Abstract
This lecture is dedicated to Max Delbrück and Seymour Benzer. Max Delbrück was our graduate advisor. He introduced us to a variety of biophysical problems, and taught us ways of thinking about these problems by example. Potassium channels was one of the topics included in his journal club in the early seventies; Max also carefully considered the feasibility of purifying potassium channels then. It was in Seymour Benzer's laboratory that we began to look for Drosophila mutants that affect synaptic transmission at the larval neuromuscular junction. Shaker was the first behavioural mutant we tested that gave a robust phenotype, a phenotype that could be mimicked by treating wild-type preparations with a potassium channel blocker. This mutant fly has led us to our subsequent molecular studies of potassium channels.
Since we settled in the University of California, San Francisco, and began to study neural development as well as potassium channels, we have settled into the pattern of each attending meetings and presenting our studies on one of these two areas so as to avoid both being away from home and our children at the same time. In following this pattern, I will be presenting the studies of potassium channels as part of our long-term collaboration. In this talk I will first briefly take you through the path that led us to the molecular studies of potassium channels and then discuss the diversity and modulation of these potassium channels at the molecular and physiological level.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A. Luteinizing hormone-releasing factor and muscarinic agonists act on the same voltage-sensitive K+-current in bullfrog sympathetic neurones. Br J Pharmacol. 1980 Mar;68(3):353–355. doi: 10.1111/j.1476-5381.1980.tb14547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiyar J., Nguyen A. N., Chandy K. G., Grissmer S. The P-region and S6 of Kv3.1 contribute to the formation of the ion conduction pathway. Biophys J. 1994 Dec;67(6):2261–2264. doi: 10.1016/S0006-3495(94)80710-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrov A., Velimirovic B., Clapham D. E. Inward rectification of the IRK1 K+ channel reconstituted in lipid bilayers. Biophys J. 1996 Jun;70(6):2680–2687. doi: 10.1016/S0006-3495(96)79837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973 Apr 13;242(5398):459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Bausch S. B., Patterson T. A., Ehrengruber M. U., Lester H. A., Davidson N., Chavkin C. Colocalization of mu opioid receptors with GIRK1 potassium channels in the rat brain: an immunocytochemical study. Receptors Channels. 1995;3(3):221–241. [PubMed] [Google Scholar]
- Bezanilla F., Stefani E. Voltage-dependent gating of ionic channels. Annu Rev Biophys Biomol Struct. 1994;23:819–846. doi: 10.1146/annurev.bb.23.060194.004131. [DOI] [PubMed] [Google Scholar]
- Bosma M. M., Bernheim L., Leibowitz M. D., Pfaffinger P. J., Hille B. Modulation of M current in frog sympathetic ganglion cells. Soc Gen Physiol Ser. 1990;45:43–59. [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A. G-proteins and potassium currents in neurons. Annu Rev Physiol. 1990;52:215–242. doi: 10.1146/annurev.ph.52.030190.001243. [DOI] [PubMed] [Google Scholar]
- Brown D. M-currents: an update. Trends Neurosci. 1988 Jul;11(7):294–299. doi: 10.1016/0166-2236(88)90089-6. [DOI] [PubMed] [Google Scholar]
- Byrne J. H. Quantitative aspects of ionic conductance mechanisms contributing to firing pattern of motor cells mediating inking behavior in Aplysia californica. J Neurophysiol. 1980 Mar;43(3):651–668. doi: 10.1152/jn.1980.43.3.651. [DOI] [PubMed] [Google Scholar]
- Choi K. L., Mossman C., Aubé J., Yellen G. The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron. 1993 Mar;10(3):533–541. doi: 10.1016/0896-6273(93)90340-w. [DOI] [PubMed] [Google Scholar]
- Chouinard S. W., Wilson G. F., Schlimgen A. K., Ganetzky B. A potassium channel beta subunit related to the aldo-keto reductase superfamily is encoded by the Drosophila hyperkinetic locus. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6763–6767. doi: 10.1073/pnas.92.15.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie M. J., North R. A., Osborne P. B., Douglass J., Adelman J. P. Heteropolymeric potassium channels expressed in Xenopus oocytes from cloned subunits. Neuron. 1990 Mar;4(3):405–411. doi: 10.1016/0896-6273(90)90052-h. [DOI] [PubMed] [Google Scholar]
