Abstract
The optimal corticosteroids dose in patients with coronavirus disease 2019 (COVID-19) requiring high oxygen remains unknown. In this retrospective study of patients with COVID-19 requiring high oxygen and receiving corticosteroids, the efficacy, safety, and duration of high-dose treatment were evaluated. The primary outcome was all-cause mortality during follow-up. Safety outcomes included infection, gastrointestinal bleeding, and venous thromboembolic events. 210 patients were included, with 126 in Group A (corticosteroids at a equivalent dose <1 mg/kg/d prednisone), 44 in Group B (corticosteroids at a equivalent dose ≥1 mg/kg/d prednisone for ≤5 days), and 40 in Group C (corticosteroids at a equivalent dose ≥1 mg/kg/d prednisone for >5 days). The all-cause mortality risk was lower in Group C but higher in Group B than in Group A. Safety outcomes did not differ significantly, except for Group C, which had the highest venous thromboembolism rate. Our results suggest that high-dose corticosteroids for a longer course decrease mortality with comparable safety outcome.
Keywords: COVID-19, Corticosteroids, Duration, High oxygen requirement
Highlights
-
•
The efficacy of different corticosteroids dosages and durations was compared in COVID-19 patients.
-
•
A longer course of high-dose corticosteroids reduced mortality in COVID-19 patients with high oxygen requirements.
-
•
Safety outcomes were not adversely affected by the higher dose and extended duration.
1. Introduction
The rapid response and management of the coronavirus disease 2019 (COVID-19) pandemic has been unprecedented in the medical history of humankind. The host inflammatory response caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection plays a significant role in organ damage; particularly, severe lung injury can lead to hypoxia, acute respiratory distress syndrome, and even death. Therefore, mitigating the inflammatory storm, characterized by elevated levels of inflammatory markers, such as ferritin, C-reactive protein (CRP), and interleukin-6, has become the mainstay therapy for COVID-19 patients with hypoxia. Treatment with 6 mg dexamethasone for up to 10 days, as proven by the early RECOVERY trial, has been widely accepted and adopted in various guidelines [1]. Other corticosteroids, including methylprednisolone, have also been effective [2,3].
The importance and rationale of identifying appropriate corticosteroids dose primarily stems from whether higher doses can reduce mortality or shorten mechanical ventilation duration. The possibility of replacing other immunomodulators to reduce costs has also been considered. Moreover, a suitable corticosteroids dosage and duration may prevent adverse chronic outcomes, such as lung injury and fibrosis. However, the optimal corticosteroids dose for COVID-19 treatment remains unclear.
The HIGHLOWDEXA-COVID trial compared high-dose (20 mg daily for 5 days followed by 10 mg daily for 5 days) with low-dose dexamethasone (6 mg daily for 10 days) in patients with COVID-19 pneumonia requiring common oxygen support [4] and concluded that the higher dose reduced clinical worsening within 11 days. However, other studies did not identify substantial benefit in patients receiving high-dose corticosteroids, compared with those receiving low-dose corticosteroids [[5], [6], [7]].
Predicting future anti-inflammatory strategies in COVID-19 patients is difficult due to the lower virulence of SARS-CoV-2 strains, increased vaccination, immunity against previous infections, and potentially fewer critically ill patients. We experienced a huge wave of Omicron infections in December 2022 as China eased its COVID-19 policies and measures. Owing to the evidence accumulated during the pandemic, managing severely and critically ill patients was considerably better than in the first wave. However, different physicians administered varied doses of corticosteroids and other anti-inflammatory agents. Therefore, we conducted a retrospective study to investigate the effect of corticosteroids dosage and duration on the prognosis of COVID-19, focusing on patients with high oxygen requirements.
2. Methods
2.1. Study design and participants
In this retrospective study, we reviewed patients diagnosed with and hospitalized for COVID-19 between November 1, 2022, and February 27, 2023, at Peking Union Medical College Hospital, a large tertiary hospital in Beijing, China. The patients were treated in multiple departments during the Omicron wave, and different physicians administered corticosteroids in a discretionary manner.
The inclusion criteria were (1) aged ≥18 years; (2) evidence of positive nucleic acid or antigen testing for COVID-19 after symptom onset; (3) admitted to Peking Union Medical College Hospital due to COVID-19 infection.
The exclusion criteria for this study were as follows: (1) patients who did not require oxygen supplementation or only required conventional oxygen support during hospitalization, which was defined as nasal cannula, simple face mask and Venturi mask; (2) patients who did not receive corticosteroids or remained at daily dose before COVID-19 during infection (the daily dose before COVID-19 was defined as the corticosteroids dose administered prior to COVID-19 infection for managing underlying comorbidities, such as autoimmune diseases or interstitial lung diseases).
