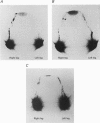
Abstract
1. The effects of dynamic and isometric muscle contractions on the lymph flow dynamics in human skeletal muscle were studied with a scintographic method. 2. Radioactively labelled human serum albumin (99mTc-HSA) was injected bilaterally into the vastus lateralis muscles of eight men (n = 16), four of whom had had an endurance training background. The subjects performed 100 submaximal contractions in 10 min as (i) dynamic knee extensions (CONS), (ii) isometric contractions with the knees at full extension (IMExt), or (iii) isometric contractions with knees fixed at 90 deg angle flexion (IMFlex). The exercises were separated by 65 min periods in supine rest. The level of radioactivity at the injection site was monitored by a gamma-camera, and the clearance rate of radioactivity (CR) was calculated as the fractional decrease during the periods of interest (CR unit = % min-1). 3. The clearance rate was low during the rest periods (0.04 +/- 0.05% min-1), though higher in the trained than in the sedentary subjects (0.06 +/- 0.05 vs. 0.03 +/- 0.03% min-1; P = 0.008). Exercise increased the clearance rate three- to sixfold, to 0.16 +/- 0.16% min-1 during CONS, 0.20 +/- 0.15% min-1 during IMExt and 0.09 +/- 0.11% min-1 during IMFlex. There were no differences between the subject subgroups. 4. The higher clearance rate during IMExt than during IMFlex (P = 0.02) demonstrates the importance of muscle deformations on lymph propulsion and experimentally confirms the current concepts of lymph formation and propulsion in voluntarily active skeletal muscle. It is suggested that lymph propulsion by working muscle is most efficient when the muscle is able to shorten close to its minimum length.
Full text
PDF


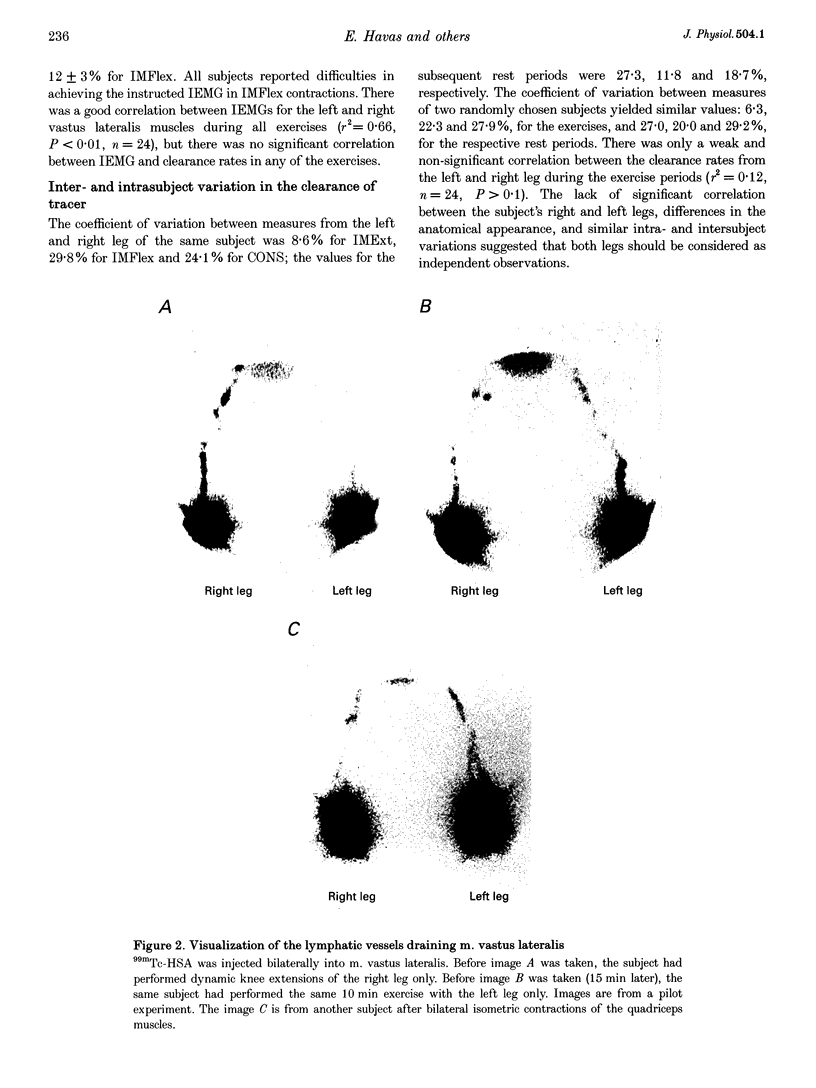
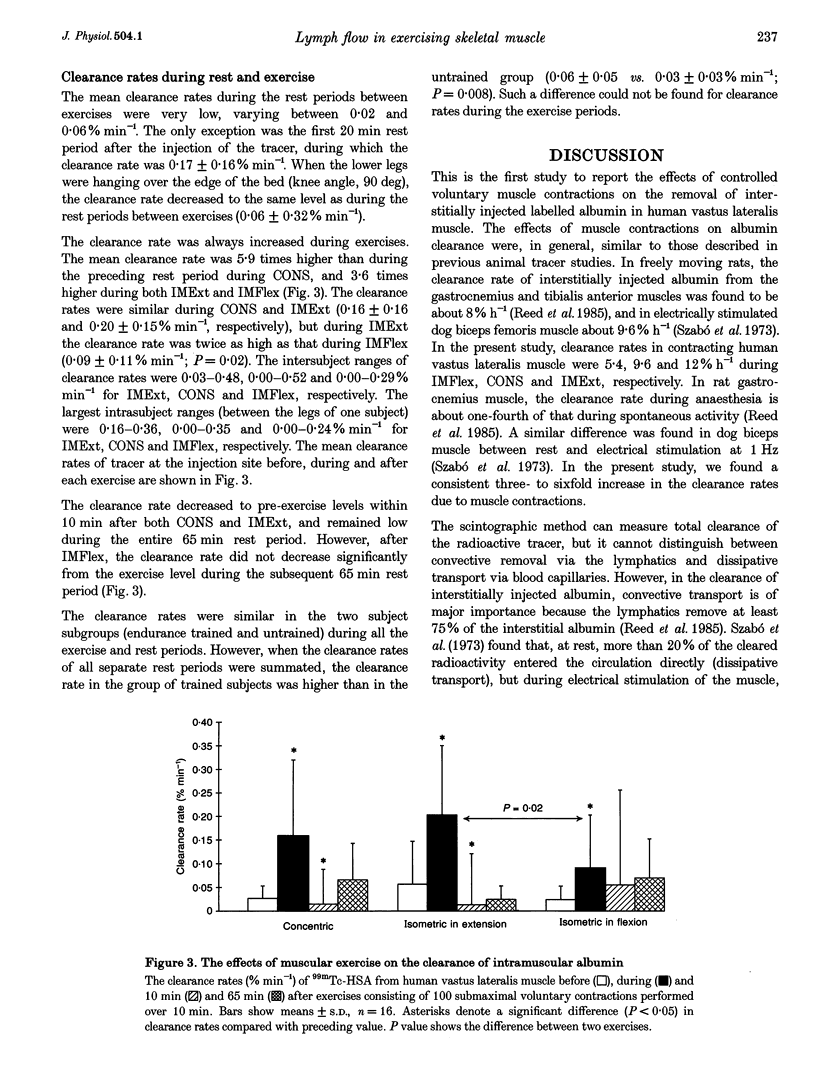


