Abstract
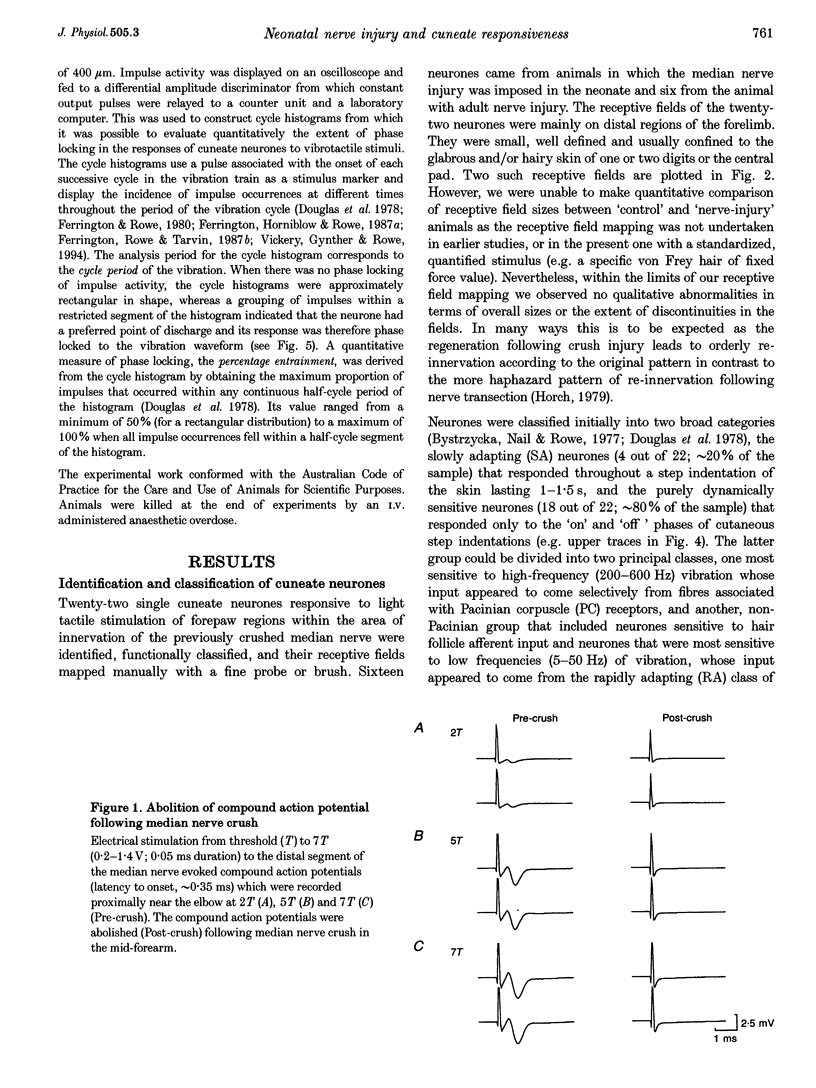
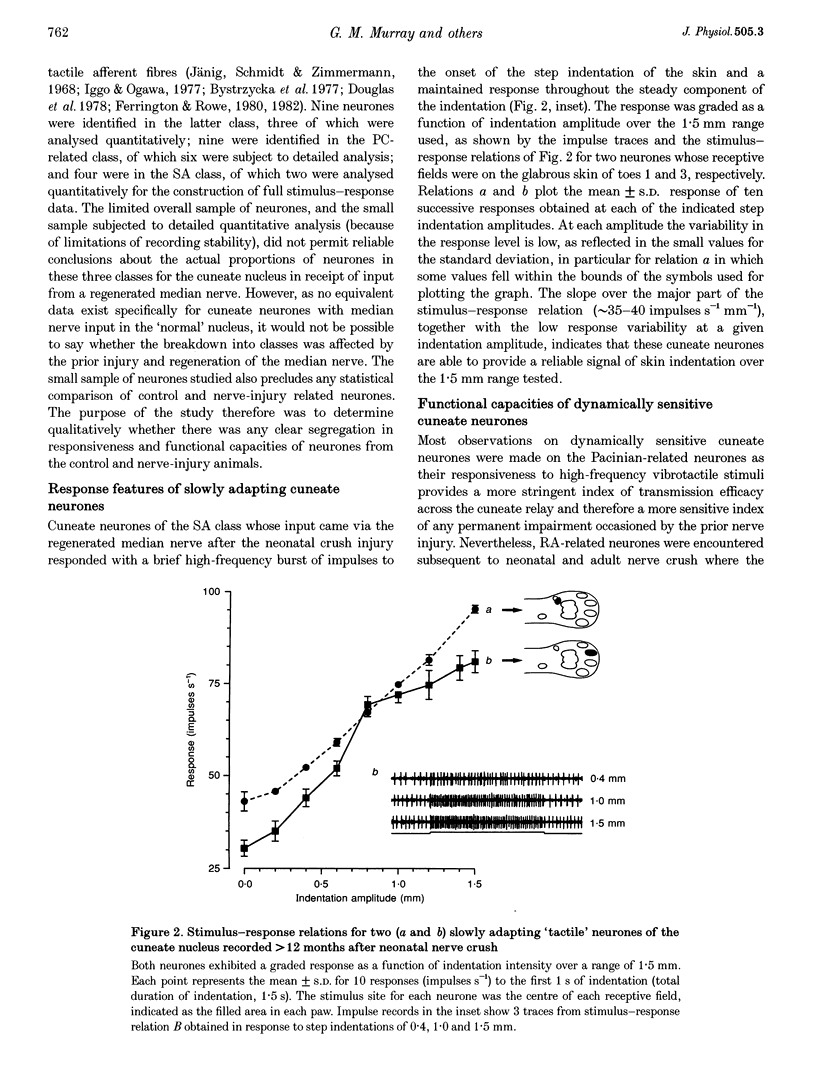
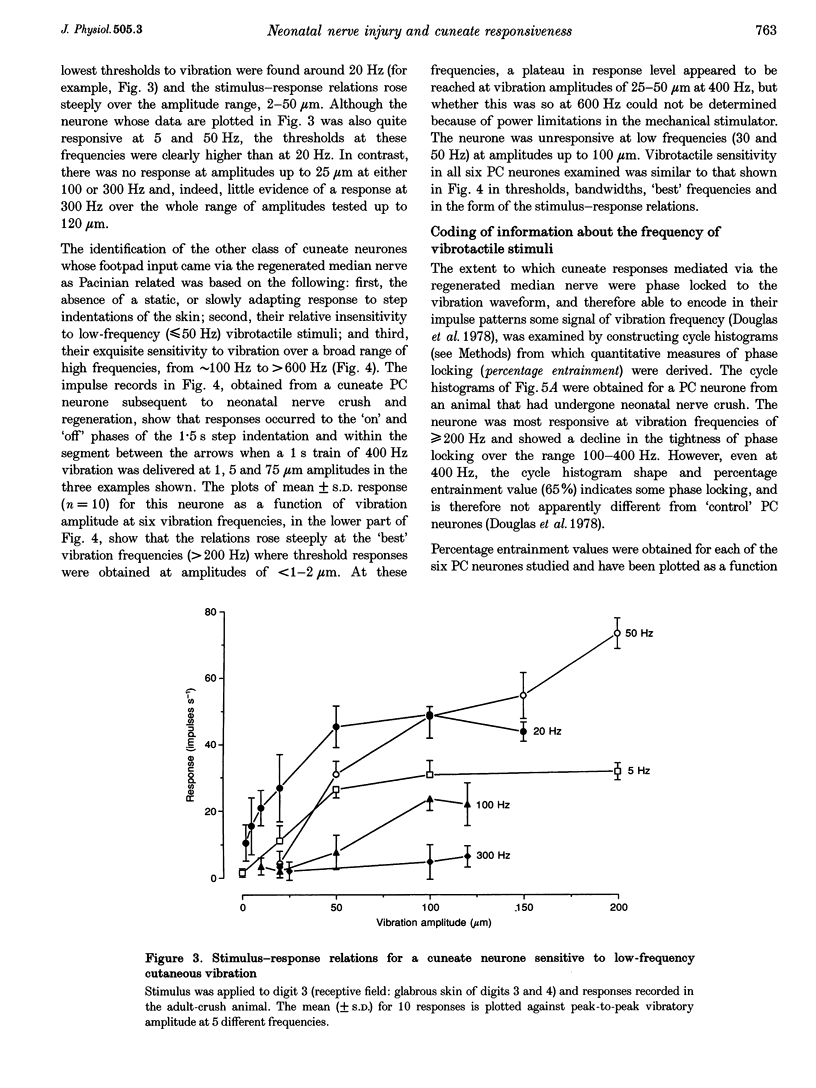
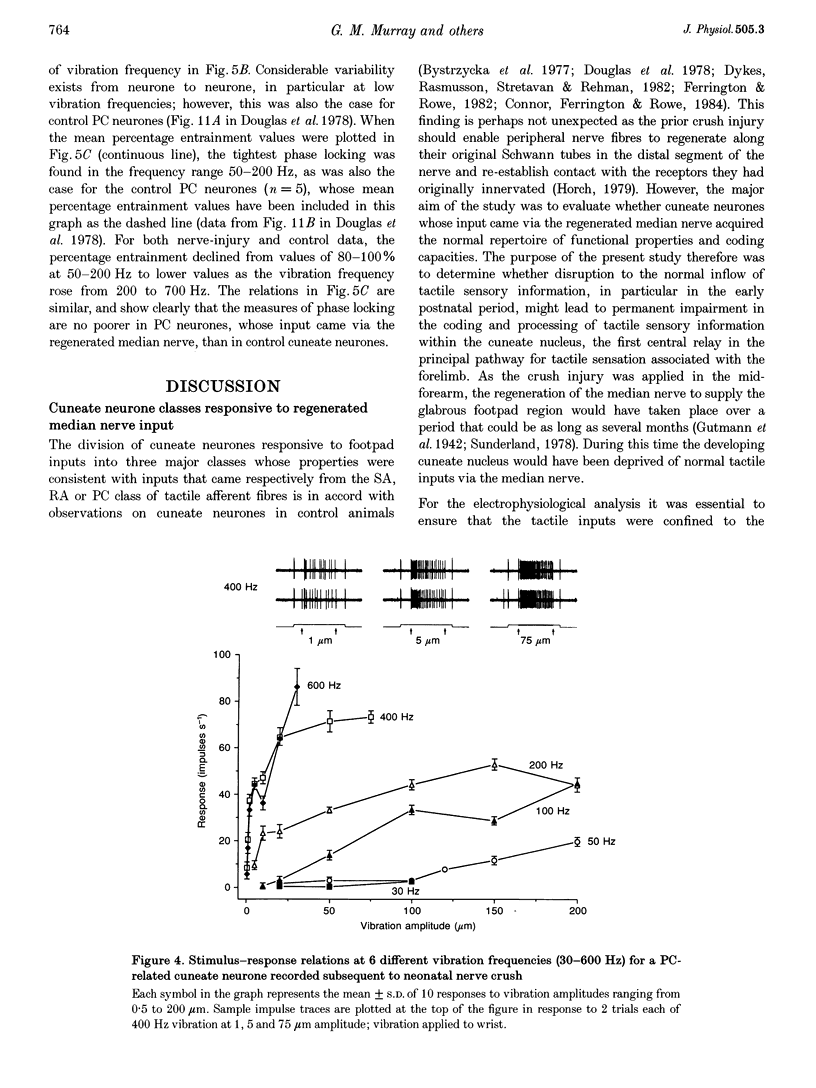
1. The capacity of cuneate neurones to attain normal functional properties following neonatal median nerve injury was investigated with single neurone recording in anaesthetized cats, 12-24 months subsequent to a controlled crush injury. Effectiveness of the peripheral nerve injury was confirmed by the abolition of the median nerve compound action potential following the crush. 2. Cuneate recording was carried out after denervation of the forearm, apart from the median nerve, to ensure that neurones studied had receptive fields within the distribution zone of the regenerated median nerve. Controlled and reproducible tactile stimuli were used to evaluate the functional capacities of neurones to determine whether they were consistent with those reported earlier for cuneate neurones in cats that had normal peripheral nerve development. 3. Twenty-two cuneate neurones with well-defined tactile receptive fields within the distribution zone of the regenerated median nerve were classified according to their adaptation characteristics and functional properties. Slowly adapting neurones responded throughout static skin indentations and had graded and approximately linear stimulus-response relations over indentation ranges up to 1.5 mm. Rapidly adapting neurones responded to the dynamic phases of skin indentations and could be divided into two broad classes, one most sensitive to vibrotactile stimuli at 200-400 Hz which appeared to receive a predominant input from Pacinian corpuscle receptors, and a non-Pacinian group that included neurones most sensitive to skin vibration at 5-50 Hz which appeared to receive glabrous skin input from the rapidly adapting class of afferent fibres. 