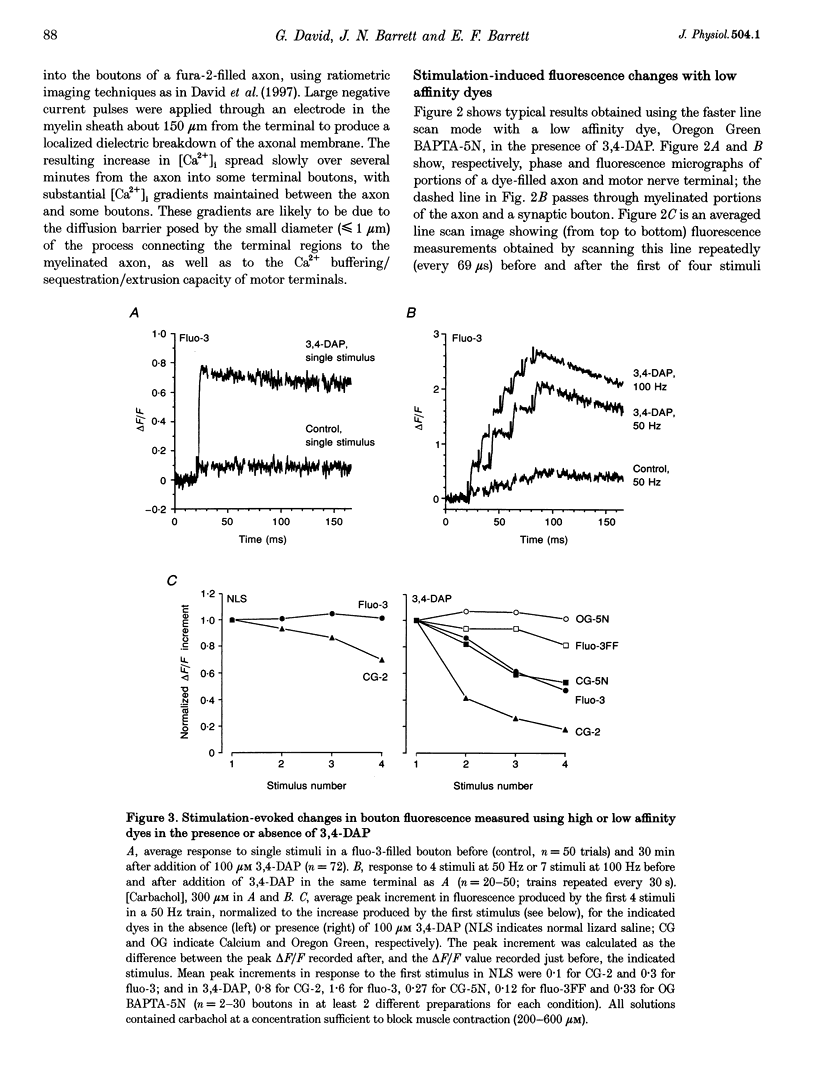
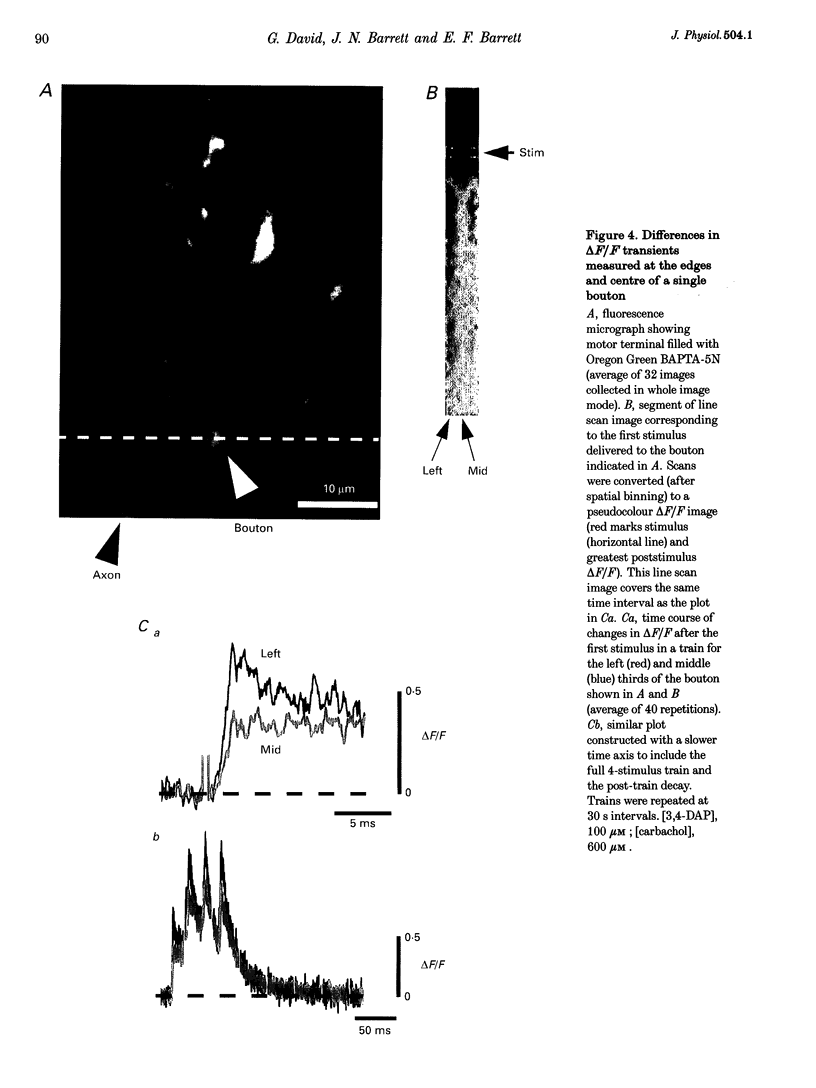
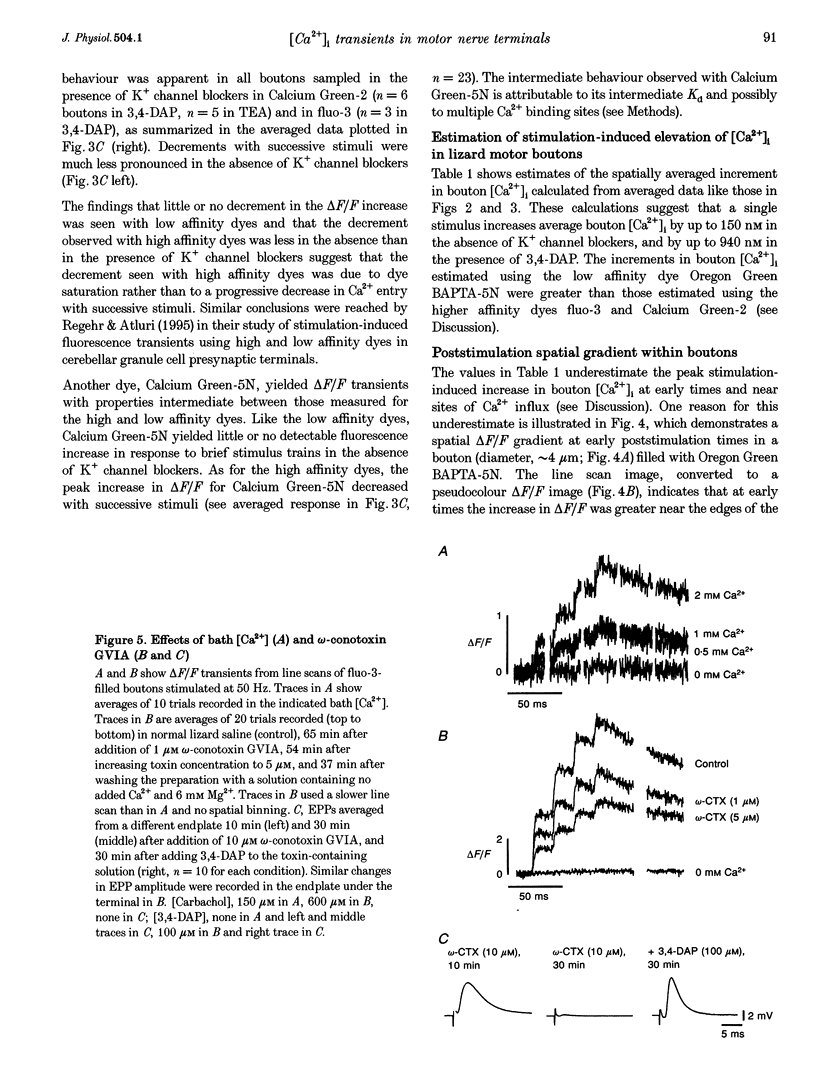
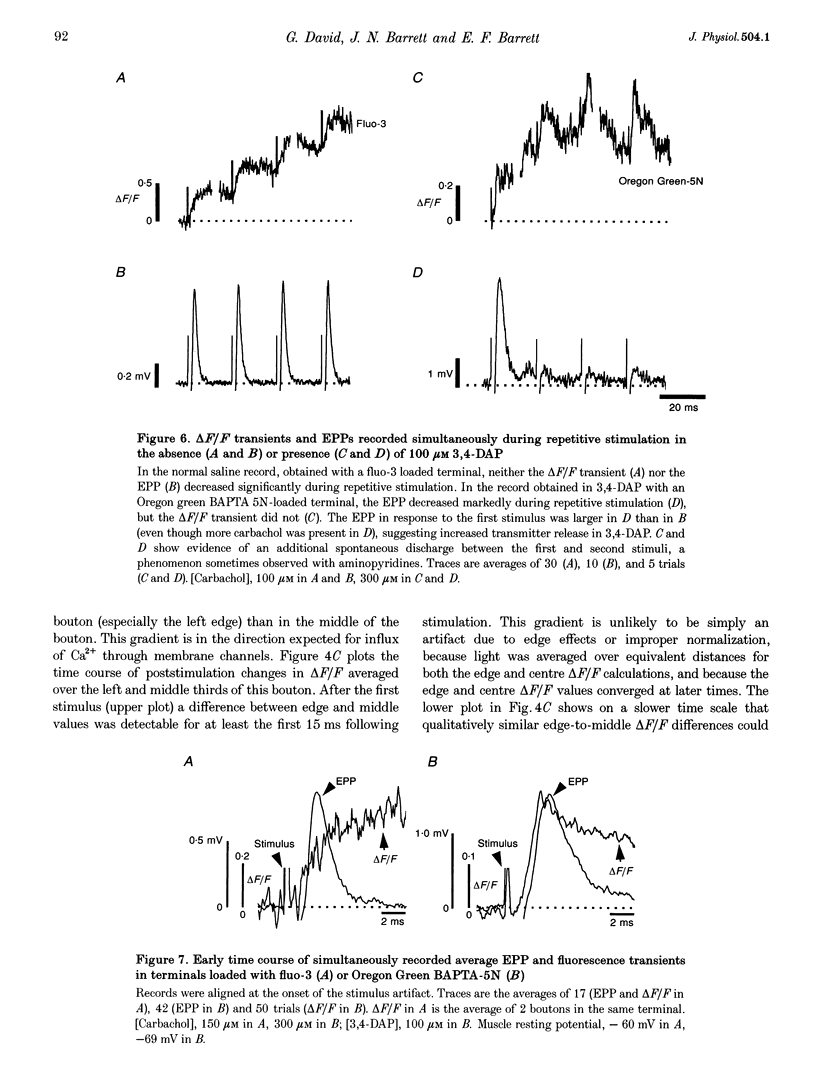
1. Motor axons were injected ionophoretically with one of five Ca(2+)-sensitive dyes (fluo-3, Calcium Green-2, Calcium Green-5N, fluo-3FF and Oregon Green BAPTA-5N). Changes in fluorescence (delta F/Frest) within motor terminal boutons following a single action potential and brief stimulus trains were monitored with high temporal resolution using a confocal microscope. 2. Stimulation-induced increases in delta F/Frest were confined primarily to boutons, with roughly uniform increases in all the boutons of a terminal. The increase in delta F/Frest began prior to, and decayed more slowly than, the endplate potential (EPP) recorded in the underlying muscle fibre. delta F/Frest was graded with bath [Ca2+]. Both delta F/Frest and the EPP were reduced, but not eliminated, by omega-conotoxin GVIA (5-10 microM). 3. For dyes with lower affinity for Ca2+ (e.g. Oregon Green BAPTA-5N, Kd approximately 60 microM) stimulation-induced increases in delta F/Frest were measured in the presence of the K+ channel blocker 3,4-diaminopyridine (3,4-DAP, 100 microM). During brief stimulus trains (4 at 50 Hz) in 3,4-DAP, the EPP exhibited profound depression, but the fluorescence increase associated with each stimulus showed little decrement, suggesting that depression was not mediated by a reduction in Ca2+ entry. 4. For dyes with a higher affinity for Ca2+ (e.g. fluo-3, Kd approximately 0.5-1 microM) stimulation-induced increases in delta F/Frest could also be measured in normal physiological saline. Increases in delta F/Frest were much greater with 3,4-DAP present, but the amplitude decreased with successive stimuli due to partial dye saturation. 