Abstract
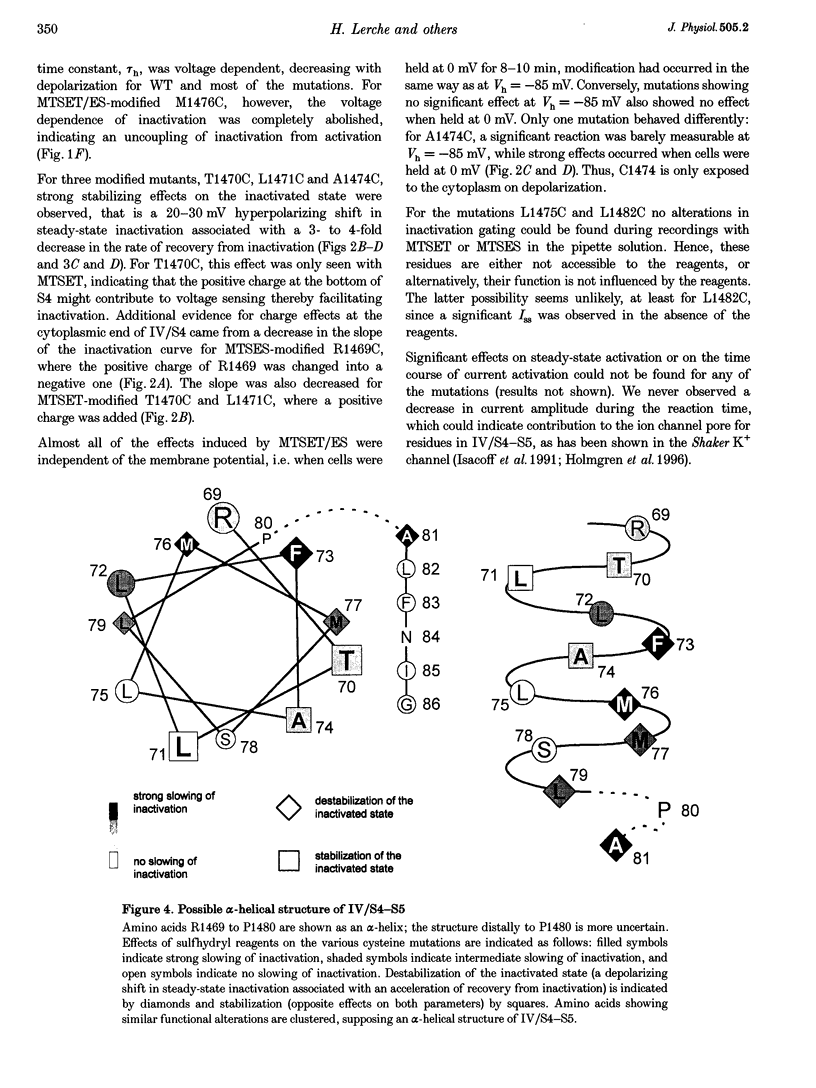
1. In order to investigate the role in fast inactivation of the cytoplasmic S4-S5 loop of the fourth domain (IV/S4-S5) within the alpha-subunit of the adult human muscle Na+ channel, every single amino acid from R1469 to G1486 was substituted by a cysteine and the mutants were studied by functional expression in human embryonic kidney cells (tsA201) using whole-cell patch clamping. Effects following intracellular application of the sulfhydryl reagents MTSET and MTSES on the mutants were investigated. 2. Sixteen of eighteen mutants resulted in the formation of functional channels. For P1480C and N1484C, no Na+ currents could be detected in transfected cells. In the absence of sulfhydryl reagents, F1473C and A1481C slowed fast Na+ channel inactivation by 2- and 1.5-fold, respectively, and L1482C induced a steady-state Na+ current (Iss) of 3% of peak current (Ipeak) (1% for wild-type). 3. Upon application of MTSET and MTSES, changes in fast inactivation gating occurred for most of the mutants. The most dramatic destabilizing effects on fast inactivation were observed for M1476C (9-fold slowing of inactivation; Iss/Ipeak, 3.6%; +15 mV shift in steady-state inactivation; 2- to 3-fold acceleration of recovery from inactivation), A1481C (3-fold; 14%; +20 mV; no change) and F1473C (2.5-fold; 2.4%; +8 mV; 1.5-fold). Less pronounced destabilizing effects were observed for M1477C and L1479C. Strongly stabilizing effects on the inactivated state, that is a 20-30 mV hyperpolarizing shift of the inactivation curve associated with a 3- to 4-fold decrease in the rate of recovery from inactivation, occurred for T1470C, L1471C and A1474C. Almost all effects were independent of the membrane potential; however, A1474C only reacted when cells were depolarized. Significant effects on activation were not observed. 4. We conclude that the IV/S4-S5 loop plays an important role in fast inactivation of the muscle Na+ channel and may contribute to the formation of a receptor for the putative inactivation particle. The effects of sulfhydryl reagents on the various mutations suggest an alpha-helical structure of IV/S4-S5 (up to P1480) with destabilizing effects on inactivation for one cluster of amino acids (1473/76/77/79) and a stabilized inactivation at the opposite side of the helix (1470/71/74).