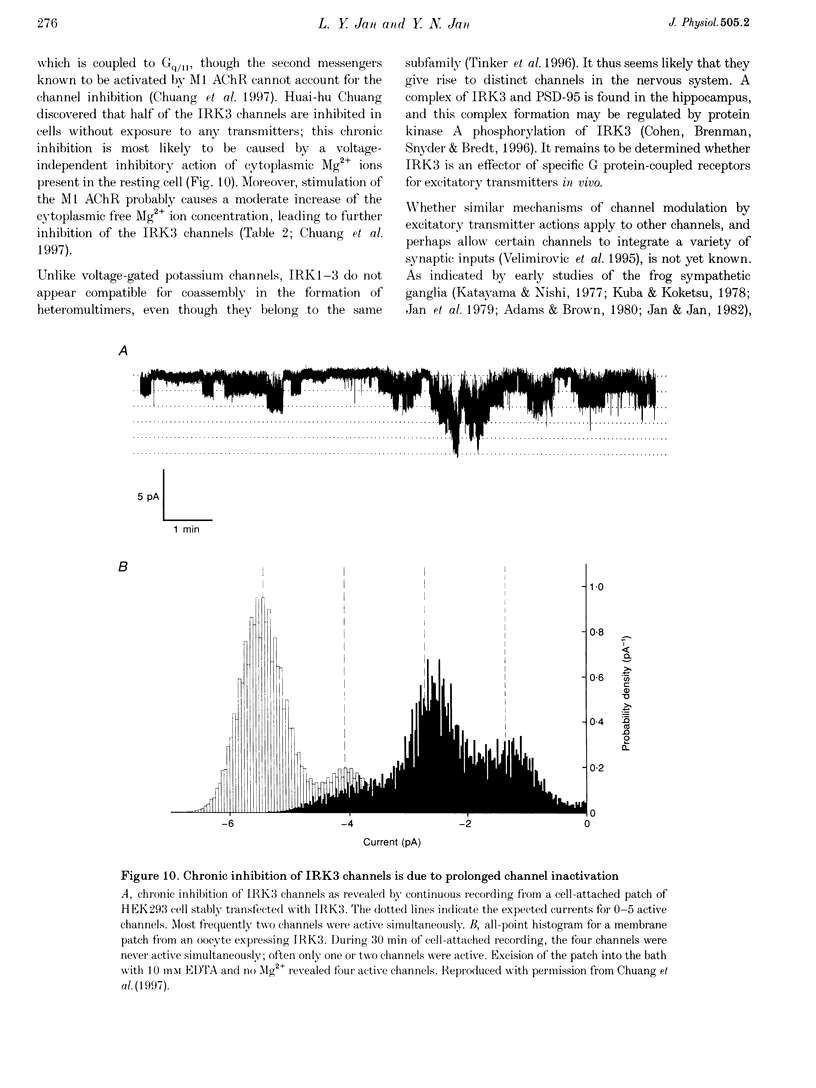
- Chuang H., Jan Y. N., Jan L. Y. Regulation of IRK3 inward rectifier K+ channel by m1 acetylcholine receptor and intracellular magnesium. Cell. 1997 Jun 27;89(7):1121–1132. doi: 10.1016/s0092-8674(00)80299-8. [DOI] [PubMed] [Google Scholar]
- Cohen N. A., Brenman J. E., Snyder S. H., Bredt D. S. Binding of the inward rectifier K+ channel Kir 2.3 to PSD-95 is regulated by protein kinase A phosphorylation. Neuron. 1996 Oct;17(4):759–767. doi: 10.1016/s0896-6273(00)80207-x. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias M., Wei A. A., Salkoff L. Shaker, Shal, Shab, and Shaw express independent K+ current systems. Neuron. 1991 Nov;7(5):763–773. doi: 10.1016/0896-6273(91)90279-9. [DOI] [PubMed] [Google Scholar]
- Dascal N., Schreibmayer W., Lim N. F., Wang W., Chavkin C., DiMagno L., Labarca C., Kieffer B. L., Gaveriaux-Ruff C., Trollinger D. Atrial G protein-activated K+ channel: expression cloning and molecular properties. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10235–10239. doi: 10.1073/pnas.90.21.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schutter E., Bower J. M. An active membrane model of the cerebellar Purkinje cell. I. Simulation of current clamps in slice. J Neurophysiol. 1994 Jan;71(1):375–400. doi: 10.1152/jn.1994.71.1.375. [DOI] [PubMed] [Google Scholar]
- Debanne D., Guérineau N. C., Gähwiler B. H., Thompson S. M. Action-potential propagation gated by an axonal I(A)-like K+ conductance in hippocampus. Nature. 1997 Sep 18;389(6648):286–289. doi: 10.1038/38502. [DOI] [PubMed] [Google Scholar]
- Ducreux C., Puizillout J. J. A-current modifies the spike of C-type neurones in the rabbit nodose ganglion. J Physiol. 1995 Jul 15;486(Pt 2):439–451. doi: 10.1113/jphysiol.1995.sp020824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler B., Brändle U., Bond C., Glowatzki E., König C., Adelman J. P., Zenner H. P., Ruppersberg J. P. A structural determinant of differential sensitivity of cloned inward rectifier K+ channels to intracellular spermine. FEBS Lett. 1994 Dec 19;356(2-3):199–203. doi: 10.1016/0014-5793(94)01258-x. [DOI] [PubMed] [Google Scholar]
- Fakler B., Brändle U., Glowatzki E., Weidemann S., Zenner H. P., Ruppersberg J. P. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 1995 Jan 13;80(1):149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- Ficker E., Taglialatela M., Wible B. A., Henley C. M., Brown A. M. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994 Nov 11;266(5187):1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- Golowasch J., Buchholtz F., Epstein I. R., Marder E. Contribution of individual ionic currents to activity of a model stomatogastric ganglion neuron. J Neurophysiol. 1992 Feb;67(2):341–349. doi: 10.1152/jn.1992.67.2.341. [DOI] [PubMed] [Google Scholar]
- Grigg J. J., Kozasa T., Nakajima Y., Nakajima S. Single-channel properties of a G-protein-coupled inward rectifier potassium channel in brain neurons. J Neurophysiol. 1996 Jan;75(1):318–328. doi: 10.1152/jn.1996.75.1.318. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO N. Membrane changes of Onchidium nerve cell in potassium-rich media. J Physiol. 1961 Mar;155:470–489. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Jaffe L. A. Electrical properties of egg cell membranes. Annu Rev Biophys Bioeng. 1979;8:385–416. doi: 10.1146/annurev.bb.08.060179.002125. [DOI] [PubMed] [Google Scholar]