This study adhered to the Strengthening the STROBE guidelines, and the STROBE checklist is available in Supplementary Table 1.
2.2. Data collection, definitions, and outcomes
Data on demographic information (age and sex), comorbidities, and previous corticosteroids use were collected. Additionally, data on height, weight, smoking status, and laboratory test results (white blood cells, neutrophils, lymphocytes, platelet count, alanine aminotransferase, serum creatinine, D-dimer, and CRP) were collected at the time of hospitalization. Patients were followed up until discharge, and treatment information during hospitalization was reviewed and recorded, including the baseline and highest respiratory support modes, corticosteroids, tocilizumab or baricitinib, antiviral drugs, and anticoagulant therapy. Diagnoses of septic shock, hospital-acquired pneumonia, ventilator-associated pneumonia, fungal infections, delirium, gastrointestinal bleeding, and venous thromboembolism were collected from medical records.
Given the significant variations in corticosteroids treatment in real-world practice, the regimens were converted into prednisone equivalents (hydrocortisone 20 mg = prednisone 5 mg = methylprednisolone 4 mg = dexamethasone 0.75 mg). All patients were divided into three groups: Group A (low dose), patients who received corticosteroids but never exceeded the equivalent dose of 1 mg/kg/d prednisone; Group B (high dose and short duration), patients who received corticosteroids at a equivalent dose ≥1 mg/kg/d predisone at maximum but continued for no more than 5 days; and Group C (high dose and long duration), patients who received corticosteroids at a equivalent dose ≥1 mg/kg/d predisone at maximum and continued for more than 5 days.
The primary outcome was all-cause mortality during follow-up until discharge. We also compared the ICU admission rate, duration of ICU stay, extubation rate, and duration of IMV between groups. Safety outcomes included bloodstream infections, hospital-acquired infections, ventilator-associated pneumonia, fungal infections, gastrointestinal bleeding, and venous thromboembolism.
2.3. Statistical analysis
Continuous variables are expressed as mean ± standard deviation or median (interquartile range) and compared using the t-test or Wilcoxon rank-sum test, as appropriate. Categorical variables are expressed as numbers (percentages) and compared using the chi-square test. Multiple imputation was performed for measures with less than 10 percent missing values, including height, weight, and laboratory tests. Predictive mean matching of mice R package (version 3.16) was used to conduct multiple imputation. Missing data on baseline height (n = 14), weight (n = 33), white blood cells (n = 3), neutrophils (n = 4), lymphocytes (n = 4), platelet counts (n = 3), alanine aminotransferase (n = 2), serum creatinine (n = 2), D-dimer (n = 11) and CRP (n = 8) were imputed by implementing the multivariate imputation by chained equations [8]. The correlation between different corticosteroids doses and all-cause mortality risk was evaluated using Kaplan–Meier survival analysis and Cox regression analysis. In addition to age, sex, and respiratory support modes, indicators associated with outcomes in the univariate regression analysis with a P-value <0.1 and factors imbalanced among groups (P < 0.1) were included for multivariable adjustment. We also applied a univariate Cox regression to the subgroups to evaluate the robustness of the estimates. ICU admission, duration of ICU stay, extubation rates, duration of IMV, and safety outcomes were compared between the groups using the chi-square test. Statistical analyses were performed using R 4.2.2 and STATA software (version 16.0, Texas 77845, USA). All statistical tests were two-sided, and a P < 0.05 was considered statistically significant.
3. Results
3.1. Clinical characteristics at baseline
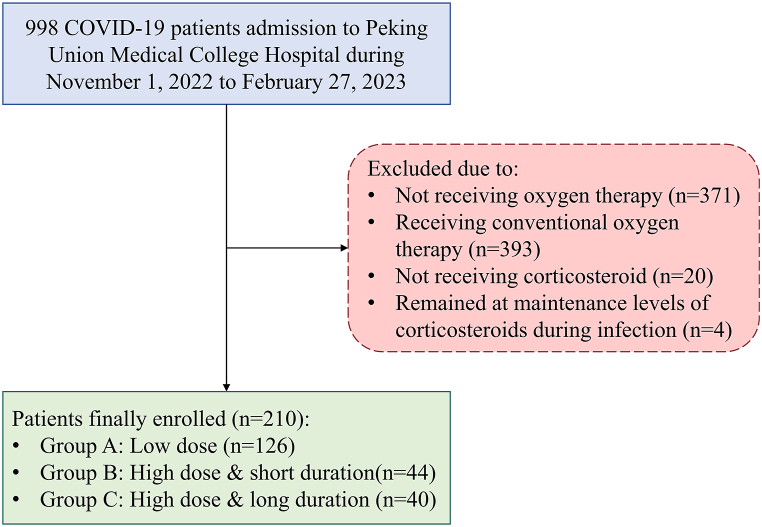
Our study included 210 patients with COVID-19 (Fig. 1), among whom 84 received a maximum dose of corticosteroids ≥1 mg/kg/d, with a median duration of 5 (4–8) days. A total of 126 (60.0 %), 44 (21.0 %), and 40 (19.0 %) patients were grouped into groups A (low dose), B (high dose and short duration), and C (high dose and long duration), respectively. The baseline clinical characteristics of the three groups are summarized in Table 1.