5. Calculations suggested that following a single action potential the average [Ca2+] within a bouton increased by up to 150 nM in normal saline and 940 nM in 3,4-DAP. With low affinity dyes the delta F/Frest measured near the membrane had a higher peak amplitude and a faster early decay than that measured in the centre of the bouton, suggesting that substantial spatial [Ca2+] gradients exist within boutons for at least 15 ms following stimulation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Koyano K., Saisu H., Nishiuchi Y., Sakakibara S. Binding of omega-conotoxin to receptor sites associated with the voltage-sensitive calcium channel. Neurosci Lett. 1986 Nov 11;71(2):203–208. doi: 10.1016/0304-3940(86)90559-8. [DOI] [PubMed] [Google Scholar]
- Adler E. M., Augustine G. J., Duffy S. N., Charlton M. P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991 Jun;11(6):1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angaut-Petit D., Benoit E., Mallart A. Membrane currents in lizard motor nerve terminals and nodes of Ranvier. Pflugers Arch. 1989 Oct;415(1):81–87. doi: 10.1007/BF00373144. [DOI] [PubMed] [Google Scholar]
- Atluri P. P., Regehr W. G. Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse. J Neurosci. 1996 Sep 15;16(18):5661–5671. doi: 10.1523/JNEUROSCI.16-18-05661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium action in synaptic transmitter release. Annu Rev Neurosci. 1987;10:633–693. doi: 10.1146/annurev.ne.10.030187.003221. [DOI] [PubMed] [Google Scholar]
- Barhanin J., Schmid A., Lazdunski M. Properties of structure and interaction of the receptor for omega-conotoxin, a polypeptide active on Ca2+ channels. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1051–1062. doi: 10.1016/0006-291x(88)90736-x. [DOI] [PubMed] [Google Scholar]
- Borst J. G., Helmchen F., Sakmann B. Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J Physiol. 1995 Dec 15;489(Pt 3):825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain K. L., Bennett M. R. Calcium in the nerve terminals of chick ciliary ganglia during facilitation, augmentation and potentiation. J Physiol. 1995 Dec 15;489(Pt 3):637–648. doi: 10.1113/jphysiol.1995.sp021079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigant J. L., Mallart A. Presynaptic currents in mouse motor endings. J Physiol. 