Abstract
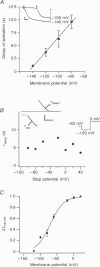
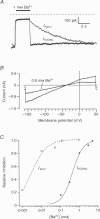
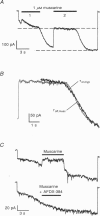
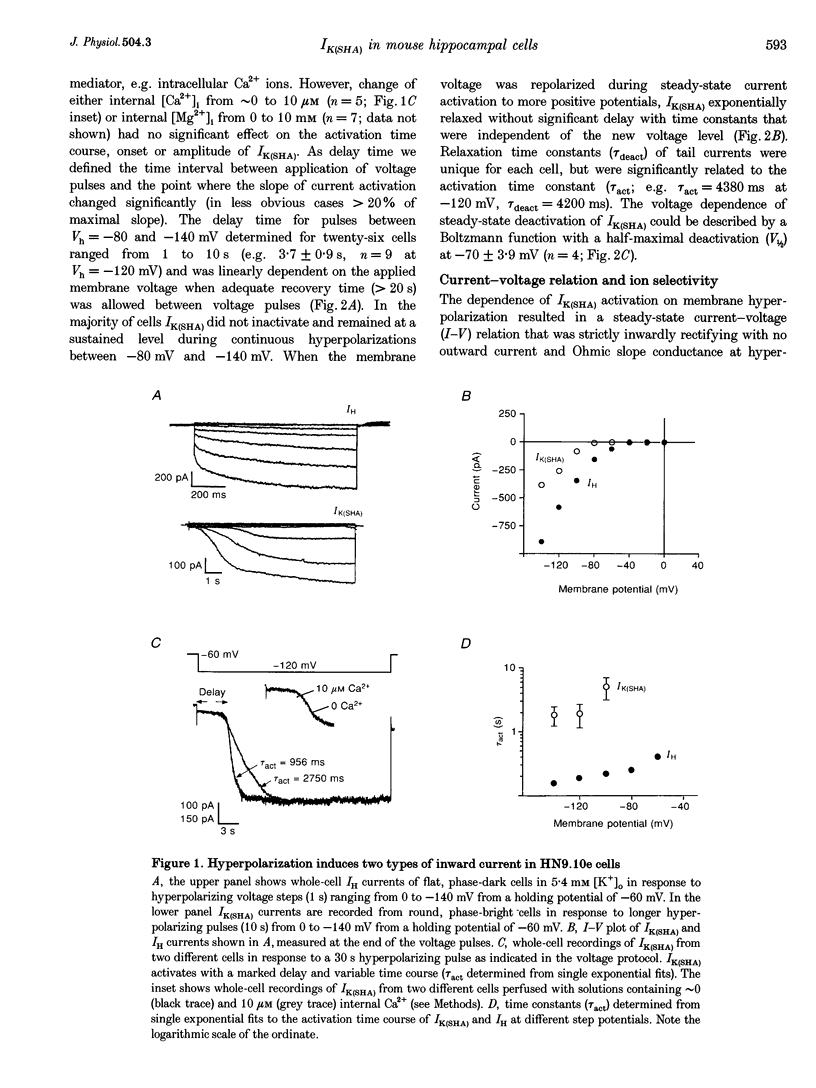
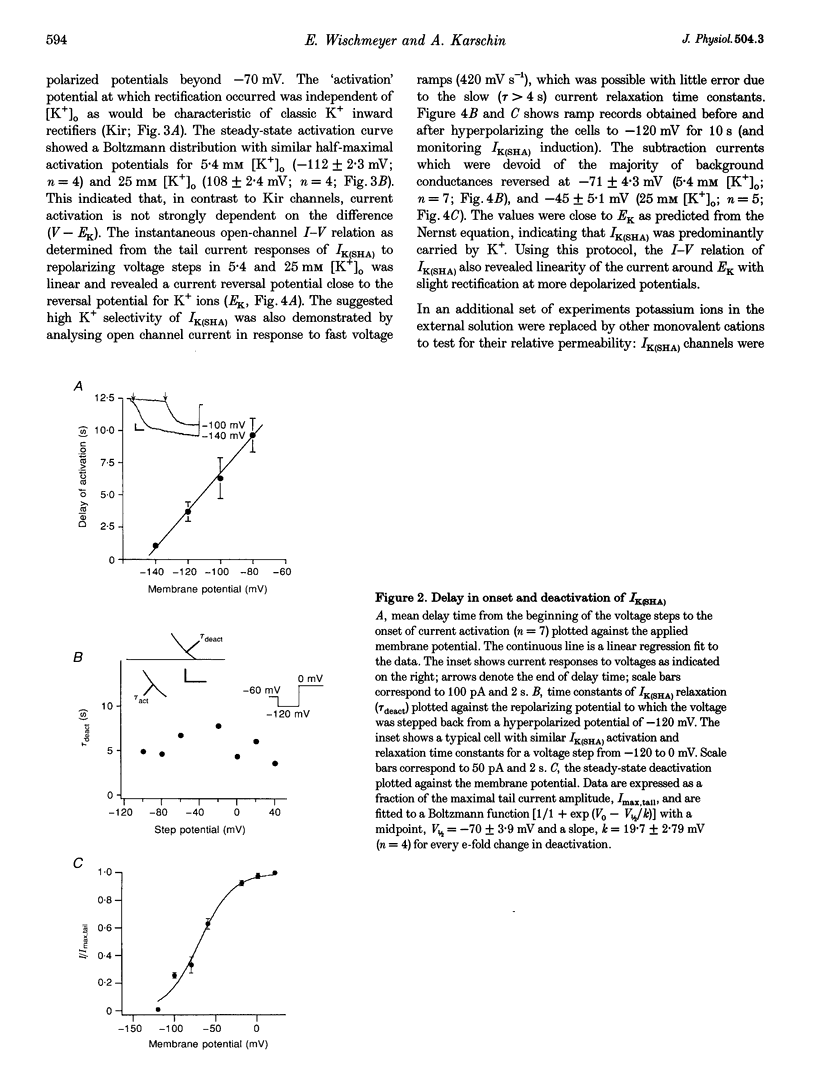
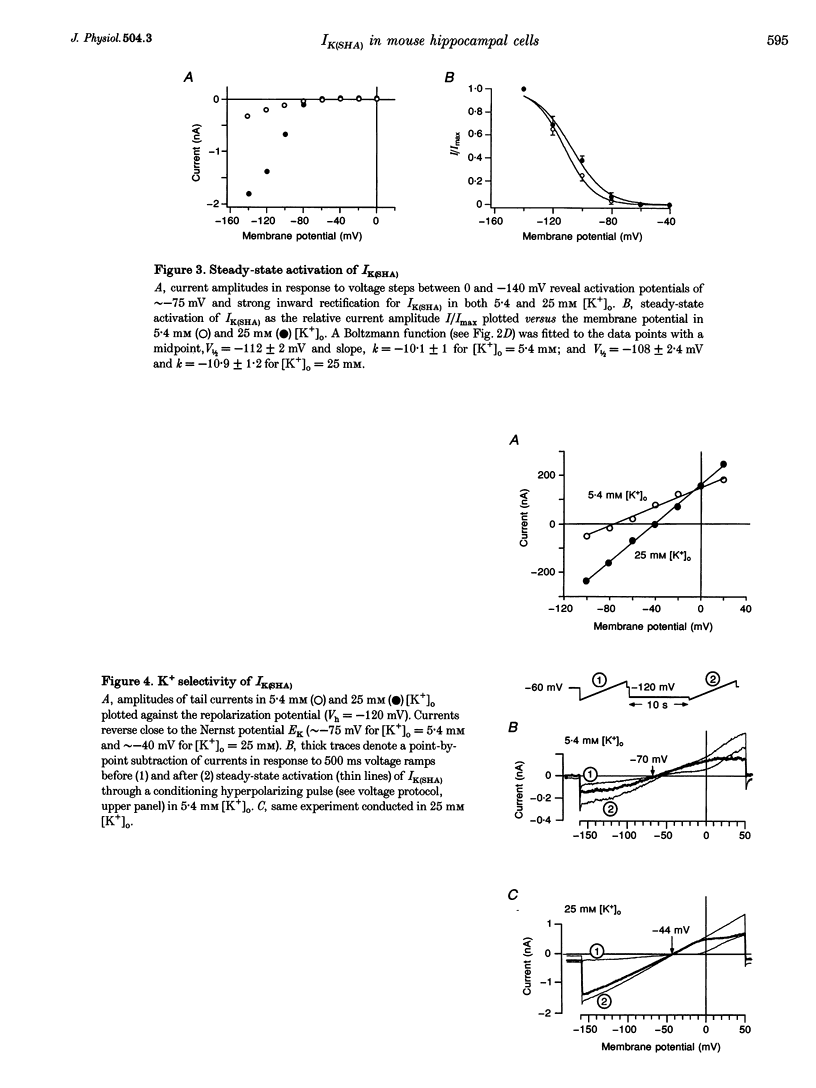
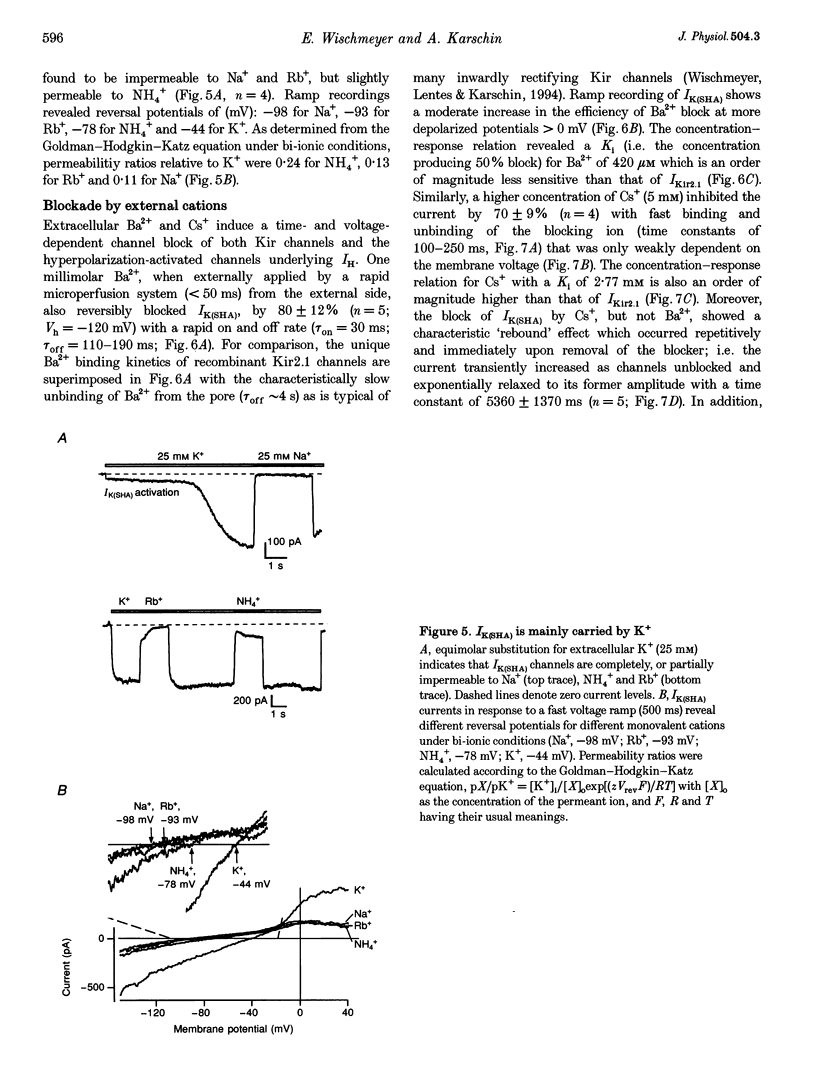
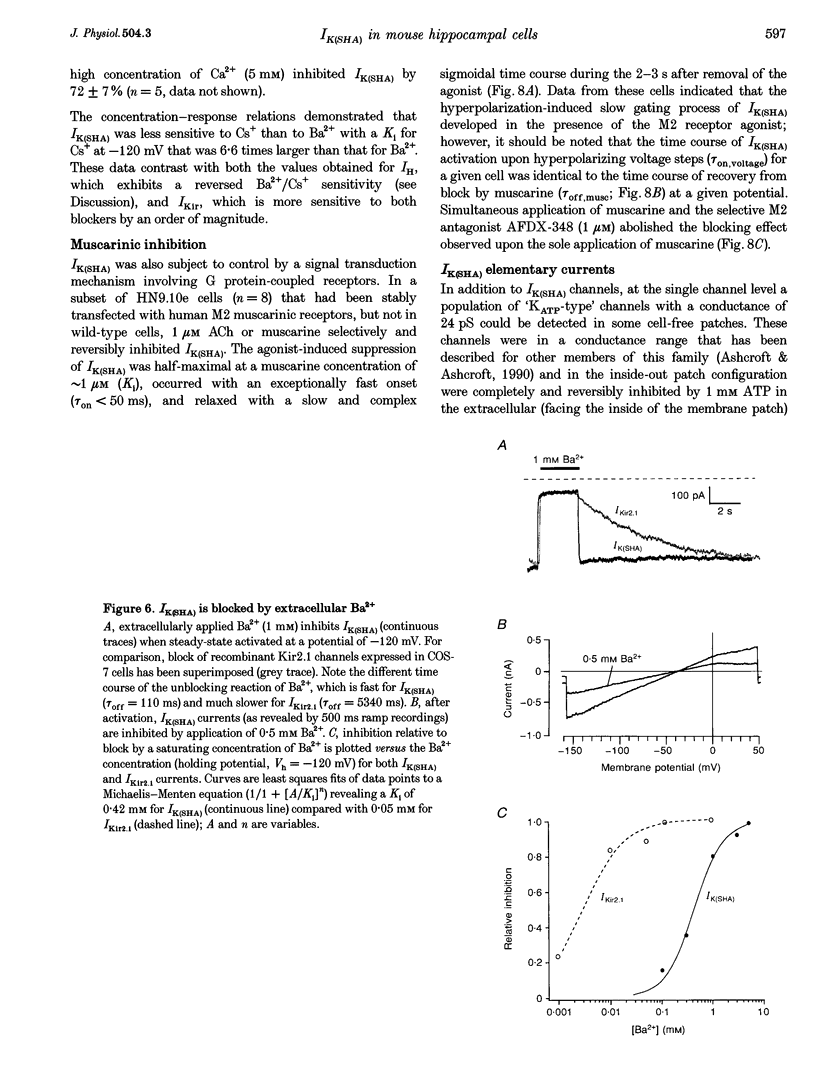
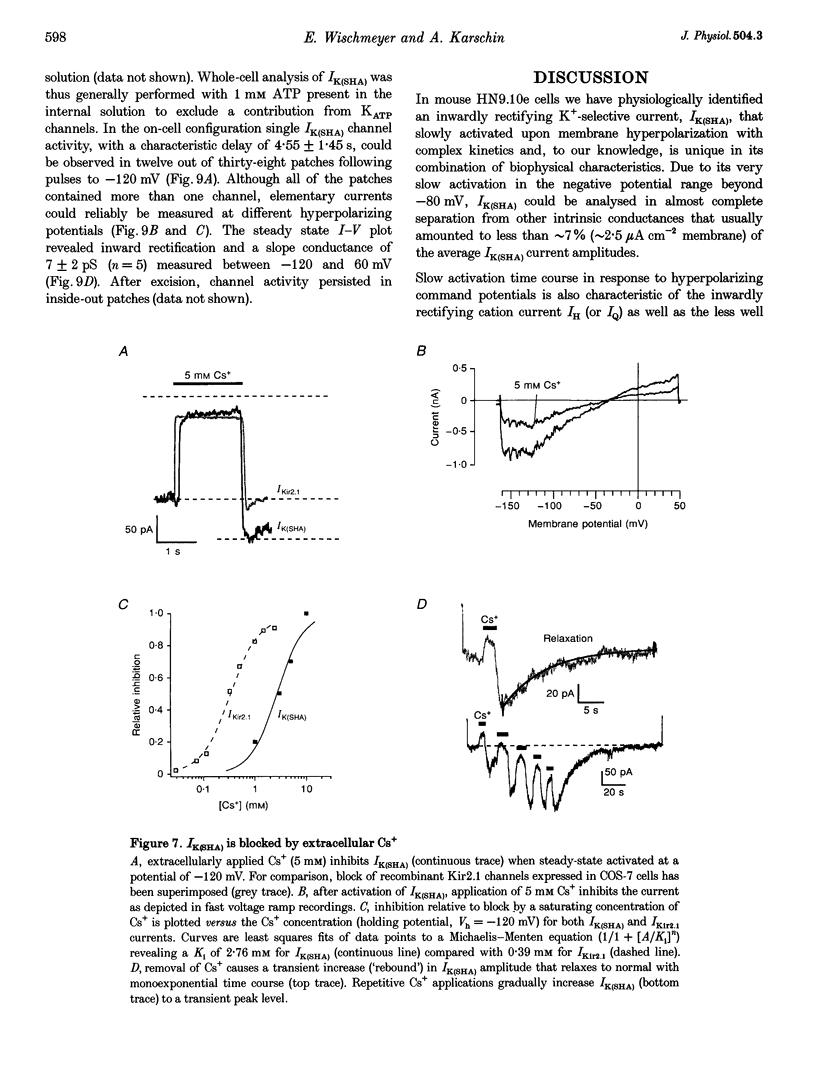
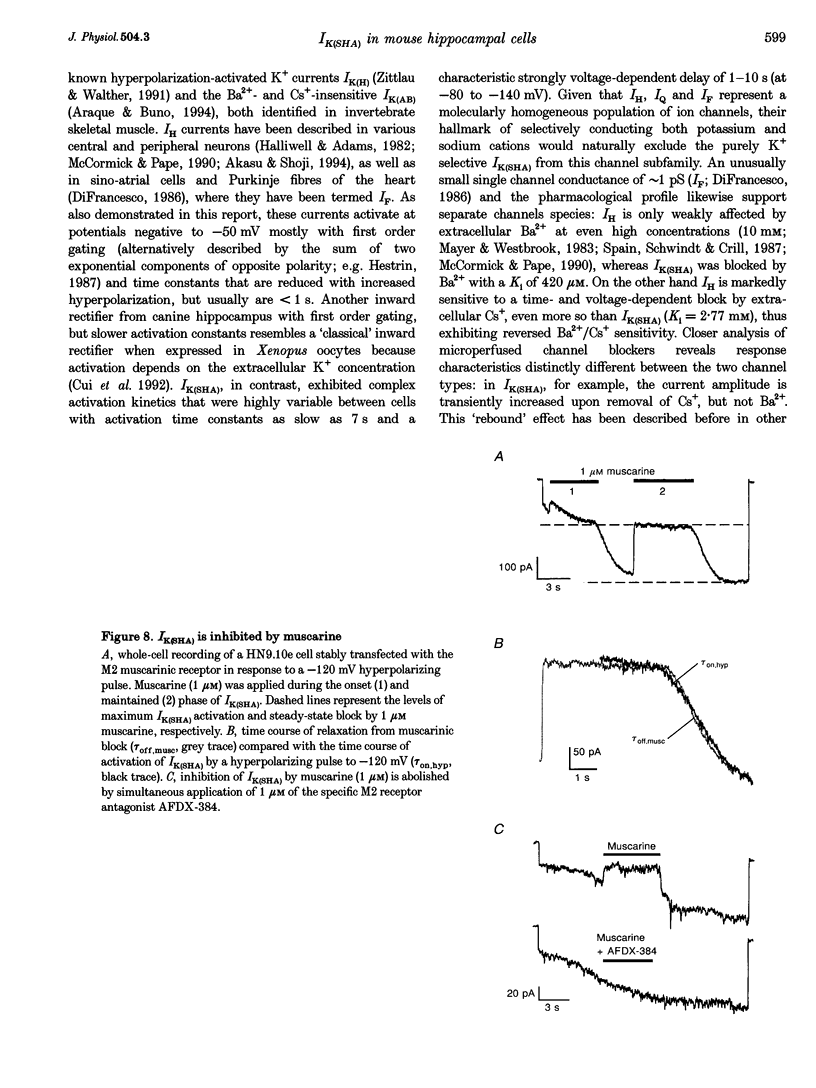
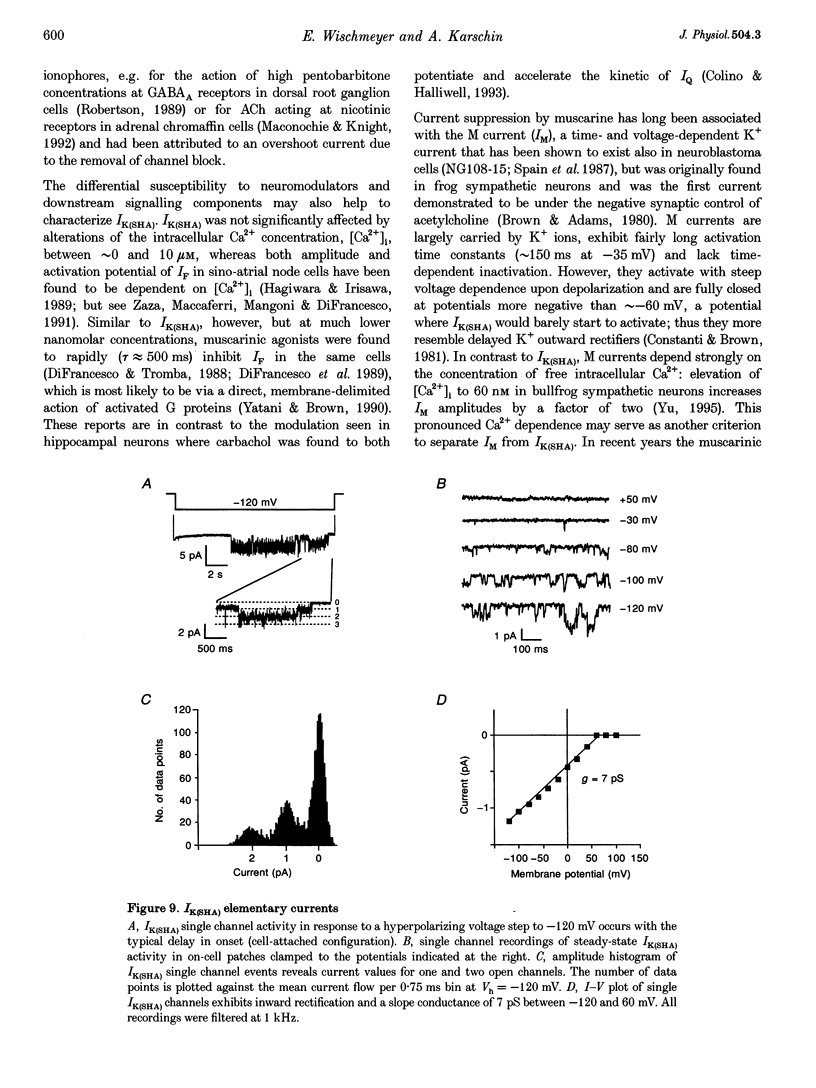
1. A slow hyperpolarization-activated inwardly rectifying K+ current (IK(SHA)) with novel characteristics was identified from the mouse embryonic hippocampus x neuroblastoma cell line HN9.10e. 2. The non-inactivating current activated negative to a membrane potential of -80 mV with slow and complex activation kinetics (tau act approximately 1-7 s) and a characteristic delay of 1-10 s (-80 to -140 mV) that was linearly dependent on the membrane potential. 3. Tail currents and instantaneous open channel currents determined through fast voltage ramps reversed at the K+ equilibrium potential (EK) indicating that primarily K+, but not Na+, permeated the channels. 