Fig. 1.
Flowchart of patients' selection.
Table 1.
Baseline characteristics, laboratory tests, and concomitant therapies.a.
| Group A (Low dose) |
Group B (High dose, short duration) |
Group C (High dose, long duration) |
P | |
|---|---|---|---|---|
| N = 126 | N = 44 | N = 40 | ||
| Age (years) | 76 (67–84) | 72.5 (63–83) | 72.5 (63.5–82.5) | 0.59 |
| Male sex | 97 (77.0 %) | 27 (61.4 %) | 27 (67.5 %) | 0.11 |
| BMI (kg/m2)c | 24.5 (22.9–26.9) | 23.0 (19.9–24.7) | 23.9 (20.8–26.4) | 0.002b1 |
| Comorbidity | ||||
| Hypertension | 83 (65.9 %) | 28 (63.6 %) | 24 (60.0 %) | 0.78 |
| Diabetes mellitus | 59 (46.8 %) | 15 (34.1 %) | 16 (40.0 %) | 0.30 |
| Hyperlipidemia | 32 (25.4 %) | 6 (13.6 %) | 7 (17.5 %) | 0.23 |
| Cardiovascular disease | 47 (37.3 %) | 12 (27.3 %) | 18 (45.0 %) | 0.25 |
| Cerebrovascular disease | 22 (17.5 %) | 3 (6.8 %) | 8 (20.0 %) | 0.17 |
| Chronic kidney disease | 26 (20.6 %) | 10 (22.7 %) | 9 (22.5 %) | 0.92 |
| Chronic liver disease | 6 (4.8 %) | 1 (2.3 %) | 2 (5.0 %) | 0.80 |
| COPDc | 6 (4.8 %) | 6 (13.6 %) | 2 (5.0 %) | 0.17 |
| Interstitial lung disease | 5 (4.0 %) | 6 (13.6 %) | 5 (12.5 %) | 0.035b1,b2 |
| Tumor | 30 (23.8 %) | 14 (31.8 %) | 11 (27.5 %) | 0.52 |
| Baseline respiratory support modes | 0.35 | |||
| No respiratory support | 24 (19.0 %) | 4 (9.1 %) | 5 (12.5 %) | |
| Conventional oxygen supplementary | 33 (26.2 %) | 15 (34.1 %) | 17 (42.5 %) | |
| Reservoir masks/HFNC/NIVc | 44 (34.9 %) | 17 (38.6 %) | 14 (35.0 %) | |
| IMV/ECMOc | 25 (19.8 %) | 8 (18.2 %) | 4 (10.0 %) | |
| Laboratory test | ||||
| White blood cells ( × 109/L) | 9.07 (6.66–13.1) | 8.83 (5.24–12.8) | 8.20 (4.89–11.1) | 0.16 |
| Lymphocytes ( × 109/L) | 0.57 (0.39–0.87) | 0.47 (0.27–0.61) | 0.55 (0.26–0.77) | 0.080b1 |
| Platelet ( × 109/L) | 195 (147–252) | 161 (96–207) | 187 (134–266) | 0.086b1 |
| Alanine aminotransferases (U/L) | 25 (15–43) | 32 (18–52) | 28 (15–62) | 0.43 |
| Serum creatinine (μmol/L) | 83 (60–148) | 73.5 (57–139) | 68.5 (57–108.5) | 0.30 |
| D-dimer (mg/L FEU) | 2.99 (1.14–9.97) | 3.16 (1.18–8.10) | 3.05 (1.10–12.7) | 0.92 |
| C-reactive protein (mg/L) | 97.4 (46.0–166) | 58.5 (42.4–107) | 71.4 (42.5–132) | 0.081b1 |
| Treatment | ||||
| History of corticosteroids use | 8 (6.3 %) | 7 (15.9 %) | 9 (22.5 %) | 0.011b2 |
| Intervals between symptoms onset to corticosteroids initiation (Days) | 9 (6–12) | 7 (3–12) | 10 (6–13) | 0.021b1,b3 |
