Abstract
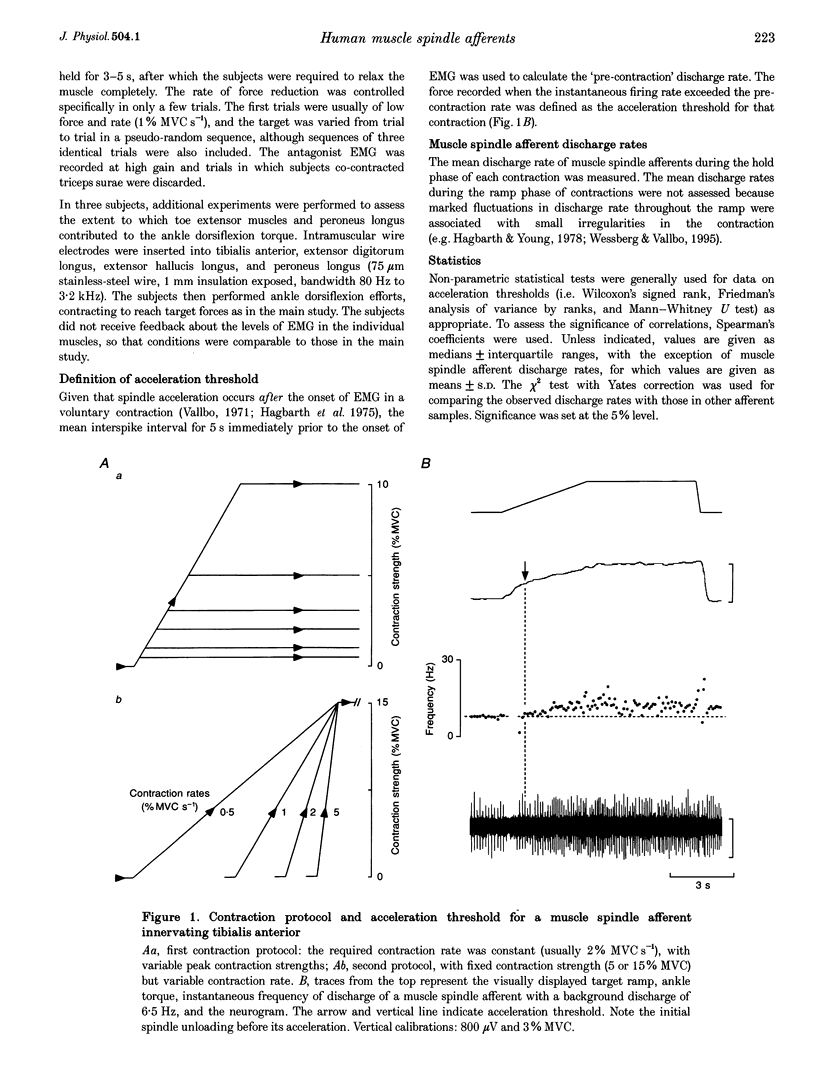
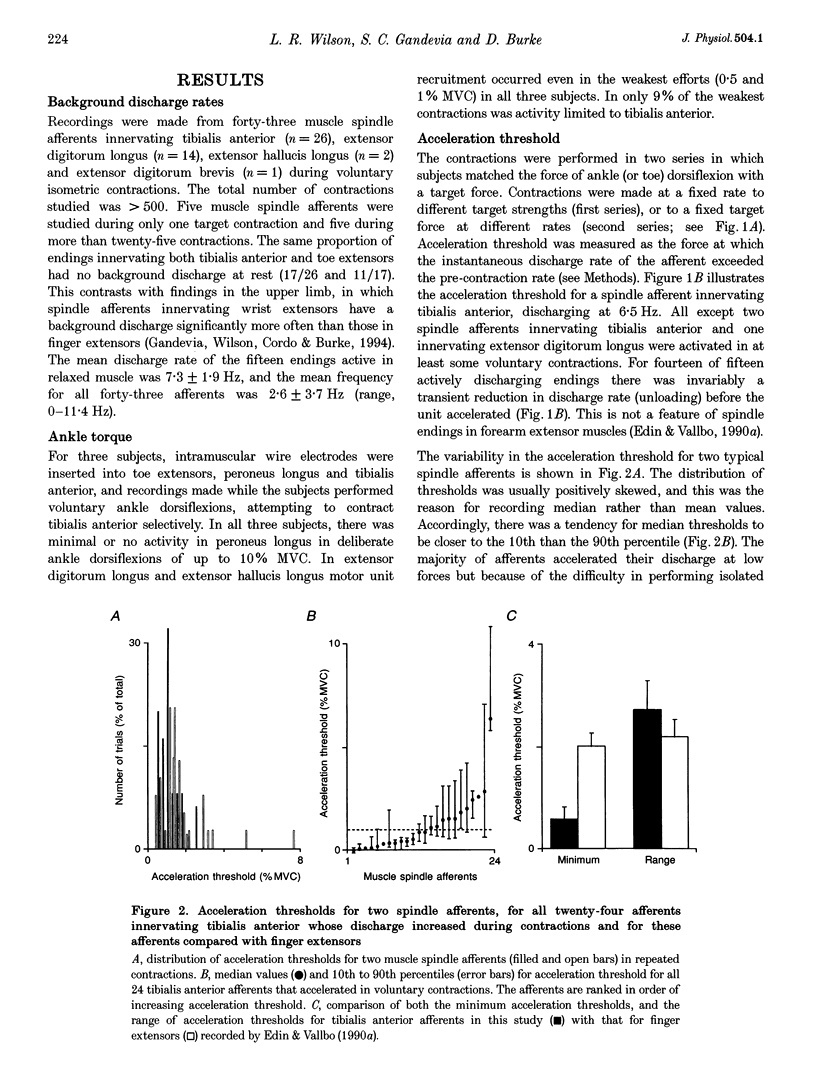
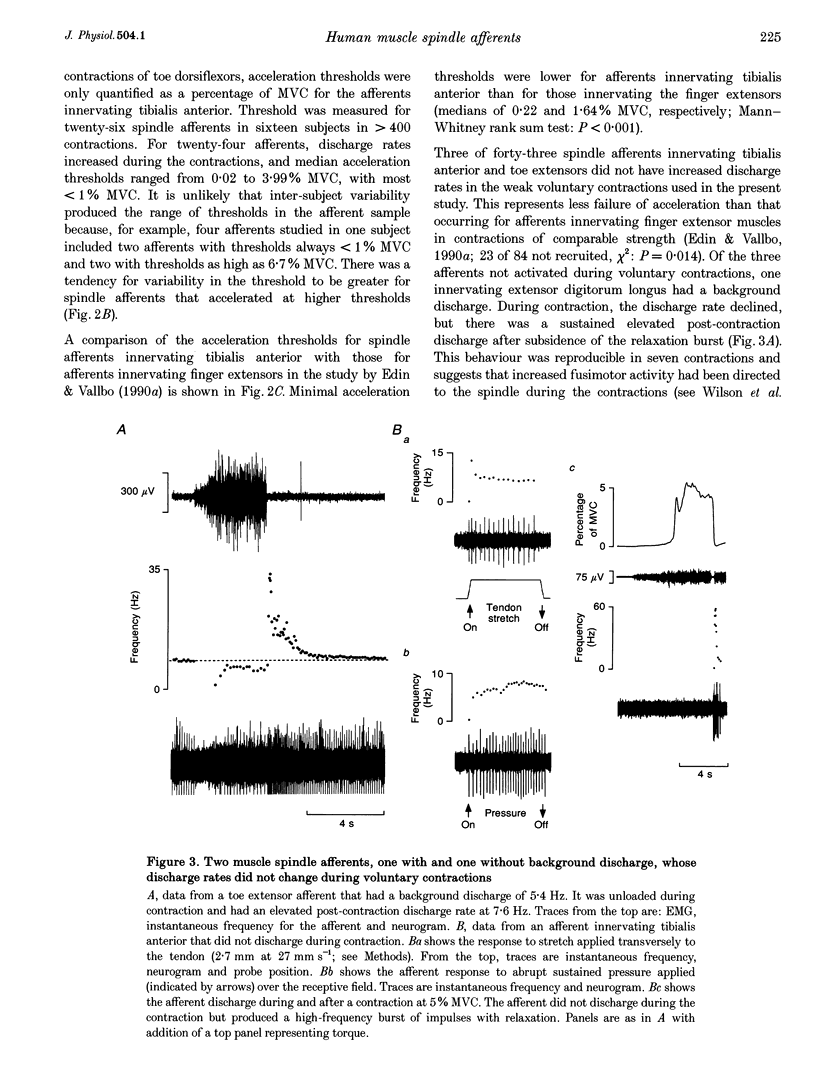
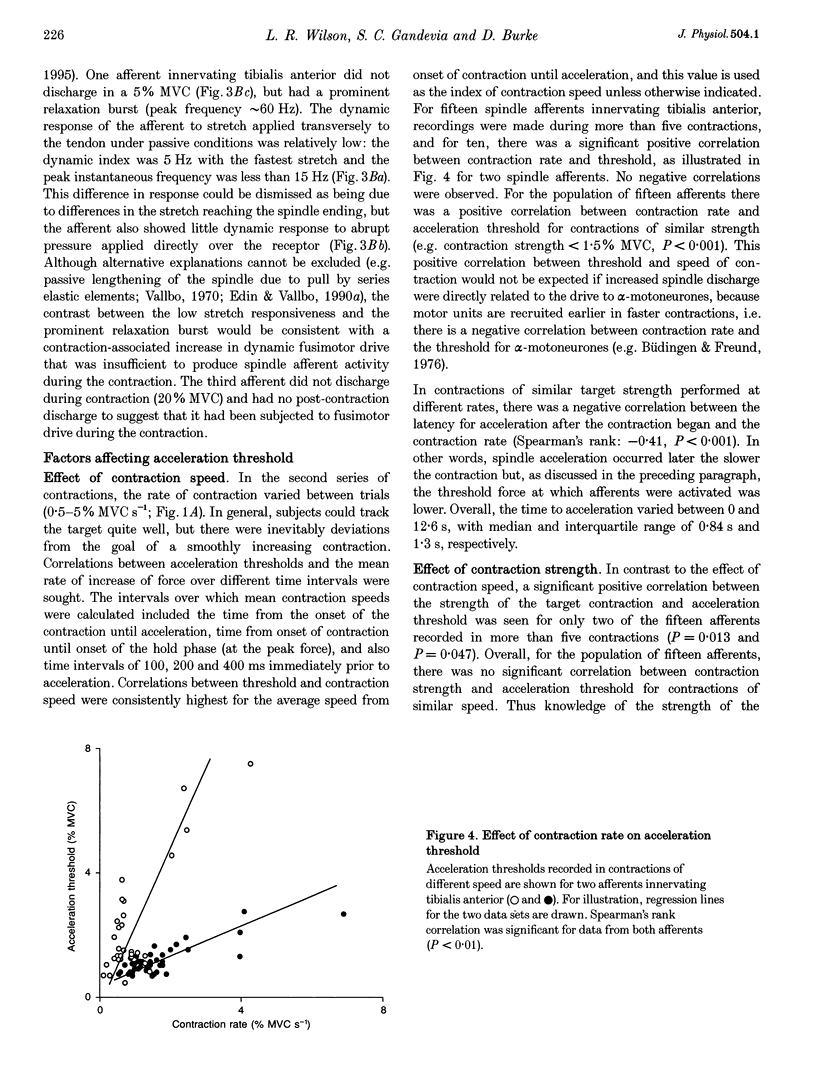
1. There are discrepancies in the literature about the reproducibility of forces at which human muscle spindle afferents accelerate their discharge during isometric voluntary contractions. The aim of this study was to determine for single muscle spindle afferents both the reproducibility of the 'acceleration threshold' and the factors contributing to variability of 'acceleration threshold'. 2. Microneurographic recordings were made from muscle spindle afferents innervating tibialis anterior while subjects performed isometric ankle dorsiflexions. Subjects matched the force of their contractions with a visually displayed 'ramp-and-hold' template. Template parameters were determined by the force of maximal isometric ankle dorsiflexion (MVC), and expressed as per cent MVC. The required 'ramp' rate and 'hold' force was adjusted between trials (range, 0.5-5% MVCs-1 and 0.5-20% MVC, respectively). The duration of the hold phase was 4 s and, following each contraction, stretch was applied transversely to the tendon to minimize the influence of any 'after-effects' on spindle afferent responses in subsequent contractions. 3. For each contraction, the force at which the rate of muscle spindle discharge increased was defined as the 'acceleration threshold'. Of twenty-six muscle spindle afferents innervating tibialis anterior, all but two increased their discharge in the test contractions. In 90% of contractions, acceleration thresholds were less than 3.2% MVC (range, 0.01-11.9% MVC). 4. Individual muscle spindle afferents increased their discharge at similar but not identical forces in repeated contractions. There was a positive correlation between the rate of contraction and the acceleration threshold (P < 0.001), but the strength of the target contraction had no effect on the threshold, and there was no trend for thresholds to change over time. 5. The results suggest, first, that most muscle spindle endings in the human pretibial muscles receive a significant