4. IK(SHA) was unaffected by altering the intracellular Ca2+ concentration between approximately 0 and 10 microM, but was susceptible to block by 5 mM extracellular Ca2+, Ba2+ (Ki = 0.42 mM), and Cs+ (Ki = 2.77 mM) 5. In cells stably transformed with M2 muscarinic receptors, IK(SHA) was rapidly, but reversibly, suppressed by application of micromolar concentrations of muscarine. 6. At the single channel level K(SHA) channel openings were observed with the characteristic delay upon membrane hyperpolarization. Analysis of unitary currents revealed an inwardly rectifying I-V profile and a channel slope conductance of 7 pS. Channel activity persisted in the inside-out configuration for many minutes. 7. It is concluded that IK(SHA) in HN9.10e cells represents a novel K+ current, which is activated upon membrane hyperpolarization. It is functionally different from both classic inwardly rectifying IKir currents and other cationic hyperpolarization-activated IH currents that have been previously described in neuronal or glial cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasu T., Shoji S. cAMP-dependent inward rectifier current in neurons of the rat suprachiasmatic nucleus. Pflugers Arch. 1994 Nov;429(1):117–125. doi: 10.1007/BF02584037. [DOI] [PubMed] [Google Scholar]
- Araque A., Buño W. Novel hyperpolarization-activated K+ current mediates anomalous rectification in crayfish muscle. J Neurosci. 1994 Jan;14(1):399–408. doi: 10.1523/JNEUROSCI.14-01-00399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft S. J., Ashcroft F. M. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2(3):197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Voltage- and calcium-activated potassium currents in mouse neuroblastoma x rat glioma hybrid cells. J Physiol. 1988 Mar;397:149–165. doi: 10.1113/jphysiol.1988.sp016993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colino A., Halliwell J. V. Carbachol potentiates Q current and activates a calcium-dependent non-specific conductance in rat hippocampus in vitro. Eur J Neurosci. 1993 Sep 1;5(9):1198–1209. doi: 10.1111/j.1460-9568.1993.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Constanti A., Brown D. A. M-Currents in voltage-clamped mammalian sympathetic neurones. Neurosci Lett. 1981 Jul 17;24(3):289–294. doi: 10.1016/0304-3940(81)90173-7. [DOI] [PubMed] [Google Scholar]
- Cui J., Mandel G., DiFrancesco D., Kline R. P., Pennefather P., Datyner N. B., Haspel H. C., Cohen I. S. Expression and characterization of a canine hippocampal inwardly rectifying K+ current in Xenopus oocytes. J Physiol. 1992 Nov;457:229–246. doi: 10.1113/jphysiol.1992.sp019375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. Characterization of single pacemaker channels in cardiac sino-atrial node cells. Nature. 