| Median corticosteroids duration until discharge (Days) | 11 (7–22) | 15 (8–25) | 25 (16–37) | <0.001b2,b3 |
| Maximum corticosteroids dose (mg/kg) | 0.63 (0.56–0.74) | 1.44 (1.18–1.63) | 1.49 (1.20–2.01) | <0.001b1,b2 |
| Duration of maximum corticosteroids use (Days) | 7 (5–10) | 4 (3–5) | 7 (5–8) | <0.001b1,b3 |
| Duration of corticosteroids dose≥1 mg/kg/d (Days) | 0 (0–0) | 4 (3–5) | 9 (7–12) | <0.001b1,b2,b3 |
| Immunomodulators (tocilizumab or baricitinib) | 61 (48.4 %) | 27 (61.4 %) | 26 (65.0 %) | 0.11 |
| Antiviral therapy | 56 (44.4 %) | 21 (47.7 %) | 18 (45.0 %) | 0.93 |
| Anticoagulation therapy | 99 (78.6 %) | 35 (79.5 %) | 31 (77.5 %) | 1.00 |
Data are presented as median (interquartile range) or n (percentage) as appropriate.
P < 0.05 between Group A and Group B.
P < 0.05 between Group A and Group C.
between Group B and Group C.
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; HFNC, high-flow nasal cannula; NIV, noninvasive mechanical ventilation; IMV, invasive mechanical ventilation; ECMO, extracorporeal membrane oxygenation.
The median age was 75 (66–83) years, and there were no significant differences among the three groups in age or sex. The most common comorbidity was hypertension (n = 135, 64.3 %), followed by diabetes (n = 90, 42.9 %) and cardiovascular disease (n = 77, 36.7 %). The proportion of interstitial lung disease was lower in Group A (4.0 %) than in Group B (13.6 %) (P = 0.035) and Group C (12.5 %) (P = 0.048). No significant differences were noted in the baseline respiratory support type. There were no significant differences in baseline white blood cell and neutrophil count, alanine aminotransferase, serum creatinine, and D-dimer levels. Group A appeared to have higher body mass index (BMI) and lymphocytes, platelet, and CRP levels; however, significant differences were only identified when compared with Group B (BMI: P < 0.001; lymphocytes: P = 0.035; platelet: P = 0.024; CRP: P = 0.033) but not with Group C (BMI: P = 0.067; lymphocytes: P = 0.20; platelet: P = 0.76; CRP: P = 0.26) (Table 1).
The proportion of patients with a history of corticosteroids use gradually increased from Group A to Group C. The median interval from symptom onset to corticosteroids initiation was 9 (6–12) days. Patients in Group B initiated corticosteroids earlier than those in Group A (P = 0.006) and Group C (P = 0.040). The three groups had no significant differences regarding tocilizumab or baricitinib use, antiviral therapy, or anticoagulation therapy.