1982 Dec;333:619–636. doi: 10.1113/jphysiol.1982.sp014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Smith S. J., Zucker R. S. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol. 1982 Feb;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- David G., Barrett J. N., Barrett E. F. Spatiotemporal gradients of intra-axonal [Na+] after transection and resealing in lizard peripheral myelinated axons. J Physiol. 1997 Jan 15;498(Pt 2):295–307. doi: 10.1113/jphysiol.1997.sp021858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney K. R., Zucker R. S., Tank D. W. Calcium in motor nerve terminals associated with posttetanic potentiation. J Neurosci. 1989 Oct;9(10):3558–3567. doi: 10.1523/JNEUROSCI.09-10-03558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Luebke J. I., Turner T. J. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995 Feb;18(2):89–98. [PubMed] [Google Scholar]
- Eilers J., Callewaert G., Armstrong C., Konnerth A. Calcium signaling in a narrow somatic submembrane shell during synaptic activity in cerebellar Purkinje neurons. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10272–10276. doi: 10.1073/pnas.92.22.10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller M. B., Delaney K. R., Tank D. W. Presynaptic calcium dynamics at the frog retinotectal synapse. J Neurophysiol. 1996 Jul;76(1):381–400. doi: 10.1152/jn.1996.76.1.381. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Heidelberger R., Heinemann C., Neher E., Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994 Oct 6;371(6497):513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- Helmchen F., Borst J. G., Sakmann B. Calcium dynamics associated with a single action potential in a CNS presynaptic terminal. Biophys J. 1997 Mar;72(3):1458–1471. doi: 10.1016/S0006-3495(97)78792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingauf J., Neher E. Modeling buffered Ca2+ diffusion near the membrane: implications for secretion in neuroendocrine cells. Biophys J. 1997 Feb;72(2 Pt 1):674–690. doi: 10.1016/s0006-3495(97)78704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landò L., Zucker R. S. Ca2+ cooperativity in neurosecretion measured using photolabile Ca2+ chelators. J Neurophysiol. 