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aukland K., Reed R. K. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993 Jan;73(1):1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- Bach C., Lewis G. P. Lymph flow and lymph protein concentration in the skin and muscle of the rabbit hind limb. J Physiol. 1973 Dec;235(2):477–492. doi: 10.1113/jphysiol.1973.sp010398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates G., O'Brodovich H., Goeree G. Hindlimb and lung lymph flows during prolonged exercise. J Appl Physiol (1985) 1993 Aug;75(2):633–638. doi: 10.1152/jappl.1993.75.2.633. [DOI] [PubMed] [Google Scholar]
- Hickner R. C., Bone D., Ungerstedt U., Jorfeldt L., Henriksson J. Muscle blood flow during intermittent exercise: comparison of the microdialysis ethanol technique and 133Xe clearance. Clin Sci (Lond) 1994 Jan;86(1):15–25. doi: 10.1042/cs0860015. [DOI] [PubMed] [Google Scholar]
- JACOBSSON S., KJELLMER I. ACCUMULATION OF FLUID IN EXERCISING SKELETAL MUSCLE. Acta Physiol Scand. 1964 Mar;60:286–292. doi: 10.1111/j.1748-1716.1964.tb02890.x. [DOI] [PubMed] [Google Scholar]
- JACOBSSON S., KJELLMER I. FLOW AND PROTEIN CONTENT OF LYMPH IN RESTING AND EXERCISING SKELETAL MUSCLE. Acta Physiol Scand. 1964 Mar;60:278–285. doi: 10.1111/j.1748-1716.1964.tb02889.x. [DOI] [PubMed] [Google Scholar]
- Leppälä J., Kallio M., Nikula T., Nikkinen P., Liewendahl K., Jäskeläinen J., Savolainen S., Gylling H., Hiltunen J., Callaway J. Accumulation of 99mTc-low-density lipoprotein in human malignant glioma. Br J Cancer. 1995 Feb;71(2):383–387. doi: 10.1038/bjc.1995.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindena J., Küpper W., Trautschold I. Enzyme activities in thoracic duct lymph and plasma of anaesthetized, conscious resting and exercising dogs. Eur J Appl Physiol Occup Physiol. 1984;52(2):188–195. doi: 10.1007/BF00433391. [DOI] [PubMed] [Google Scholar]
- Mazzoni M. C., Skalak T. C., Schmid-Schönbein G. W. Effects of skeletal muscle fiber deformation on lymphatic volumes. Am J Physiol. 1990 Dec;259(6 Pt 2):H1860–H1868. doi: 10.1152/ajpheart.1990.259.6.H1860. [DOI] [PubMed] [Google Scholar]