3.2. Primary outcome
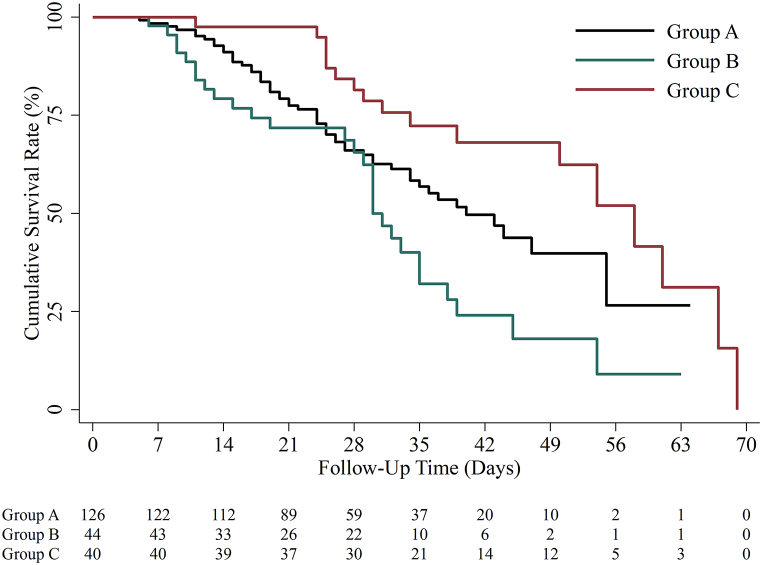
During a median follow-up period of 29 (20–39) days, 99/210 patients (47.1 %) died, and the median time from symptom onset to death was 25 (15–34) days (Table 2). The all-cause mortality risk was significantly lower in Group C who received corticosteroids at a equivalent dose ≥1 mg/kg/d predisone over 5 days (HR: 0.54; 95 % CI: 0.30–0.97; P = 0.038) and higher in Group B who received corticosteroids at a equivalent dose ≥1 mg/kg/d predisone for a duration of ≤5 days (HR: 1.64; 95 % CI: 1.04–2.60; P = 0.034) than in patients in Group A who received low-dose corticosteroids (Fig. 2). We further applied a multivariate Cox regression using three models. Besides age, sex, and baseline respiratory support modes, Model 1 included imbalanced factors among groups with P < 0.1 (BMI, interstitial lung disease, history of corticosteroids, intervals between symptom onset to corticosteroids initiation, lymphocytes, platelet, and CRP); Model 2 included indicators associated with primary outcomes in univariate regression models with P < 0.1 (hyperlipidemia, chronic kidney disease, BMI, intervals between symptom onset to corticosteroids initiation, anticoagulation therapy and immunomodulators use); and Model 3 included all abovementioned factors. The lower mortality risk in Group C than in Group A remained in all three multivariable-adjusted models (Table 2).
Table 2.
Multivariable cox regression for associations between different corticosteroids groups and overall mortality risks
| No. of subjects | No. of events | Model 1a |
Model 2b |
Model 3c |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95 % CI | P | HR | 95 % CI | P | HR | 95 % CI | P | |||
| Group A | 126 | 54 | 1(Reference) | 1(Reference) | 1(Reference) | ||||||
| Group B | 44 | 28 | 1.4 | 0.88–2.4 | 0.15 | 1.4 | 0.87–2.4 | 0.16 | 1.5 | 0.92–2.6 | 0.099 |
| Group C | 40 | 17 | 0.50 | 0.27–0.91 | 0.024 | 0.54 | 0.30–0.97 | 0.041 | 0.49 | 0.26–0.91 | 0.024 |
Model 1: age, sex, baseline respiratory support modes, body mass index (BMI), interstitial lung disease, lymphocytes, platelet, history of corticosteroids using, and intervals between symptoms onset and initiation of corticosteroids therapy.
Model 2: age, sex, baseline respiratory support modes, body mass index (BMI), hyperlipidemia, chronic kidney disease, intervals between symptoms onset and initiation of corticosteroids therapy, immunomodulators, and anticoagulation.
Model 3: all covariates in Model 1 and Model 2.
Fig. 2.
Kaplan-Meier curve of mortality risks among different corticosteroids group.
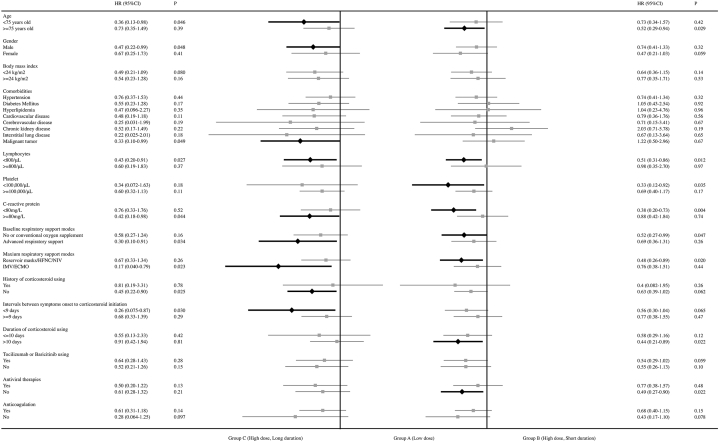
We further applied the univariate regression model to different subgroups. The associations between Group C and higher survival probability, compared with Group A, remained in patients younger than 75 years old, with male sex or history of tumor, without history of corticosteroids use, with lymphocytes <0.8×109/L or CRP ≥80 mg/L, and those who started corticosteroids within 9 days from symptom onset (Fig. 3). Interestingly, the lower mortality risk of Group C compared with Group A remained in patients with advanced respiratory support at baseline (HR: 0.30; 95 % CI 0.10–0.91; P = 0.034) and those who progressed to receive IMV or ECMO (HR: 0.18; 95 % CI 0.040–0.79; P = 0.023). However, the significant differences between Group C and Group A disappeared in patients requiring no or only conventional oxygen supplement (nasal cannula, Venturi masks, or <10 L/min of reservoir masks) at baseline or those who required reservoir masks, high-flow nasal cannula or noninvasive mechanical ventilation during follow-up. However, in these patients, the mortality risk in Group B was significantly higher than that in Group A (Fig. 3).