1994 Aug;72(2):825–830. doi: 10.1152/jn.1994.72.2.825. [DOI] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr, Bartschat D. K. The effect of pH on rate constants, ion selectivity and thermodynamic properties of fluorescent calcium and magnesium indicators. Biochem Biophys Res Commun. 1991 May 31;177(1):184–191. doi: 10.1016/0006-291x(91)91966-g. [DOI] [PubMed] [Google Scholar]
- Lindgren C. A., Moore J. W. Calcium current in motor nerve endings of the lizard. Ann N Y Acad Sci. 1991;635:58–69. doi: 10.1111/j.1749-6632.1991.tb36481.x. [DOI] [PubMed] [Google Scholar]
- Lindgren C. A., Moore J. W. Identification of ionic currents at presynaptic nerve endings of the lizard. J Physiol. 1989 Jul;414:201–222. doi: 10.1113/jphysiol.1989.sp017684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Silver R. B. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992 May 1;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- Mallart A. A calcium-activated potassium current in motor nerve terminals of the mouse. J Physiol. 1985 Nov;368:577–591. doi: 10.1113/jphysiol.1985.sp015877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed N., Rahamimoff R. Confocal microscopy of the lizard motor nerve terminals. J Basic Clin Physiol Pharmacol. 1991 Jan-Jun;2(1-2):63–85. doi: 10.1515/jbcpp.1991.2.1-2.63. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Calcium transients recorded with arsenazo III in the presynaptic terminal of the squid giant synapse. Proc R Soc Lond B Biol Sci. 1981 May 22;212(1187):197–211. doi: 10.1098/rspb.1981.0034. [DOI] [PubMed] [Google Scholar]
- Morita K., Barrett E. F. Calcium-dependent depolarizations originating in lizard motor nerve terminals. J Neurosci. 1989 Sep;9(9):3359–3369. doi: 10.1523/JNEUROSCI.09-09-03359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., Barrett E. F. Evidence for two calcium-dependent potassium conductances in lizard motor nerve terminals. J Neurosci. 1990 Aug;10(8):2614–2625. doi: 10.1523/JNEUROSCI.10-08-02614.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas H., Parnas I. Neurotransmitter release at fast synapses. J Membr Biol. 1994 Dec;142(3):267–279. doi: 10.1007/BF00233434. [DOI] [PubMed] [Google Scholar]