- Hartmann H. A., Kirsch G. E., Drewe J. A., Taglialatela M., Joho R. H., Brown A. M. Exchange of conduction pathways between two related K+ channels. Science. 1991 Feb 22;251(4996):942–944. doi: 10.1126/science.2000495. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994 Dec;17(12):531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K., Nichols C. G., Lederer W. J., Lytton J., Vassilev P. M., Kanazirska M. V., Hebert S. C. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993 Mar 4;362(6415):31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Hoffman D. A., Magee J. C., Colbert C. M., Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997 Jun 26;387(6636):869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Hopkins W. F., Demas V., Tempel B. L. Both N- and C-terminal regions contribute to the assembly and functional expression of homo- and heteromultimeric voltage-gated K+ channels. J Neurosci. 1994 Mar;14(3 Pt 1):1385–1393. doi: 10.1523/JNEUROSCI.14-03-01385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L., Jan Y. N., Jan L. Y. Binding of the G protein betagamma subunit to multiple regions of G protein-gated inward-rectifying K+ channels. FEBS Lett. 1997 Apr 1;405(3):291–298. doi: 10.1016/s0014-5793(97)00197-x. [DOI] [PubMed] [Google Scholar]
- Huang C. L., Slesinger P. A., Casey P. J., Jan Y. N., Jan L. Y. Evidence that direct binding of G beta gamma to the GIRK1 G protein-gated inwardly rectifying K+ channel is important for channel activation. Neuron. 1995 Nov;15(5):1133–1143. doi: 10.1016/0896-6273(95)90101-9. [DOI] [PubMed] [Google Scholar]
- Huang X. Y., Morielli A. D., Peralta E. G. Tyrosine kinase-dependent suppression of a potassium channel by the G protein-coupled m1 muscarinic acetylcholine receptor. Cell. 1993 Dec 17;75(6):1145–1156. doi: 10.1016/0092-8674(93)90324-j. [DOI] [PubMed] [Google Scholar]
- Inanobe A., Ito H., Ito M., Hosoya Y., Kurachi Y. Immunological and physical characterization of the brain G protein-gated muscarinic potassium channel. Biochem Biophys Res Commun. 1995 Dec 26;217(3):1238–1244. doi: 10.1006/bbrc.1995.2901. [DOI] [PubMed] [Google Scholar]
- Isacoff E. Y., Jan Y. N., Jan L. Y. Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes. Nature. 1990 Jun 7;345(6275):530–534. doi: 10.1038/345530a0. [DOI] [PubMed] [Google Scholar]
- Isacoff E. Y., Jan Y. N., Jan L. Y. Putative receptor for the cytoplasmic inactivation gate in the Shaker K+ channel. Nature. 1991 Sep 5;353(6339):86–90. doi: 10.1038/353086a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. L-glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol. 1976 Oct;262(1):215–236. doi: 10.1113/jphysiol.1976.sp011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Peptidergic transmission in sympathetic ganglia of the frog. J Physiol. 1982 Jun;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Potassium channels and their evolving gates. Nature. 1994 Sep 8;371(6493):119–122. doi: 10.1038/371119a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol. 1976 Oct;262(1):189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Dennis M. J. Two mutations of synaptic transmission in Drosophila. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):87–108. doi: 10.1098/rspb.1977.0087. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Kuffler S. W. A peptide as a possible transmitter in sympathetic ganglia of the frog. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1501–1505. doi: 10.1073/pnas.76.3.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegla T., Salkoff L. A novel subunit for shal K+ channels radically alters activation and inactivation. J Neurosci. 1997 Jan 1;17(1):32–44. doi: 10.1523/JNEUROSCI.17-01-00032.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaang B. K., Pfaffinger P. J., Grant S. G., Kandel E. R., Furukawa Y. Overexpression of an Aplysia shaker K+ channel gene modifies the electrical properties and synaptic efficacy of identified Aplysia neurons. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1133–1137. doi: 10.1073/pnas.89.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamb A., Tseng-Crank J., Tanouye M. A. Multiple products of the Drosophila Shaker gene may contribute to potassium channel diversity. Neuron. 1988 Jul;1(5):421–430. doi: 10.1016/0896-6273(88)90192-4. [DOI] [PubMed] [Google Scholar]
- Karschin C., Dissmann E., Stühmer W., Karschin A. IRK(1-3) and GIRK(1-4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci. 1996 Jun 1;16(11):3559–3570. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh M. P., Hurst R. S., Yakel J., Varnum M. D., Adelman J. P., North R. A. Multiple subunits of a voltage-dependent potassium channel contribute to the binding site for tetraethylammonium. Neuron. 1992 Mar;8(3):493–497. doi: 10.1016/0896-6273(92)90277-k. [DOI] [PubMed] [Google Scholar]