Fig. 3.
Subgroup Cox regression of mortality risks among different corticosteroids groups.
3.3. Corticosteroids therapy and other outcomes
Table 3 compares secondary and safety outcomes among groups. We observed the lowest ICU admission rates and shortest ICU stay in Group C, but without statistical significance, compared with Group A and Group B. No differences were found among groups on extubation rates and duration of IMV.
Table 3.
Comparison of secondary outcomes and safety outcomes among groups.a.
| Group A (Low dose) |
Group B (High dose, short duration) |
Group C (High dose, long duration) |
P | |
|---|---|---|---|---|
| N = 126 | N = 44 | N = 40 | ||
| Intensive Care Unit admission | 42 (33.3 %) | 17 (38.6 %) | 10 (25.0 %) | 0.42 |
| Duration of Intensive Care Unit stay (Days) | 13 (6–18) | 16 (9–26) | 8 (4–16) | 0.12 |
| Extutbaion | 24 (56 %) | 5 (26 %) | 3 (38 %) | 0.081 |
| Extubation in 28 days | 22 (51 %) | 5 (26 %) | 3 (38 %) | 0.2 |
| Duration of invasive mechanical ventilation (Days) | 11 (5–19) | 13 (8–26) | 12 (5–21) | 0.60 |
| Safety outcomes | ||||
| Shock | 35 (27.8 %) | 17 (38.6 %) | 9 (22.5 %) | 0.26 |
| Infection | 32 (25.4 %) | 18 (40.9 %) | 13 (32.5 %) | 0.14 |
| Bloodstream infection | 10 (7.9 %) | 6 (13.6 %) | 3 (7.5 %) | 0.50 |
| Hospital acquired pneumonia | 20 (15.9 %) | 10 (22.7 %) | 6 (15.0 %) | 0.56 |
| Ventilator-associated pneumonia | 10 (7.9 %) | 5 (11.4 %) | 1 (2.5 %) | 0.31 |
| Fungal infection | 17 (13.5 %) | 11 (25.0 %) | 9 (22.5 %) | 0.13 |
| Gastrointestinal bleeding | 8 (6.3 %) | 3 (6.8 %) | 2 (5.0 %) | 1.00 |
| Delirium | 4 (3.2 %) | 3 (6.8 %) | 0 (0.0 %) | 0.21 |
| Venous thromboembolism | 14 (11.1 %) | 8 (18.2 %) | 11 (27.5 %) | 0.042b2 |
Data are presented as median (interquartile range) or n (percentage) as appropriate.
P < 0.05 between Group A and Group C.
The proportion of patients in Group C who experienced septic shock, bloodstream infections, hospital-acquired infections, or fungal infections did not increase. Moreover, the proportion of patients experiencing gastrointestinal bleeding in Group C was the lowest among the three groups (5.0 %), although the difference was not significant (P = 1.00). Notably, the incidence of venous thromboembolism was 11.1 % in Group A, 18.2 % in Group B, and 27.5 % in Group C, with P = 0.042 among groups.
4. Discussion
This study included a cohort of 210 patients with COVID-19 in the Omicron wave with advanced oxygen requirements in China. A high dose (>1 mg/kg/d prednisone) of corticosteroids with a longer duration (>5 days) showed benefit over low-dose or high-dose but a shorter duration of corticosteroids. Additionally, after adjusting for covariates, large doses and long durations of corticosteroids treatment were found to significantly reduce the risk of death, which is consistent with different analysis models. Moreover, like other trials (RECOVERY, HIGHLOWDEXA-COVID, and COVID STEROID 2) [4,6,7], we did not find differences in adverse reactions such as hospital-acquired pneumonia, ventilator-associated pneumonia, and gastrointestinal bleeding among the three groups.