1986 Dec 4;324(6096):470–473. doi: 10.1038/324470a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ducouret P., Robinson R. B. Muscarinic modulation of cardiac rate at low acetylcholine concentrations. Science. 1989 Feb 3;243(4891):669–671. doi: 10.1126/science.2916119. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Inhibition of the hyperpolarization-activated current (if) induced by acetylcholine in rabbit sino-atrial node myocytes. J Physiol. 1988 Nov;405:477–491. doi: 10.1113/jphysiol.1988.sp017343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupnik C. A., Davidson N., Lester H. A. The inward rectifier potassium channel family. Curr Opin Neurobiol. 1995 Jun;5(3):268–277. doi: 10.1016/0959-4388(95)80038-7. [DOI] [PubMed] [Google Scholar]
- Fakler B., Brändle U., Bond C., Glowatzki E., König C., Adelman J. P., Zenner H. P., Ruppersberg J. P. A structural determinant of differential sensitivity of cloned inward rectifier K+ channels to intracellular spermine. FEBS Lett. 1994 Dec 19;356(2-3):199–203. doi: 10.1016/0014-5793(94)01258-x. [DOI] [PubMed] [Google Scholar]
- Ficker E., Taglialatela M., Wible B. A., Henley C. M., Brown A. M. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994 Nov 11;266(5187):1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- Glitsch M., Wischmeyer E., Karschin A. Functional characterization of two 5-HT3 receptor splice variants isolated from a mouse hippocampal cell line. Pflugers Arch. 1996 May;432(1):134–143. doi: 10.1007/s004240050115. [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J Physiol. 1989 Feb;409:121–141. doi: 10.1113/jphysiol.1989.sp017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hestrin S. The properties and function of inward rectification in rod photoreceptors of the tiger salamander. J Physiol. 1987 Sep;390:319–333. doi: 10.1113/jphysiol.1987.sp016703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin A., Wischmeyer E., Davidson N., Lester H. A. Fast inhibition of inwardly rectifying K+ channels by multiple neurotransmitter receptors in oligodendroglia. Eur J Neurosci. 1994 Nov 1;6(11):1756–1764. doi: 10.1111/j.1460-9568.1994.tb00568.x. [DOI] [PubMed] [Google Scholar]
- Kofuji P., Doupnik C. A., Davidson N., Lester H. A. A unique P-region residue is required for slow voltage-dependent gating of a G protein-activated inward rectifier K+ channel expressed in Xenopus oocytes. J Physiol. 