- Petrozzino J. J., Pozzo Miller L. D., Connor J. A. Micromolar Ca2+ transients in dendritic spines of hippocampal pyramidal neurons in brain slice. Neuron. 1995 Jun;14(6):1223–1231. doi: 10.1016/0896-6273(95)90269-4. [DOI] [PubMed] [Google Scholar]
- Redman R. S., Silinsky E. M. ATP released together with acetylcholine as the mediator of neuromuscular depression at frog motor nerve endings. J Physiol. 1994 May 15;477(Pt 1):117–127. doi: 10.1113/jphysiol.1994.sp020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr W. G., Atluri P. P. Calcium transients in cerebellar granule cell presynaptic terminals. Biophys J. 1995 May;68(5):2156–2170. doi: 10.1016/S0006-3495(95)80398-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala F., Hernández-Cruz A. Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys J. 1990 Feb;57(2):313–324. doi: 10.1016/S0006-3495(90)82533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. Imaging of calcium variations in living dendritic spines of cultured rat hippocampal neurons. J Physiol. 1995 Jul 15;486(Pt 2):283–295. doi: 10.1113/jphysiol.1995.sp020811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J. Calcium ions, active zones and synaptic transmitter release. Trends Neurosci. 1988 Oct;11(10):458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Svoboda K., Tank D. W., Denk W. Direct measurement of coupling between dendritic spines and shafts. Science. 1996 May 3;272(5262):716–719. doi: 10.1126/science.272.5262.716. [DOI] [PubMed] [Google Scholar]
- Tucker T. R., Fettiplace R. Monitoring calcium in turtle hair cells with a calcium-activated potassium channel. J Physiol. 1996 Aug 1;494(Pt 3):613–626. doi: 10.1113/jphysiol.1996.sp021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker T., Fettiplace R. Confocal imaging of calcium microdomains and calcium extrusion in turtle hair cells. Neuron. 1995 Dec;15(6):1323–1335. doi: 10.1016/0896-6273(95)90011-x. [DOI] [PubMed] [Google Scholar]