Multiple studies have previously evaluated the outcomes of different corticosteroids doses in patients with COVID-19; however, the results have been controversial. As mentioned before, The HIGHLOWDEXA-COVID trial identified that high-dose dexamethasone (20 mg daily for 5 days followed by 10 mg daily for 5 days) mitigating clinical worsening within 11 days of COVID-19 patients requiring common oxygen support compared with low-dose dexamethasone (6 mg daily for 10 days) (16.3 % vs. 31.4 %; RR 0.427; 95 % CI 0.216–0.842; P = 0.014) [4]. However, Langer-Gould et al. found no differences in the propensity score-matched odds of death between low-dose (6–10 mg daily) and high-dose (>10–20 mg daily) dexamethasone group (OR 1.17; 95 % CI 0.72–1.90) [9]. The same conclusion was obtained by another study conducted by Katz et al. [10]. Furthermore, Kumar G et al. [11] found higher in-hospital mortality in high-dose corticosteroids group (>40 mg/d methylprednisolone equivalent dose) than standard dose corticosteroids (<40 mg/d methylprednisolone equivalent dose) (40.7 % vs. 18.6 %; P < 0.001). The effect of corticosteroids also varied in patients with different severity of disease. In a recent meta-analysis by Qiao et al. [12], corticosteroids reduced mortality of patients with severe and critical COVID-19 (OR 0.85; 95 % CI 0.76–0.94; P = 0.002), but did not benefit patients with mild symptoms.
This study had some limitations. First, the retrospective nature of the study limits its capacity to establish causality, and relatively small sample size in Group B and C compared to Group A may affect the statistical power of the comparison. Second, patients were treated by different doctors with a discretionary manner, this variability could introduce bias. However, the real-world data used in this study provides a more generalizable conclusion regarding how treatment affects individuals. Third, characteristics of each group at baseline were not perfectly controlled, which may also introduce bias. To address this concern, we used Cox regression and different models for the statistical analysis, and the main conclusions remained consistent. Finally, as maximum dose and duration were used to sketch the regimens in the different groups, we were unable to ascertain whether the dosing and corticosteroids type changed during hospitalization due to superimposed infections and changing illness severity.
Our results strongly suggest that the beneficial effect of corticosteroids in patients with COVID-19 depends on the selection of the correct dose and course. However, this conclusion should be evaluated in future clinical trials [7]. There's still a lot to explore about corticosteroids using in COVID-19. For example, dexamethasone was recommended by numerous clinical guidelines for COVID-19 patients, based on the results of RECOVERY trial [1]. However, methylprednisolone was more commonly used in clinical practice for its better anti-inflammatory effect. The effect of methylprednisolone has been confirmed in the previous study conducted by Ko J.J. et al. [13]. Due to limited number of patients included, we did not discuss this topic. Moreover, most randomized controlled trials with different corticosteroids doses used mortality or survival as the primary outcome; however, among surviving patients, other outcomes such as fibrosis, pulmonary function decline, and long-term impact of corticosteroids were not recorded. From the perspective of interstitial lung disease management, a longer duration of corticosteroids use is rational, but consequently side effects should also be focused. Therefore, further prospective randomized controlled trials are required to determine the optimal dose and duration of corticosteroids use to improve survival and long-term pulmonary outcomes.
5. Conclusions
In summary, this single-center, retrospective study demonstrated that using higher doses of corticosteroids for a longer course reduced mortality in COVID-19 patients with high oxygen requirements. The beneficial effect of corticosteroids in COVID-19 patients may depend on choosing the correct combination of both dose and course.
CRediT authorship contribution statement
Junping Fan: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Huaiya Xie: Writing – review & editing, Writing – original draft, Visualization, Validation, Investigation, Formal analysis, Data curation. Yaqi Wang: Writing – review & editing, Visualization, Validation, Investigation, Formal analysis, Data curation. Siqi Pan: Writing – review & editing, Investigation. Tingyu Wang: Writing – review & editing, Investigation. Chuan Shi: Writing – review & editing, Investigation. Xinjie Hui: Writing – review & editing, Investigation. Huan Hou: Writing – review & editing, Investigation. Xiaoxing Gao: Writing – review & editing, Investigation. Wangji Zhou: Writing – review & editing, Investigation. Xiangning Liu: Writing – review & editing, Investigation. Yunxin Liu: Writing – review & editing, Investigation. Jinglan Wang: Writing – review & editing, Supervision, Project administration, Conceptualization. Xinlun Tian: Writing – review & editing, Supervision, Project administration, Investigation, Funding acquisition, Conceptualization.
Ethics statement
This study was reviewed by the Ethics Committee of Peking Union Medical College Hospital (ethics approval number I-23PJ946). Considering the nature of this retrospective research without intervention, the requirement for informed consent from individual patients was waived.
Data availability statement
Data will be made available on request.