- Kim E., Niethammer M., Rothschild A., Jan Y. N., Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995 Nov 2;378(6552):85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- Kim K. M., Nakajima Y., Nakajima S. G protein-coupled inward rectifier modulated by dopamine agonists in cultured substantia nigra neurons. Neuroscience. 1995 Dec;69(4):1145–1158. doi: 10.1016/0306-4522(95)00326-e. [DOI] [PubMed] [Google Scholar]
- Kofuji P., Davidson N., Lester H. A. Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by G beta gamma subunits and function as heteromultimers. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6542–6546. doi: 10.1073/pnas.92.14.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P., Hofer M., Millen K. J., Millonig J. H., Davidson N., Lester H. A., Hatten M. E. Functional analysis of the weaver mutant GIRK2 K+ channel and rescue of weaver granule cells. Neuron. 1996 May;16(5):941–952. doi: 10.1016/s0896-6273(00)80117-8. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G., Gordon E. A., Wickman K., Velimirović B., Krapivinsky L., Clapham D. E. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature. 1995 Mar 9;374(6518):135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G., Krapivinsky L., Wickman K., Clapham D. E. G beta gamma binds directly to the G protein-gated K+ channel, IKACh. J Biol Chem. 1995 Dec 8;270(49):29059–29062. doi: 10.1074/jbc.270.49.29059. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Synaptic events in sympathetic ganglia. Prog Neurobiol. 1978;11(2):77–169. doi: 10.1016/0301-0082(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Kubo Y., Baldwin T. J., Jan Y. N., Jan L. Y. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993 Mar 11;362(6416):127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Kubo Y. Effects of extracellular cations and mutations in the pore region on the inward rectifier K+ channel IRK1. Receptors Channels. 1996;4(2):73–83. [PubMed] [Google Scholar]
- Kubo Y., Reuveny E., Slesinger P. A., Jan Y. N., Jan L. Y. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 1993 Aug 26;364(6440):802–806. doi: 10.1038/364802a0. [DOI] [PubMed] [Google Scholar]
- Kunkel M. T., Peralta E. G. Identification of domains conferring G protein regulation on inward rectifier potassium channels. Cell. 1995 Nov 3;83(3):443–449. doi: 10.1016/0092-8674(95)90122-1. [DOI] [PubMed] [Google Scholar]
- Kurachi Y. G protein regulation of cardiac muscarinic potassium channel. Am J Physiol. 1995 Oct;269(4 Pt 1):C821–C830. doi: 10.1152/ajpcell.1995.269.4.C821. [DOI] [PubMed] [Google Scholar]
- Larsson H. P., Baker O. S., Dhillon D. S., Isacoff E. Y. Transmembrane movement of the shaker K+ channel S4. Neuron. 1996 Feb;16(2):387–397. doi: 10.1016/s0896-6273(00)80056-2. [DOI] [PubMed] [Google Scholar]
- Li M., Jan Y. N., Jan L. Y. Specification of subunit assembly by the hydrophilic amino-terminal domain of the Shaker potassium channel. Science. 1992 Aug 28;257(5074):1225–1230. doi: 10.1126/science.1519059. [DOI] [PubMed] [Google Scholar]
- Li M., Unwin N., Stauffer K. A., Jan Y. N., Jan L. Y. Images of purified Shaker potassium channels. Curr Biol. 1994 Feb 1;4(2):110–115. doi: 10.1016/s0960-9822(94)00026-6. [DOI] [PubMed] [Google Scholar]
- Liao Y. J., Jan Y. N., Jan L. Y. Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J Neurosci. 1996 Nov 15;16(22):7137–7150. doi: 10.1523/JNEUROSCI.16-22-07137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman E. R., Tytgat J., Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992 Nov;9(5):861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987 Jan 22;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Lopatin A. N., Makhina E. N., Nichols C. G. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994 Nov 24;372(6504):366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- Lopez G. A., Jan Y. N., Jan L. Y. Evidence that the S6 segment of the Shaker voltage-gated K+ channel comprises part of the pore. Nature. 1994 Jan 13;367(6459):179–182. doi: 10.1038/367179a0. [DOI] [PubMed] [Google Scholar]