4. Based on the stimulus-response relations and on measures of phase locking in the responses to vibrotactile stimuli, it appears that the functional properties of cuneate neurones activated from the field of a regenerated median nerve subsequent to a neonatal nerve crush injury were consistent with those reported previously for 'control' cuneate neurones. The results indicate that cuneate neurones can acquire normal tactile coding capacities despite the disruption caused by prior crush injury to their peripheral nerve source.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUEKER E. K., MEYERS C. E. The maturity of peripheral nerves at the time of injury as a factor in nerve regeneration. Anat Rec. 1951 Apr;109(4):723–743. doi: 10.1002/ar.1091090409. [DOI] [PubMed] [Google Scholar]
- Basbaum A. I., Wall P. D. Chronic changes in the response of cells in adult cat dorsal horn following partial deafferentation: the appearance of responding cells in a previously non-responsive region. Brain Res. 1976 Nov 5;116(2):181–204. doi: 10.1016/0006-8993(76)90899-4. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Brown P. B., Fyffe R. E., Pubols L. M. Effects of dorsal root section on spinocervical tract neurones in the cat. J Physiol. 1983 Apr;337:589–608. doi: 10.1113/jphysiol.1983.sp014644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E., Noble R., Rowe M. J. Effects of hind limb nerve section on lumbosacral dorsal horn neurones in the cat. J Physiol. 1984 Sep;354:375–394. doi: 10.1113/jphysiol.1984.sp015382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P. R., Horch K. W. Specific regeneration of cutaneous fibers in the cat. J Neurophysiol. 1973 Jan;36(1):101–114. doi: 10.1152/jn.1973.36.1.101. [DOI] [PubMed] [Google Scholar]
- Bystrzycka E., NAil B. S., Rowe M. Inhibition of cuneate neurones: its afferent source and influence on dynamically sensitive "tactile" neurones. J Physiol. 1977 Jun;268(1):251–270. doi: 10.1113/jphysiol.1977.sp011856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford M. B., Tweedale R. Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature. 1988 Mar 31;332(6163):446–448. doi: 10.1038/332446a0. [DOI] [PubMed] [Google Scholar]