increase in fusimotor drive during relatively weak isometric efforts and secondly, that when fusimotor after-effects are controlled, much of the residual variability in 'acceleration threshold' for any one spindle in repeated contractions is due to extrafusal factors, particularly variability in contraction rate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. W., Gandevia S. C., Skuse N. F. The distribution of muscle weakness in upper motoneuron lesions affecting the lower limb. Brain. 1990 Oct;113(Pt 5):1459–1476. doi: 10.1093/brain/113.5.1459. [DOI] [PubMed] [Google Scholar]
- Al-Falahe N. A., Vallbo A. B. Role of the human fusimotor system in a motor adaptation task. J Physiol. 1988 Jul;401:77–95. doi: 10.1113/jphysiol.1988.sp017152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann T. K., Hulliger M. The dependence of the response of cat spindle Ia afferents to sinusoidal stretch on the velocity of concomitant movement. J Physiol. 1991 Aug;439:325–350. doi: 10.1113/jphysiol.1991.sp018669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Goodwin G. M., Matthews P. B. After-effects of fusimotor stimulation on the response of muscle spindle primary afferent endings. J Physiol. 1969 Dec;205(3):677–694. doi: 10.1113/jphysiol.1969.sp008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Aniss A. M., Gandevia S. C. In-parallel and in-series behavior of human muscle spindle endings. J Neurophysiol. 1987 Aug;58(2):417–426. doi: 10.1152/jn.1987.58.2.417. [DOI] [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Skuse N. F. Recruitment order of human spindle endings in isometric voluntary contractions. J Physiol. 1978 Dec;285:101–112. doi: 10.1113/jphysiol.1978.sp012560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Skuse N. F. Voluntary activation of spindle endings in human muscles temporarily paralysed by nerve pressure. J Physiol. 1979 Feb;287:329–336. doi: 10.1113/jphysiol.1979.sp012662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., McKeon B., Westerman R. A. Induced changes in the thresholds for voluntary activation of human spindle endings. J Physiol. 1980 May;302:171–181. doi: 10.1113/jphysiol.1980.sp013236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büdingen H. J., Freund H. J. The relationship between the rate of rise of isometric tension and motor unit recruitment in a human forearm muscle. Pflugers Arch. 1976 Mar 11;362(1):61–67. doi: 10.1007/BF00588682. [DOI] [PubMed] [Google Scholar]