1996 Feb 1;490(Pt 3):633–645. doi: 10.1113/jphysiol.1996.sp021173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Hammond D. N., Large T. H., Roback J. D., Sim J. A., Brown D. A., Otten U. H., Wainer B. H. Neuronal properties and trophic activities of immortalized hippocampal cells from embryonic and young adult mice. J Neurosci. 1990 Jun;10(6):1779–1787. doi: 10.1523/JNEUROSCI.10-06-01779.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Hammond D. N., Large T. H., Wainer B. H. Immortalized young adult neurons from the septal region: generation and characterization. Brain Res Dev Brain Res. 1990 Mar 1;52(1-2):219–228. doi: 10.1016/0165-3806(90)90238-t. [DOI] [PubMed] [Google Scholar]
- Lopez H. S. Kinetics of G protein-mediated modulation of the potassium M-current in bullfrog sympathetic neurons. Neuron. 1992 Apr;8(4):725–736. doi: 10.1016/0896-6273(92)90093-s. [DOI] [PubMed] [Google Scholar]
- Maconochie D. J., Knight D. E. A study of the bovine adrenal chromaffin nicotinic receptor using patch clamp and concentration-jump techniques. J Physiol. 1992 Aug;454:129–153. doi: 10.1113/jphysiol.1992.sp019257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990 Dec;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J., Marsh S. J., Brown D. A. On the mechanism of M-current inhibition by muscarinic m1 receptors in DNA-transfected rodent neuroblastoma x glioma cells. J Physiol. 