- Lu Z., MacKinnon R. Electrostatic tuning of Mg2+ affinity in an inward-rectifier K+ channel. Nature. 1994 Sep 15;371(6494):243–246. doi: 10.1038/371243a0. [DOI] [PubMed] [Google Scholar]
- Ma M., Koester J. The role of K+ currents in frequency-dependent spike broadening in Aplysia R20 neurons: a dynamic-clamp analysis. J Neurosci. 1996 Jul 1;16(13):4089–4101. doi: 10.1523/JNEUROSCI.16-13-04089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991 Mar 21;350(6315):232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- Mannuzzu L. M., Moronne M. M., Isacoff E. Y. Direct physical measure of conformational rearrangement underlying potassium channel gating. Science. 1996 Jan 12;271(5246):213–216. doi: 10.1126/science.271.5246.213. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- McCormack K., Lin J. W., Iverson L. E., Rudy B. Shaker K+ channel subunits from heteromultimeric channels with novel functional properties. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1361–1371. doi: 10.1016/0006-291x(90)90836-c. [DOI] [PubMed] [Google Scholar]
- Miyashita T., Kubo Y. Localization and developmental changes of the expression of two inward rectifying K(+)-channel proteins in the rat brain. Brain Res. 1997 Mar 7;750(1-2):251–263. doi: 10.1016/s0006-8993(96)01365-0. [DOI] [PubMed] [Google Scholar]
- Nair L. A., Inglese J., Stoffel R., Koch W. J., Lefkowitz R. J., Kwatra M. M., Grant A. O. Cardiac muscarinic potassium channel activity is attenuated by inhibitors of G beta gamma. Circ Res. 1995 May;76(5):832–838. doi: 10.1161/01.res.76.5.832. [DOI] [PubMed] [Google Scholar]
- Nakahira K., Shi G., Rhodes K. J., Trimmer J. S. Selective interaction of voltage-gated K+ channel beta-subunits with alpha-subunits. J Biol Chem. 1996 Mar 22;271(12):7084–7089. doi: 10.1074/jbc.271.12.7084. [DOI] [PubMed] [Google Scholar]
- Nakajima S. Analysis of K inactivation and TEA action in the supramedullary cells of puffer. J Gen Physiol. 1966 Mar;49(4):629–640. doi: 10.1085/jgp.49.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y., Nakajima S., Inoue M. Pertussis toxin-insensitive G protein mediates substance P-induced inhibition of potassium channels in brain neurons. Proc Natl Acad Sci U S A. 1988 May;85(10):3643–3647. doi: 10.1073/pnas.85.10.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro B., Kennedy M. E., Velimirovíc B., Bhat D., Peterson A. S., Clapham D. E. Nonselective and G betagamma-insensitive weaver K+ channels. Science. 1996 Jun 28;272(5270):1950–1953. doi: 10.1126/science.272.5270.1950. [DOI] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Quayle J. M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995 Apr;268(4 Pt 1):C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- North R. A. Twelfth Gaddum memorial lecture. Drug receptors and the inhibition of nerve cells. Br J Pharmacol. 1989 Sep;98(1):13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Uchimura N. 5-Hydroxytryptamine acts at 5-HT2 receptors to decrease potassium conductance in rat nucleus accumbens neurones. J Physiol. 1989 Oct;417:1–12. doi: 10.1113/jphysiol.1989.sp017786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian D. M., Schwarz T. L., Tempel B. L., Jan Y. N., Jan L. Y. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science. 1987 Aug 14;237(4816):749–753. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- Parcej D. N., Scott V. E., Dolly J. O. Oligomeric properties of alpha-dendrotoxin-sensitive potassium ion channels purified from bovine brain. Biochemistry. 1992 Nov 17;31(45):11084–11088. doi: 10.1021/bi00160a018. [DOI] [PubMed] [Google Scholar]