- Cordo P., Gandevia S. C., Hales J. P., Burke D., Laird G. Force and displacement-controlled tendon vibration in humans. Electroencephalogr Clin Neurophysiol. 1993 Feb;89(1):45–53. doi: 10.1016/0168-5597(93)90084-3. [DOI] [PubMed] [Google Scholar]
- Edin B. B., Vallbo A. B. Classification of human muscle stretch receptor afferents: a Bayesian approach. J Neurophysiol. 1990 Jun;63(6):1314–1322. doi: 10.1152/jn.1990.63.6.1314. [DOI] [PubMed] [Google Scholar]
- Edin B. B., Vallbo A. B. Muscle afferent responses to isometric contractions and relaxations in humans. J Neurophysiol. 1990 Jun;63(6):1307–1313. doi: 10.1152/jn.1990.63.6.1307. [DOI] [PubMed] [Google Scholar]
- Fukunaga T., Roy R. R., Shellock F. G., Hodgson J. A., Day M. K., Lee P. L., Kwong-Fu H., Edgerton V. R. Physiological cross-sectional area of human leg muscles based on magnetic resonance imaging. J Orthop Res. 1992 Nov;10(6):928–934. doi: 10.1002/jor.1100100623. [DOI] [PubMed] [Google Scholar]
- Gandevia S. C., Macefield G., Burke D., McKenzie D. K. Voluntary activation of human motor axons in the absence of muscle afferent feedback. The control of the deafferented hand. Brain. 1990 Oct;113(Pt 5):1563–1581. doi: 10.1093/brain/113.5.1563. [DOI] [PubMed] [Google Scholar]
- Gandevia S. C., Wilson L., Cordo P. J., Burke D. Fusimotor reflexes in relaxed forearm muscles produced by cutaneous afferents from the human hand. J Physiol. 1994 Sep 15;479(Pt 3):499–508. doi: 10.1113/jphysiol.1994.sp020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNEMAN E., SOMJEN G., CARPENTER D. O. FUNCTIONAL SIGNIFICANCE OF CELL SIZE IN SPINAL MOTONEURONS. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Hagbarth K. E., Wallen G., Löfstedt L. Muscle spindle activity in man during voluntary fast alternating movements. J Neurol Neurosurg Psychiatry. 