1993 Sep;469:153–178. doi: 10.1113/jphysiol.1993.sp019809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. Actions of anaesthetics and avermectin on GABAA chloride channels in mammalian dorsal root ganglion neurones. Br J Pharmacol. 1989 Sep;98(1):167–176. doi: 10.1111/j.1476-5381.1989.tb16878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K. Z., North R. A. Muscarine increases cation conductance and decreases potassium conductance in rat locus coeruleus neurones. J Physiol. 1992 Sep;455:471–485. doi: 10.1113/jphysiol.1992.sp019312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain W. J., Schwindt P. C., Crill W. E. Anomalous rectification in neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1987 May;57(5):1555–1576. doi: 10.1152/jn.1987.57.5.1555. [DOI] [PubMed] [Google Scholar]
- Stansfeld C. E., Marsh S. J., Gibb A. J., Brown D. A. Identification of M-channels in outside-out patches excised from sympathetic ganglion cells. Neuron. 1993 Apr;10(4):639–654. doi: 10.1016/0896-6273(93)90166-o. [DOI] [PubMed] [Google Scholar]
- Wang H. S., McKinnon D. Modulation of inwardly rectifying currents in rat sympathetic neurones by muscarinic receptors. J Physiol. 1996 Apr 15;492(Pt 2):467–478. doi: 10.1113/jphysiol.1996.sp021322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischmeyer E., Karschin A. Receptor stimulation causes slow inhibition of IRK1 inwardly rectifying K+ channels by direct protein kinase A-mediated phosphorylation. Proc Natl Acad Sci U S A. 1996 Jun 11;93(12):5819–5823. doi: 10.1073/pnas.93.12.5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Brown A. M. Regulation of cardiac pacemaker current If in excised membranes from sinoatrial node cells. Am J Physiol. 1990 Jun;258(6 Pt 2):H1947–H1951. doi: 10.1152/ajpheart.1990.258.6.H1947. [DOI] [PubMed] [Google Scholar]
- Yu S. P. Roles of arachidonic acid, lipoxygenases and phosphatases in calcium-dependent modulation of M-current in bullfrog sympathetic neurons. J Physiol. 1995 Sep 15;487(Pt 3):797–811. doi: 10.1113/jphysiol.1995.sp020919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaza A., Maccaferri G., Mangoni M., DiFrancesco D. Intracellular calcium does not directly modulate cardiac pacemaker (if) channels. Pflugers Arch. 1991 Dec;419(6):662–664. doi: 10.1007/BF00370312. [DOI] [PubMed] [Google Scholar]
- Zittlau K. E., Walther C. Hyperpolarization slowly activates a potassium current in locust skeletal muscle. Pflugers Arch. 1991 Mar;418(1-2):190–192. doi: 10.1007/BF00370470. [DOI] [PubMed] [Google Scholar]