- Patil N., Cox D. R., Bhat D., Faham M., Myers R. M., Peterson A. S. A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nat Genet. 1995 Oct;11(2):126–129. doi: 10.1038/ng1095-126. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Ponce A., Bueno E., Kentros C., Vega-Saenz de Miera E., Chow A., Hillman D., Chen S., Zhu L., Wu M. B., Wu X. G-protein-gated inward rectifier K+ channel proteins (GIRK1) are present in the soma and dendrites as well as in nerve terminals of specific neurons in the brain. J Neurosci. 1996 Mar 15;16(6):1990–2001. doi: 10.1523/JNEUROSCI.16-06-01990.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O., Kecskemethy N., Müller R., Krah-Jentgens I., Baumann A., Kiltz H. H., Canal I., Llamazares S., Ferrus A. Shaker encodes a family of putative potassium channel proteins in the nervous system of Drosophila. EMBO J. 1988 Apr;7(4):1087–1096. doi: 10.1002/j.1460-2075.1988.tb02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar L. S., Gage P. W. Potassium channels activated by GABAB agonists and serotonin in cultured hippocampal neurons. J Neurophysiol. 1994 Jun;71(6):2570–2575. doi: 10.1152/jn.1994.71.6.2570. [DOI] [PubMed] [Google Scholar]
- Rakic P., Sidman R. L. Sequence of developmental abnormalities leading to granule cell deficit in cerebellar cortex of weaver mutant mice. J Comp Neurol. 1973 Nov 15;152(2):103–132. doi: 10.1002/cne.901520202. [DOI] [PubMed] [Google Scholar]
- Rettig J., Heinemann S. H., Wunder F., Lorra C., Parcej D. N., Dolly J. O., Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature. 1994 May 26;369(6478):289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- Reuveny E., Jan Y. N., Jan L. Y. Contributions of a negatively charged residue in the hydrophobic domain of the IRK1 inwardly rectifying K+ channel to K(+)-selective permeation. Biophys J. 1996 Feb;70(2):754–761. doi: 10.1016/S0006-3495(96)79615-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveny E., Slesinger P. A., Inglese J., Morales J. M., Iñiguez-Lluhi J. A., Lefkowitz R. J., Bourne H. R., Jan Y. N., Jan L. Y. Activation of the cloned muscarinic potassium channel by G protein beta gamma subunits. Nature. 1994 Jul 14;370(6485):143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Sewing S., Pongs O. Heteromultimeric channels formed by rat brain potassium-channel proteins. Nature. 1990 Jun 7;345(6275):535–537. doi: 10.1038/345535a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Noma A., Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983 May 19;303(5914):250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- Salkoff L., Baker K., Butler A., Covarrubias M., Pak M. D., Wei A. An essential 'set' of K+ channels conserved in flies, mice and humans. Trends Neurosci. 1992 May;15(5):161–166. doi: 10.1016/0166-2236(92)90165-5. [DOI] [PubMed] [Google Scholar]
- Schwarz T. L., Tempel B. L., Papazian D. M., Jan Y. N., Jan L. Y. Multiple potassium-channel components are produced by alternative splicing at the Shaker locus in Drosophila. Nature. 1988 Jan 14;331(6152):137–142. doi: 10.1038/331137a0. [DOI] [PubMed] [Google Scholar]
- Scott V. E., Rettig J., Parcej D. N., Keen J. N., Findlay J. B., Pongs O., Dolly J. O. Primary structure of a beta subunit of alpha-dendrotoxin-sensitive K+ channels from bovine brain. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1637–1641. doi: 10.1073/pnas.91.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoh S. A., Sigg D., Papazian D. M., Bezanilla F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron. 1996 Jun;16(6):1159–1167. doi: 10.1016/s0896-6273(00)80142-7. [DOI] [PubMed] [Google Scholar]
- Sewing S., Roeper J., Pongs O. Kv beta 1 subunit binding specific for shaker-related potassium channel alpha subunits. Neuron. 1996 Feb;16(2):455–463. doi: 10.1016/s0896-6273(00)80063-x. [DOI] [PubMed] [Google Scholar]
- Shen K. Z., North R. A. Muscarine increases cation conductance and decreases potassium conductance in rat locus coeruleus neurones. J Physiol. 1992 Sep;455:471–485. doi: 10.1113/jphysiol.1992.sp019312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen N. V., Chen X., Boyer M. M., Pfaffinger P. J. Deletion analysis of K+ channel assembly. Neuron. 1993 Jul;11(1):67–76. doi: 10.1016/0896-6273(93)90271-r. [DOI] [PubMed] [Google Scholar]
- Shen N. V., Pfaffinger P. J. Molecular recognition and assembly sequences involved in the subfamily-specific assembly of voltage-gated K+ channel subunit proteins. Neuron. 1995 Mar;14(3):625–633. doi: 10.1016/0896-6273(95)90319-4. [DOI] [PubMed] [Google Scholar]
- Sheng M., Liao Y. J., Jan Y. N., Jan L. Y. Presynaptic A-current based on heteromultimeric K+ channels detected in vivo. Nature. 1993 Sep 2;365(6441):72–75. doi: 10.1038/365072a0. [DOI] [PubMed] [Google Scholar]