- Connor K. M., Ferrington D. G., Rowe M. J. Tactile sensory coding during development: signaling capacities of neurons in kitten dorsal column nuclei. J Neurophysiol. 1984 Jul;52(1):86–98. doi: 10.1152/jn.1984.52.1.86. [DOI] [PubMed] [Google Scholar]
- Devor M., Wall P. D. Effect of peripheral nerve injury on receptive fields of cells in the cat spinal cord. J Comp Neurol. 1981 Jun 20;199(2):277–291. doi: 10.1002/cne.901990209. [DOI] [PubMed] [Google Scholar]
- Dostrovsky J. O., Millar J., Wall P. D. The immediate shift of afferent drive to dorsal column nucleus cells following deafferentation: a comparison of acute and chronic deafferentation in gracile nucleus and spinal cord. Exp Neurol. 1976 Sep;52(3):480–495. doi: 10.1016/0014-4886(76)90219-3. [DOI] [PubMed] [Google Scholar]
- Douglas P. R., Ferrington D. G., Rowe M. Coding of information about tactile stimuli by neurones of the cuneate nucleus. J Physiol. 1978 Dec;285:493–513. doi: 10.1113/jphysiol.1978.sp012585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykes R. W., Rasmusson D. D., Sretavan D., Rehman N. B. Submodality segregation and receptive-field sequences in cuneate, gracile, and external cuneate nuclei of the cat. J Neurophysiol. 1982 Mar;47(3):389–416. doi: 10.1152/jn.1982.47.3.389. [DOI] [PubMed] [Google Scholar]
- Dykes R. W., Terzis J. K. Reinnervation of glabrous skin in baboons: properties of cutaneous mechanoreceptors subsequent to nerve crush. J Neurophysiol. 1979 Sep;42(5):1461–1478. doi: 10.1152/jn.1979.42.5.1461. [DOI] [PubMed] [Google Scholar]
- Ferrington D. G., Hora M. O., Rowe M. J. Functional maturation of tactile sensory fibers in the kitten. J Neurophysiol. 1984 Jul;52(1):74–85. doi: 10.1152/jn.1984.52.1.74. [DOI] [PubMed] [Google Scholar]