1975 Jul;38(7):625–635. doi: 10.1136/jnnp.38.7.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbreath S. L., Gandevia S. C. Limited independent flexion of the thumb and fingers in human subjects. J Physiol. 1994 Sep 15;479(Pt 3):487–497. doi: 10.1113/jphysiol.1994.sp020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau W. M. American Neurological Association. Presidential Address. Trans Am Neurol Assoc. 1978;103:1–2. [PubMed] [Google Scholar]
- Macefield G., Hagbarth K. E., Gorman R., Gandevia S. C., Burke D. Decline in spindle support to alpha-motoneurones during sustained voluntary contractions. J Physiol. 1991;440:497–512. doi: 10.1113/jphysiol.1991.sp018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield V. G., Gandevia S. C., Bigland-Ritchie B., Gorman R. B., Burke D. The firing rates of human motoneurones voluntarily activated in the absence of muscle afferent feedback. J Physiol. 1993 Nov;471:429–443. doi: 10.1113/jphysiol.1993.sp019908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol. 1986 May;374:73–90. doi: 10.1113/jphysiol.1986.sp016066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon B., Burke D. Identification of muscle spindle afferents during in vivo recordings in man. Electroencephalogr Clin Neurophysiol. 1980 May;48(5):606–608. doi: 10.1016/0013-4694(80)90297-7. [DOI] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol. 1973 Apr;230(2):359–370. doi: 10.1113/jphysiol.1973.sp010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. L., Prochazka A., Proske U. The after-effects of stretch and fusimotor stimulation on the responses of primary endings of cat muscle spindles. J Physiol. 1984 Nov;356:465–477. doi: 10.1113/jphysiol.1984.sp015477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P. R. The recruitment order of gamma-motoneurones in the decerebrate rabbit. J Physiol. 1981 Jun;315:59–67. doi: 10.1113/jphysiol.1981.sp013732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A., Hulliger M., Zangger P., Appenteng K. 'Fusimotor set': new evidence for alpha-independent control of gamma-motoneurones during movement in the awake cat. Brain Res. 1985 Jul 22;339(1):136–140. doi: 10.1016/0006-8993(85)90632-8. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Trend P., Hulliger M., Vincent S. Ensemble proprioceptive activity in the cat step cycle: towards a representative look-up chart. Prog Brain Res. 1989;80:61–60. doi: 10.1016/s0079-6123(08)62200-1. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Westerman R. A., Ziccone S. P. Ia afferent activity during a variety of voluntary movements in the cat. J Physiol. 1977 Jun;268(2):423–448. doi: 10.1113/jphysiol.1977.sp011864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo A. B. Discharge patterns in human muscle spindle afferents during isometric voluntary contractions. Acta Physiol Scand. 1970 Dec;80(4):552–566. doi: 10.1111/j.1748-1716.1970.tb04823.x. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B. Human muscle spindle discharge during isometric voluntary contractions. Amplitude relations between spindle frequency and torque. Acta Physiol Scand. 1974 Feb;90(2):319–336. doi: 10.1111/j.1748-1716.1974.tb05594.x. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B. Muscle spindle response at the onset of isometric voluntary contractions in man. Time difference between fusimotor and skeletomotor effects. J Physiol. 1971 Oct;218(2):405–431. doi: 10.1113/jphysiol.1971.sp009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss H. Tabelle der abosluten und relativen Muskelspindelzahlen der menschlichen Skelettmuskulatur. Anat Anz. 1971;129(5):562–572. [PubMed] [Google Scholar]
- Wessberg J., Vallbo A. B. Coding of pulsatile motor output by human muscle afferents during slow finger movements. J Physiol. 1995 May 15;485(Pt 1):271–282. doi: 10.1113/jphysiol.1995.sp020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L. R., Gandevia S. C., Burke D. Increased resting discharge of human spindle afferents following voluntary contractions. J Physiol. 1995 Nov 1;488(Pt 3):833–840. doi: 10.1113/jphysiol.1995.sp021015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leva P. Adjustments to Zatsiorsky-Seluyanov's segment inertia parameters. J Biomech. 1996 Sep;29(9):1223–1230. doi: 10.1016/0021-9290(95)00178-6. [DOI] [PubMed] [Google Scholar]