Funding statement
This work was supported by the National High Level Hospital Clinical Research Funding (grant number 2022-PUMCH-A-129); The Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (grant number 2021-I2M-1–048). These funding sources had no role in the study design or execution, analyses, interpretation of the data, or decision to submit results.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e40059.
Contributor Information
Jinglan Wang, Email: wangjinglan@aliyun.com.
Xinlun Tian, Email: xinlun_t@sina.com.
Abbreviations
- coronavirus disease 2019
COVID-19
- severe acute respiratory syndrome coronavirus 2
SARS-CoV-2
- C-reactive protein
CRP
- intensive care unit
ICU
- odds ratio
OR
- hazard ratio
HR
- relative risk
RR
- confidence interval
CI
- extracorporeal membrane oxygenation
ECMO
- invasive mechanical ventilation
IMV
- body mass index
BMI
- Strengthening the Reporting of Observational Studies in Epidemiology
STROBE
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papamanoli A., Yoo J., Grewal P., Predun W., Hotelling J., Jacob R., et al. High-dose methylprednisolone in nonintubated patients with severe COVID-19 pneumonia. Eur. J. Clin. Invest. 2021;51 doi: 10.1111/eci.13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson B.C., Laracy J., Shoucri S., Dietz D., Zucker J., Patel N., et al. Clinical outcomes associated with methylprednisolone in mechanically ventilated patients with COVID-19. Clin. Infect. Dis. 2021;72:e367–e372. doi: 10.1093/cid/ciaa1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taboada M., Rodríguez N., Varela P.M., Rodríguez M.T., Abelleira R., González A., et al. Effect of high versus low dose of dexamethasone on clinical worsening in patients hospitalised with moderate or severe COVID-19 pneumonia: an open-label, randomised clinical trial. Eur. Respir. J. 2022;60 doi: 10.1183/13993003.02518-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouadma L., Mekontso-Dessap A., Burdet C., Merdji H., Poissy J., Dupuis C., et al. High-dose dexamethasone and oxygen support strategies in Intensive Care Unit patients with severe COVID-19 acute hypoxemic respiratory failure: the COVIDICUS randomized clinical trial. JAMA Intern. Med. 2022;182:906–916. doi: 10.1001/jamainternmed.2022.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.COVID STEROID 2 Trial Group. Munch M.W., Myatra S.N., Vijayaraghavan B.K.T., Saseedharan S., Benfield T., Wahlin R.R., et al. Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and severe hypoxemia: the COVID STEROID 2 randomized trial. JAMA. 2021;326:1807–1817. doi: 10.1001/jama.2021.18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abani O., Abbas A., Abbas F., Abbas J., Abbas K., Abbas M., et al. Higher dose corticosteroids in patients admitted to hospital with COVID-19 who are hypoxic but not requiring ventilatory support (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2023;401:1499–1507. doi: 10.1016/S0140-6736(23)00510-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Royston P., White I. Multiple imputation by chained equations (MICE): implementation in Stata. J Stat Soft. 2011;45:1–20. doi: 10.18637/jss.v045.i04. [DOI] [Google Scholar]
- 9.Langer-Gould A., Xu S., Myers L.C., Chen A., Greene J.D., Creekmur B., et al. High-dose corticosteroids in patients hospitalized for COVID-19 pneumonia: an observational study of comparative effectiveness. Int. J. Infect. Dis. 2022;125:184–191. doi: 10.1016/j.ijid.2022.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz A., Altshuler D., Papadopoulos J., Amoroso N., Goldenberg R., Tarras E., et al. The use of high-dose corticosteroids versus low-dose corticosteroids with and without tocilizumab in COVID-19 acute respiratory distress syndrome. Ann. Pharmacother. 2023;57:5–15. doi: 10.1177/10600280221094571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar G., Patel D., Hererra M., Jefferies D., Sakhuja A., Meersman M., et al. Do high-dose corticosteroids improve outcomes in hospitalized COVID-19 patients? J. Med. Virol. 2022;94:372–379. doi: 10.1002/jmv.27357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiao W., Meng L., Zhang Y., Li D., Chen J., Wang J., et al. Safety and efficacy of glucocorticoids in the treatment of COVID-19: a meta-analysis of randomized control trials. Expert Rev Respir Med. 2023;17:81–96. doi: 10.1080/17476348.2023.2177155. [DOI] [PubMed] [Google Scholar]
- 13.Ko J.J., Wu C., Mehta N., Wald-Dickler N., Yang W., Qiao R. A comparison of methylprednisolone and dexamethasone in intensive care patients with COVID-19. J. Intensive Care Med. 2021;36(6):673–680. doi: 10.1177/0885066621994057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.