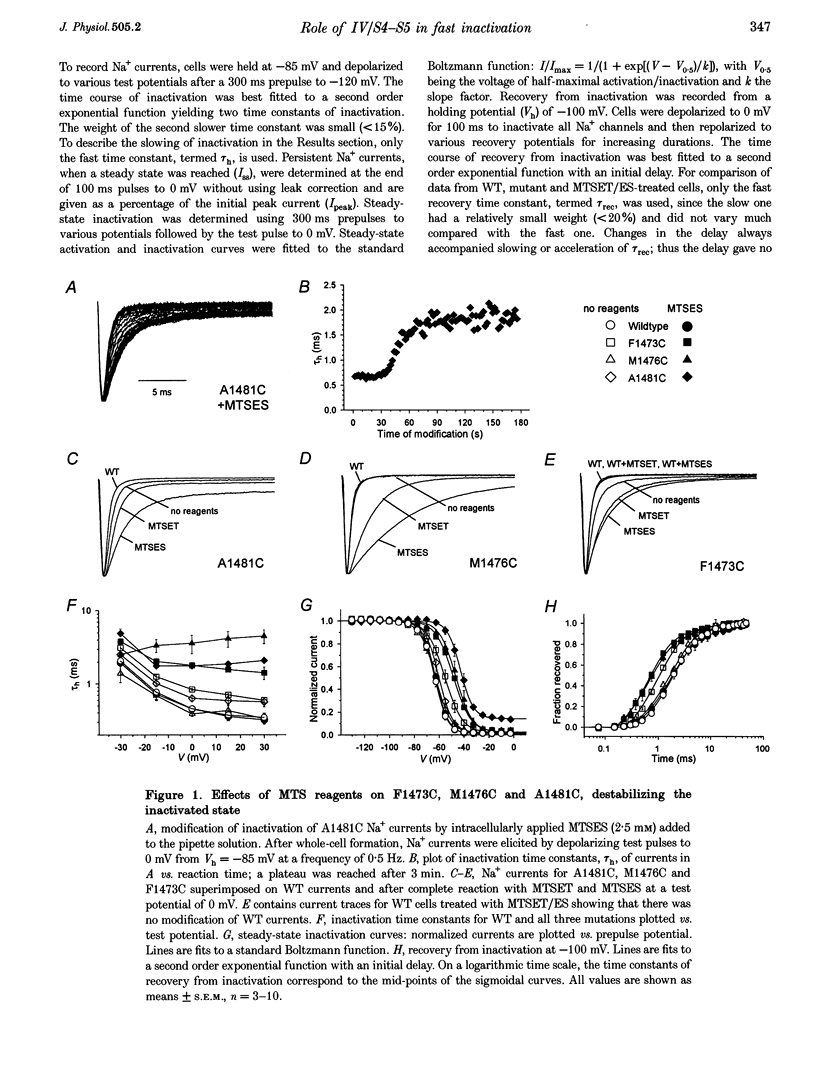
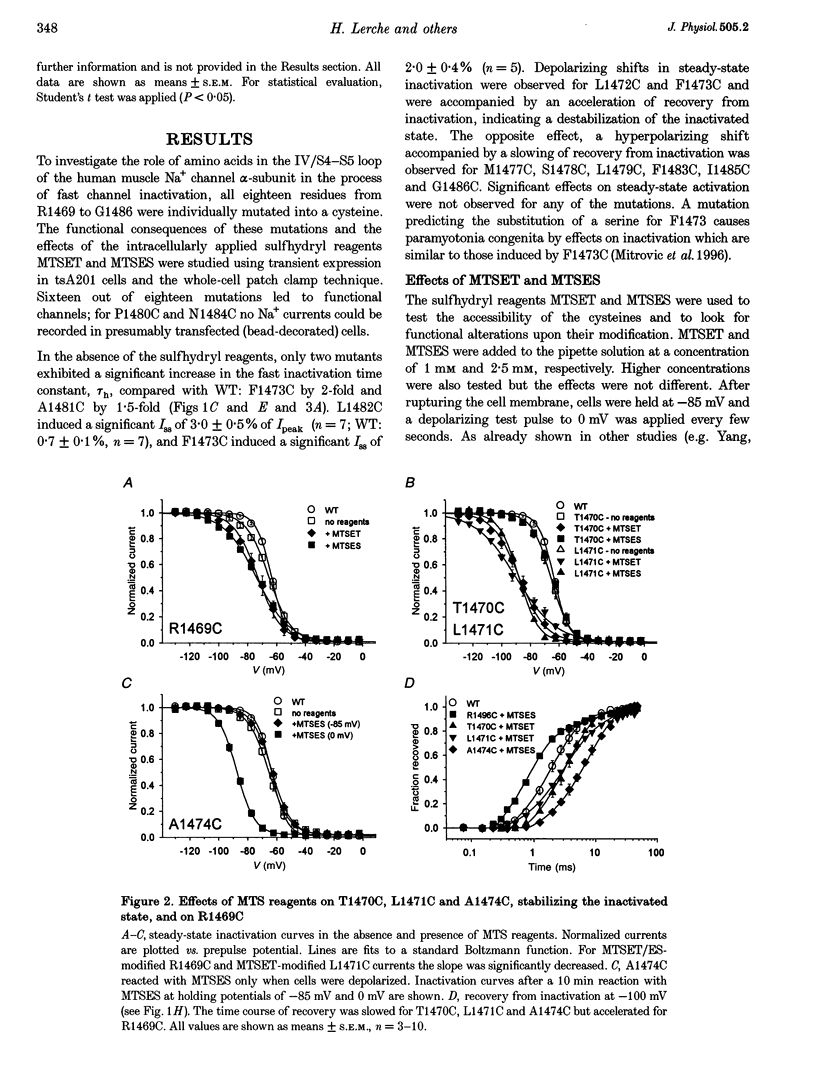
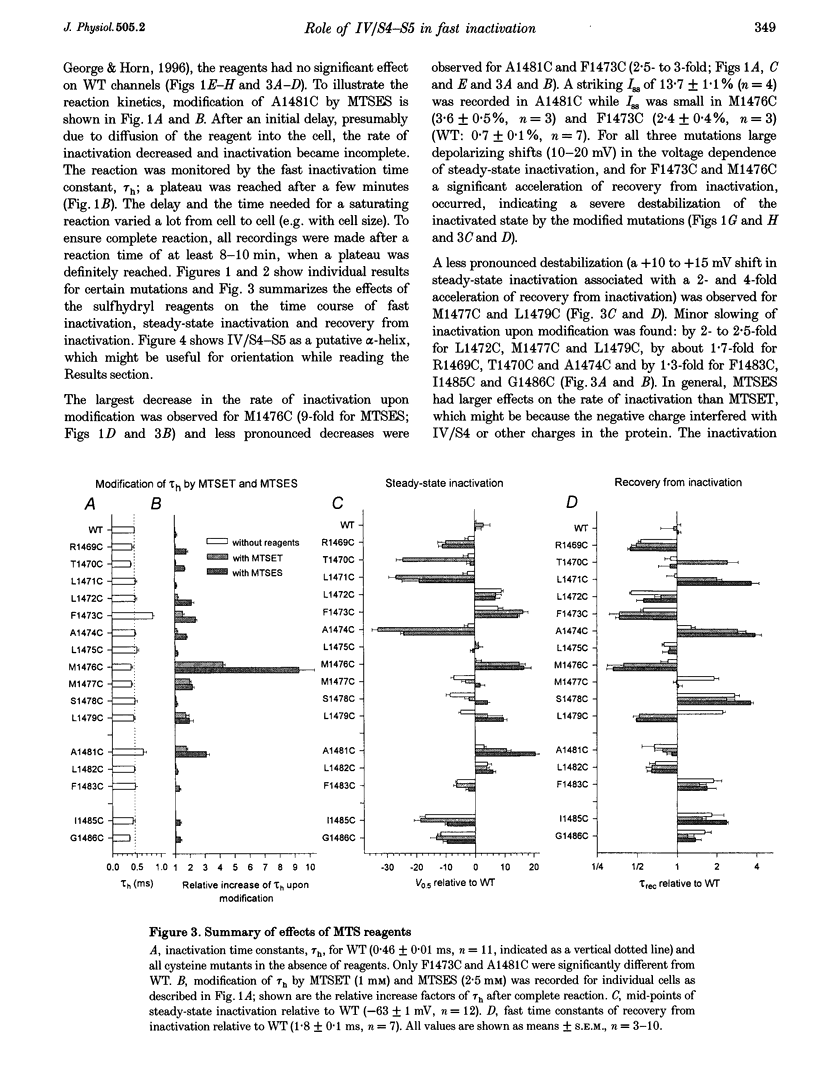
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine M., George A. L., Jr, Zhou M., Ji S., Sun W., Barchi R. L., Horn R. Sodium channel mutations in paramyotonia congenita uncouple inactivation from activation. Neuron. 1994 Feb;12(2):281–294. doi: 10.1016/0896-6273(94)90271-2. [DOI] [PubMed] [Google Scholar]
- Chen L. Q., Santarelli V., Horn R., Kallen R. G. A unique role for the S4 segment of domain 4 in the inactivation of sodium channels. J Gen Physiol. 1996 Dec;108(6):549–556. doi: 10.1085/jgp.108.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren M., Jurman M. E., Yellen G. N-type inactivation and the S4-S5 region of the Shaker K+ channel. J Gen Physiol. 1996 Sep;108(3):195–206. doi: 10.1085/jgp.108.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990 Oct 26;250(4980):533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Isacoff E. Y., Jan Y. N., Jan L. Y. Putative receptor for the cytoplasmic inactivation gate in the Shaker K+ channel. Nature. 