- Sheng M. PDZs and receptor/channel clustering: rounding up the latest suspects. Neuron. 1996 Oct;17(4):575–578. doi: 10.1016/s0896-6273(00)80190-7. [DOI] [PubMed] [Google Scholar]
- Sheng M., Tsaur M. L., Jan Y. N., Jan L. Y. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 1992 Aug;9(2):271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- Shi G., Nakahira K., Hammond S., Rhodes K. J., Schechter L. E., Trimmer J. S. Beta subunits promote K+ channel surface expression through effects early in biosynthesis. Neuron. 1996 Apr;16(4):843–852. doi: 10.1016/s0896-6273(00)80104-x. [DOI] [PubMed] [Google Scholar]
- Shieh R. C., John S. A., Lee J. K., Weiss J. N. Inward rectification of the IRK1 channel expressed in Xenopus oocytes: effects of intracellular pH reveal an intrinsic gating mechanism. J Physiol. 1996 Jul 15;494(Pt 2):363–376. doi: 10.1113/jphysiol.1996.sp021498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorini S., Liao Y. J., Duncan S. A., Jan L. Y., Stoffel M. Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc Natl Acad Sci U S A. 1997 Feb 4;94(3):923–927. doi: 10.1073/pnas.94.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. Voltage gating of ion channels. Q Rev Biophys. 1994 Feb;27(1):1–40. doi: 10.1017/s0033583500002894. [DOI] [PubMed] [Google Scholar]
- Slesinger P. A., Jan Y. N., Jan L. Y. The S4-S5 loop contributes to the ion-selective pore of potassium channels. Neuron. 1993 Oct;11(4):739–749. doi: 10.1016/0896-6273(93)90083-4. [DOI] [PubMed] [Google Scholar]
- Slesinger P. A., Patil N., Liao Y. J., Jan Y. N., Jan L. Y., Cox D. R. Functional effects of the mouse weaver mutation on G protein-gated inwardly rectifying K+ channels. Neuron. 1996 Feb;16(2):321–331. doi: 10.1016/s0896-6273(00)80050-1. [DOI] [PubMed] [Google Scholar]
- Slesinger P. A., Reuveny E., Jan Y. N., Jan L. Y. Identification of structural elements involved in G protein gating of the GIRK1 potassium channel. Neuron. 1995 Nov;15(5):1145–1156. doi: 10.1016/0896-6273(95)90102-7. [DOI] [PubMed] [Google Scholar]
- Smeyne R. J., Goldowitz D. Development and death of external granular layer cells in the weaver mouse cerebellum: a quantitative study. J Neurosci. 1989 May;9(5):1608–1620. doi: 10.1523/JNEUROSCI.09-05-01608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodickson D. L., Bean B. P. GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J Neurosci. 1996 Oct 15;16(20):6374–6385. doi: 10.1523/JNEUROSCI.16-20-06374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R., Davies N. W., Shelton P. A., Sutcliffe M. J., Khan I. A., Brammar W. J., Conley E. C. A single aspartate residue is involved in both intrinsic gating and blockage by Mg2+ of the inward rectifier, IRK1. J Physiol. 1994 Jul 1;478(Pt 1):1–6. doi: 10.1113/jphysiol.1994.sp020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R., Nakajima Y., Yamaguchi K. Substance P raises neuronal membrane excitability by reducing inward rectification. Nature. 1985 Jun 6;315(6019):498–501. doi: 10.1038/315498a0. [DOI] [PubMed] [Google Scholar]
- Taglialatela M., Champagne M. S., Drewe J. A., Brown A. M. Comparison of H5, S6, and H5-S6 exchanges on pore properties of voltage-dependent K+ channels. J Biol Chem. 1994 May 13;269(19):13867–13873. [PubMed] [Google Scholar]
- Taglialatela M., Ficker E., Wible B. A., Brown A. M. C-terminus determinants for Mg2+ and polyamine block of the inward rectifier K+ channel IRK1. EMBO J. 1995 Nov 15;14(22):5532–5541. doi: 10.1002/j.1460-2075.1995.tb00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela M., Wible B. A., Caporaso R., Brown A. M. Specification of pore properties by the carboxyl terminus of inwardly rectifying K+ channels. Science. 1994 May 6;264(5160):844–847. doi: 10.1126/science.8171340. [DOI] [PubMed] [Google Scholar]