- Ferrington D. G., Horniblow S., Rowe M. J. Temporal patterning in the responses of gracile and cuneate neurones in the cat to cutaneous vibration. J Physiol. 1987 May;386:277–291. doi: 10.1113/jphysiol.1987.sp016534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J. Functional capacities of tactile afferent fibres in neonatal kittens. J Physiol. 1980 Oct;307:335–353. doi: 10.1113/jphysiol.1980.sp013438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J. Specificity of connections and tactile coding capacities in cuneate nucleus of the neonatal kitten. J Neurophysiol. 1982 Apr;47(4):622–640. doi: 10.1152/jn.1982.47.4.622. [DOI] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J., Tarvin R. P. Actions of single sensory fibres on cat dorsal column nuclei neurones: vibratory signalling in a one-to-one linkage. J Physiol. 1987 May;386:293–309. doi: 10.1113/jphysiol.1987.sp016535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTH L. Regeneration in the mammalian peripheral nervous system. Physiol Rev. 1956 Oct;36(4):441–478. doi: 10.1152/physrev.1956.36.4.441. [DOI] [PubMed] [Google Scholar]
- Horch K. Guidance of regrowing sensory axons after cutaneous nerve lesions in the cat. J Neurophysiol. 1979 Sep;42(5):1437–1449. doi: 10.1152/jn.1979.42.5.1437. [DOI] [PubMed] [Google Scholar]
- Iggo A., Ogawa H. Correlative physiological and morphological studies of rapidly adapting mechanoreceptors in cat's glabrous skin. J Physiol. 1977 Apr;266(2):275–296. doi: 10.1113/jphysiol.1977.sp011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenq C. B., Jenq L. L., Coggeshall R. E. Numerical patterns of axon regeneration that follow sciatic nerve crush in the neonatal rat. Exp Neurol. 1987 Feb;95(2):492–499. doi: 10.1016/0014-4886(87)90155-5. [DOI] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Single unit responses and the total afferent outflow from the cat's foot pad upon mechanical stimulation. Exp Brain Res. 1968;6(2):100–115. doi: 10.1007/BF00239165. [DOI] [PubMed] [Google Scholar]
- Koerber H. R., Brown P. B. Quantitative analysis of dorsal horn cell receptive fields following limited deafferentation. J Neurophysiol. 1995 Nov;74(5):2065–2076. doi: 10.1152/jn.1995.74.5.2065. [DOI] [PubMed] [Google Scholar]