- McGeown J. G., McHale N. G., Thornbury K. D. Effects of varying patterns of external compression on lymph flow in the hindlimb of the anaesthetized sheep. J Physiol. 1988 Mar;397:449–457. doi: 10.1113/jphysiol.1988.sp017011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeown J. G., McHale N. G., Thornbury K. D. The role of external compression and movement in lymph propulsion in the sheep hind limb. J Physiol. 1987 Jun;387:83–93. doi: 10.1113/jphysiol.1987.sp016564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski W. L., Engeset A., Sokolowski J. Lymph flow and protein in the normal male leg during lying, getting up, and walking. Lymphology. 1977 Sep;10(3):178–183. [PubMed] [Google Scholar]
- Reed R. K., Johansen S., Noddeland H. Turnover rate of interstitial albumin in rat skin and skeletal muscle. Effects of limb movements and motor activity. Acta Physiol Scand. 1985 Dec;125(4):711–718. doi: 10.1111/j.1748-1716.1985.tb07774.x. [DOI] [PubMed] [Google Scholar]
- Reed R. K. Transcapillary extravasation rate of albumin in rat skeletal muscle. Effect of motor activity. Acta Physiol Scand. 1985 Dec;125(4):719–725. doi: 10.1111/j.1748-1716.1985.tb07775.x. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W. Microlymphatics and lymph flow. Physiol Rev. 1990 Oct;70(4):987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- Skalak T. C., Schmid-Schönbein G. W., Zweifach B. W. New morphological evidence for a mechanism of lymph formation in skeletal muscle. Microvasc Res. 1984 Jul;28(1):95–112. doi: 10.1016/0026-2862(84)90032-3. [DOI] [PubMed] [Google Scholar]
- Szabó G., Magyar Z., Molnár G. Lymphatic and venous transport of colloids from the tissues. Lymphology. 1973 Jun;6(2):69–79. [PubMed] [Google Scholar]
- Thakur M. L., DeFulvio J. D. Technetium-99m-labeled monoclonal antibodies for immunoscintigraphy. Simplified preparation and evaluation. J Immunol Methods. 1991 Mar 21;137(2):217–224. doi: 10.1016/0022-1759(91)90027-d. [DOI] [PubMed] [Google Scholar]
- Tönnesen K. H., Sejrsen P. Washout of 133Xenon after intramuscular injection and direct measurement of blood flow in skeletal muscle. Scand J Clin Lab Invest. 1970 Jan;25(1):71–81. doi: 10.3109/00365517009046193. [DOI] [PubMed] [Google Scholar]