- Tanouye M. A., Ferrus A., Fujita S. C. Abnormal action potentials associated with the Shaker complex locus of Drosophila. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6548–6552. doi: 10.1073/pnas.78.10.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor F. J., Bokhari A., Rogero O., Gorczyca M., Zhang J., Kim E., Sheng M., Budnik V. Essential role for dlg in synaptic clustering of Shaker K+ channels in vivo. J Neurosci. 1997 Jan 1;17(1):152–159. doi: 10.1523/JNEUROSCI.17-01-00152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempel B. L., Papazian D. M., Schwarz T. L., Jan Y. N., Jan L. Y. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila. Science. 1987 Aug 14;237(4816):770–775. doi: 10.1126/science.2441471. [DOI] [PubMed] [Google Scholar]
- Terzic A., Jahangir A., Kurachi Y. Cardiac ATP-sensitive K+ channels: regulation by intracellular nucleotides and K+ channel-opening drugs. Am J Physiol. 1995 Sep;269(3 Pt 1):C525–C545. doi: 10.1152/ajpcell.1995.269.3.C525. [DOI] [PubMed] [Google Scholar]
- Tierney A. J., Harris-Warrick R. M. Physiological role of the transient potassium current in the pyloric circuit of the lobster stomatogastric ganglion. J Neurophysiol. 1992 Mar;67(3):599–609. doi: 10.1152/jn.1992.67.3.599. [DOI] [PubMed] [Google Scholar]
- Timpe L. C., Schwarz T. L., Tempel B. L., Papazian D. M., Jan Y. N., Jan L. Y. Expression of functional potassium channels from Shaker cDNA in Xenopus oocytes. Nature. 1988 Jan 14;331(6152):143–145. doi: 10.1038/331143a0. [DOI] [PubMed] [Google Scholar]
- Tinker A., Jan Y. N., Jan L. Y. Regions responsible for the assembly of inwardly rectifying potassium channels. Cell. 1996 Nov 29;87(5):857–868. doi: 10.1016/s0092-8674(00)81993-5. [DOI] [PubMed] [Google Scholar]
- Vandenberg C. A. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velimirovic B. M., Koyano K., Nakajima S., Nakajima Y. Opposing mechanisms of regulation of a G-protein-coupled inward rectifier K+ channel in rat brain neurons. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1590–1594. doi: 10.1073/pnas.92.5.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Kunkel D. D., Martin T. M., Schwartzkroin P. A., Tempel B. L. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993 Sep 2;365(6441):75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Wible B. A., Taglialatela M., Ficker E., Brown A. M. Gating of inwardly rectifying K+ channels localized to a single negatively charged residue. Nature. 1994 Sep 15;371(6494):246–249. doi: 10.1038/371246a0. [DOI] [PubMed] [Google Scholar]
- Wickman K. D., Iñiguez-Lluhl J. A., Davenport P. A., Taussig R., Krapivinsky G. B., Linder M. E., Gilman A. G., Clapham D. E. Recombinant G-protein beta gamma-subunits activate the muscarinic-gated atrial potassium channel. Nature. 1994 Mar 17;368(6468):255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- Wickman K., Clapham D. E. Ion channel regulation by G proteins. Physiol Rev. 1995 Oct;75(4):865–885. doi: 10.1152/physrev.1995.75.4.865. [DOI] [PubMed] [Google Scholar]
- Xu J., Yu W., Jan Y. N., Jan L. Y., Li M. Assembly of voltage-gated potassium channels. Conserved hydrophilic motifs determine subfamily-specific interactions between the alpha-subunits. J Biol Chem. 1995 Oct 20;270(42):24761–24768. doi: 10.1074/jbc.270.42.24761. [DOI] [PubMed] [Google Scholar]
- Yang J., Jan Y. N., Jan L. Y. Control of rectification and permeation by residues in two distinct domains in an inward rectifier K+ channel. Neuron. 1995 May;14(5):1047–1054. doi: 10.1016/0896-6273(95)90343-7. [DOI] [PubMed] [Google Scholar]
- Yang J., Jan Y. N., Jan L. Y. Determination of the subunit stoichiometry of an inwardly rectifying potassium channel. Neuron. 1995 Dec;15(6):1441–1447. doi: 10.1016/0896-6273(95)90021-7. [DOI] [PubMed] [Google Scholar]
- Yang J., Yu M., Jan Y. N., Jan L. Y. Stabilization of ion selectivity filter by pore loop ion pairs in an inwardly rectifying potassium channel. Proc Natl Acad Sci U S A. 1997 Feb 18;94(4):1568–1572. doi: 10.1073/pnas.94.4.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yool A. J., Schwarz T. L. Alteration of ionic selectivity of a K+ channel by mutation of the H5 region. Nature. 1991 Feb 21;349(6311):700–704. doi: 10.1038/349700a0. [DOI] [PubMed] [Google Scholar]
- Yu W., Xu J., Li M. NAB domain is essential for the subunit assembly of both alpha-alpha and alpha-beta complexes of shaker-like potassium channels. Neuron. 1996 Feb;16(2):441–453. doi: 10.1016/s0896-6273(00)80062-8. [DOI] [PubMed] [Google Scholar]