1991 Sep 5;353(6339):86–90. doi: 10.1038/353086a0. [DOI] [PubMed] [Google Scholar]
- Kellenberger S., Scheuer T., Catterall W. A. Movement of the Na+ channel inactivation gate during inactivation. J Biol Chem. 1996 Nov 29;271(48):30971–30979. doi: 10.1074/jbc.271.48.30971. [DOI] [PubMed] [Google Scholar]
- Lerche H., Mitrovic N., Dubowitz V., Lehmann-Horn F. Paramyotonia congenita: the R1448P Na+ channel mutation in adult human skeletal muscle. Ann Neurol. 1996 May;39(5):599–608. doi: 10.1002/ana.410390509. [DOI] [PubMed] [Google Scholar]
- Mitrovic N., Lerche H., Heine R., Fleischhauer R., Pika-Hartlaub U., Hartlaub U., George A. L., Jr, Lehmann-Horn F. Role in fast inactivation of conserved amino acids in the IV/S4-S5 loop of the human muscle Na+ channel. Neurosci Lett. 1996 Aug 16;214(1):9–12. doi: 10.1016/0304-3940(96)12866-4. [DOI] [PubMed] [Google Scholar]
- Mitrović N., George A. L., Jr, Heine R., Wagner S., Pika U., Hartlaub U., Zhou M., Lerche H., Fahlke C., Lehmann-Horn F. K(+)-aggravated myotonia: destabilization of the inactivated state of the human muscle Na+ channel by the V1589M mutation. J Physiol. 1994 Aug 1;478(Pt 3):395–402. doi: 10.1113/jphysiol.1994.sp020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton D. E., West J. W., Catterall W. A., Goldin A. L. A peptide segment critical for sodium channel inactivation functions as an inactivation gate in a potassium channel. Neuron. 1993 Nov;11(5):967–974. doi: 10.1016/0896-6273(93)90125-b. [DOI] [PubMed] [Google Scholar]
- Stauffer D. A., Karlin A. Electrostatic potential of the acetylcholine binding sites in the nicotinic receptor probed by reactions of binding-site cysteines with charged methanethiosulfonates. Biochemistry. 1994 Jun 7;33(22):6840–6849. doi: 10.1021/bi00188a013. [DOI] [PubMed] [Google Scholar]
- West J. W., Patton D. E., Scheuer T., Wang Y., Goldin A. L., Catterall W. A. A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]