- Ukhanov K. Y., Flores T. M., Hsiao H. S., Mohapatra P., Pitts C. H., Payne R. Measurement of cytosolic Ca2+ concentration in Limulus ventral photoreceptors using fluorescent dyes. J Gen Physiol. 1995 Jan;105(1):95–116. doi: 10.1085/jgp.105.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemiya M., Berger A. J. Single-channel properties of four calcium channel types in rat motoneurons. J Neurosci. 1995 Mar;15(3 Pt 2):2218–2224. doi: 10.1523/JNEUROSCI.15-03-02218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. A., Snowman A. M., Biswas A., Olivera B. M., Snyder S. H. Omega-conotoxin GVIA binding to a high-affinity receptor in brain: characterization, calcium sensitivity, and solubilization. J Neurosci. 1988 Sep;8(9):3354–3359. doi: 10.1523/JNEUROSCI.08-09-03354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walrond J. P., Reese T. S. Structure of axon terminals and active zones at synapses on lizard twitch and tonic muscle fibers. J Neurosci. 1985 May;5(5):1118–1131. doi: 10.1523/JNEUROSCI.05-05-01118.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witcher D. R., De Waard M., Campbell K. P. Characterization of the purified N-type Ca2+ channel and the cation sensitivity of omega-conotoxin GVIA binding. Neuropharmacology. 1993 Nov;32(11):1127–1139. doi: 10.1016/0028-3908(93)90007-p. [DOI] [PubMed] [Google Scholar]
- Yamada W. M., Zucker R. S. Time course of transmitter release calculated from simulations of a calcium diffusion model. Biophys J. 1992 Mar;61(3):671–682. doi: 10.1016/S0006-3495(92)81872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H., Chuhma N. Omega-conotoxin-sensitive and -resistant transmitter release from the chick ciliary presynaptic terminal. J Physiol. 1994 Jun 15;477(Pt 3):437–448. doi: 10.1113/jphysiol.1994.sp020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikami D., Bagabaldo Z., Olivera B. M. The inhibitory effects of omega-conotoxins on Ca channels and synapses. Ann N Y Acad Sci. 1989;560:230–248. doi: 10.1111/j.1749-6632.1989.tb24100.x. [DOI] [PubMed] [Google Scholar]