- Lisney S. J. Changes in the somatotopic organization of the cat lumbar spinal cord following peripheral nerve transection and regeneration. Brain Res. 1983 Jan 17;259(1):31–39. doi: 10.1016/0006-8993(83)91064-8. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Kaas J. H., Wall J., Nelson R. J., Sur M., Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983 Jan;8(1):33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Nicolelis M. A., Lin R. C., Woodward D. J., Chapin J. K. Induction of immediate spatiotemporal changes in thalamic networks by peripheral block of ascending cutaneous information. Nature. 1993 Feb 11;361(6412):533–536. doi: 10.1038/361533a0. [DOI] [PubMed] [Google Scholar]
- Pettit M. J., Schwark H. D. Receptive field reorganization in dorsal column nuclei during temporary denervation. Science. 1993 Dec 24;262(5142):2054–2056. doi: 10.1126/science.8266104. [DOI] [PubMed] [Google Scholar]
- Risling M., Aldskogius H., Hildebrand C. Effects of sciatic nerve crush on the L7 spinal roots and dorsal root ganglia in kittens. Exp Neurol. 1983 Jan;79(1):176–187. doi: 10.1016/0014-4886(83)90389-8. [DOI] [PubMed] [Google Scholar]
- Talbot W. H., Darian-Smith I., Kornhuber H. H., Mountcastle V. B. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- Vickery R. M., Gynther B. D., Rowe M. J. Synaptic transmission between single slowly adapting type I fibres and their cuneate target neurones in cat. J Physiol. 1994 Feb 1;474(3):379–392. doi: 10.1113/jphysiol.1994.sp020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J. T., Huerta M. F., Kaas J. H. Changes in the cortical map of the hand following postnatal median nerve injury in monkeys: modification of somatotopic aggregates. J Neurosci. 1992 Sep;12(9):3445–3455. doi: 10.1523/JNEUROSCI.12-09-03445.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P. Absence of mediolateral reorganization of dorsal horn somatotopy after peripheral deafferentation in the cat. Exp Neurol. 1987 Feb;95(2):432–447. doi: 10.1016/0014-4886(87)90150-6. [DOI] [PubMed] [Google Scholar]


