Abstract
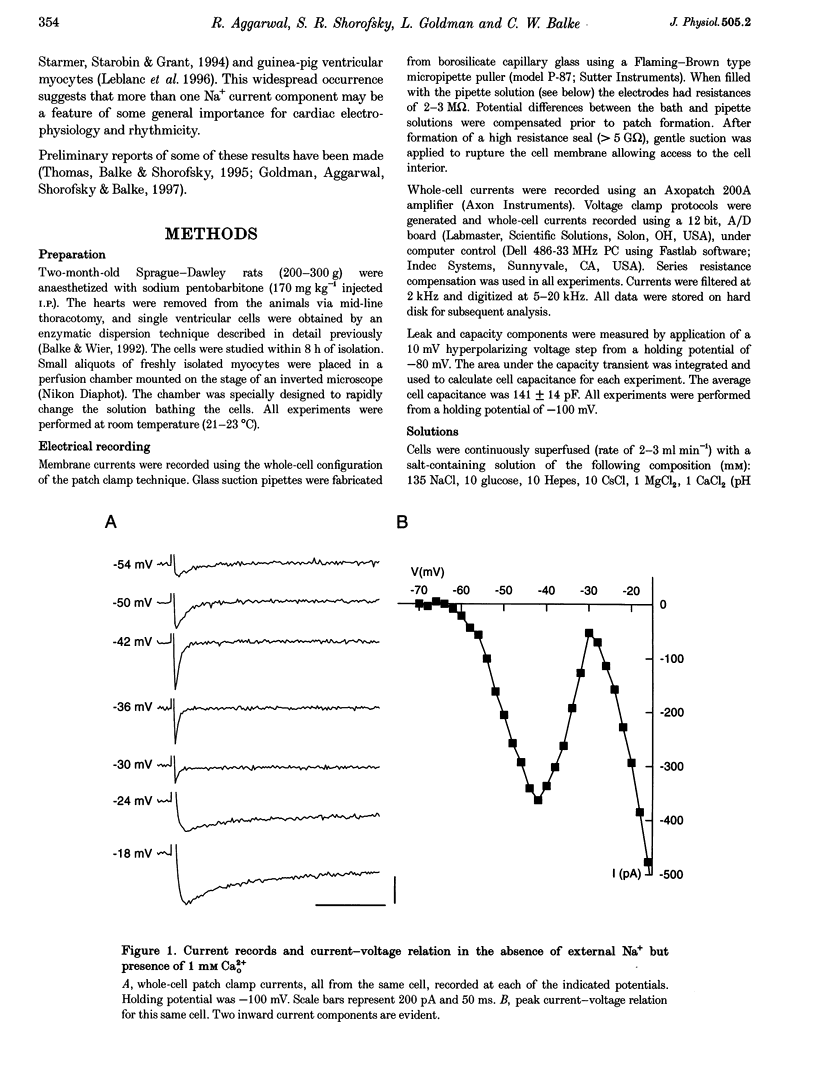
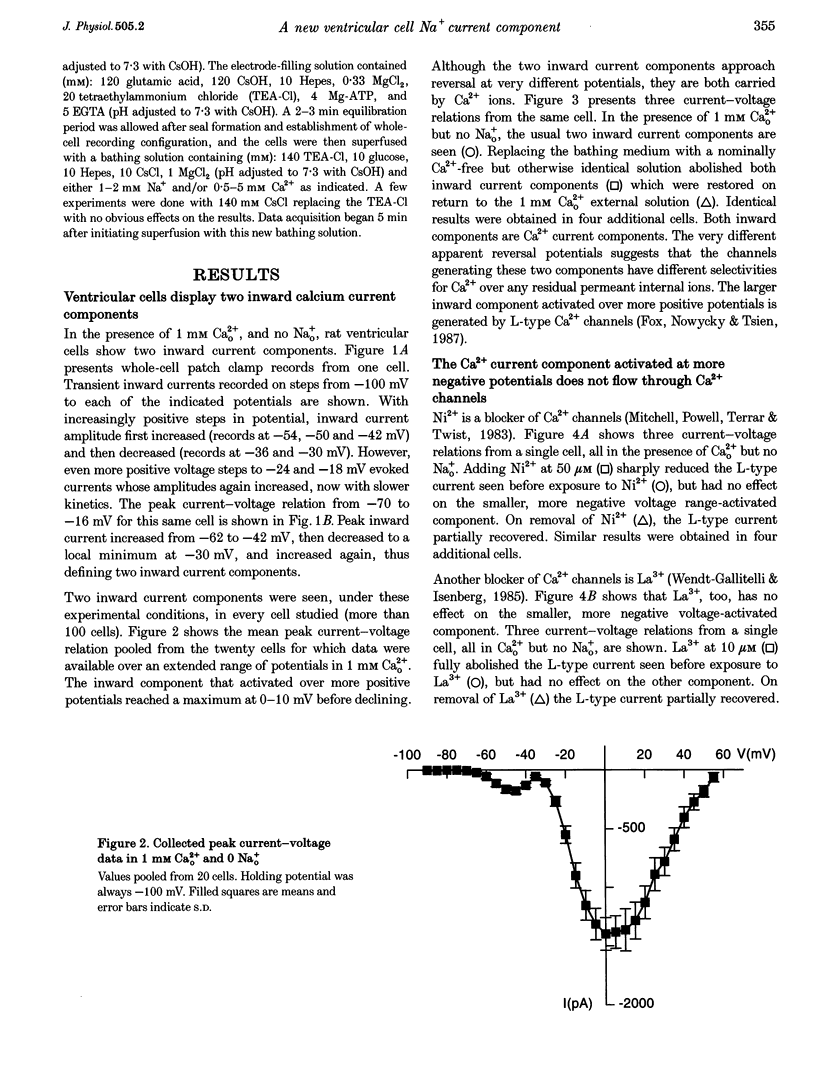
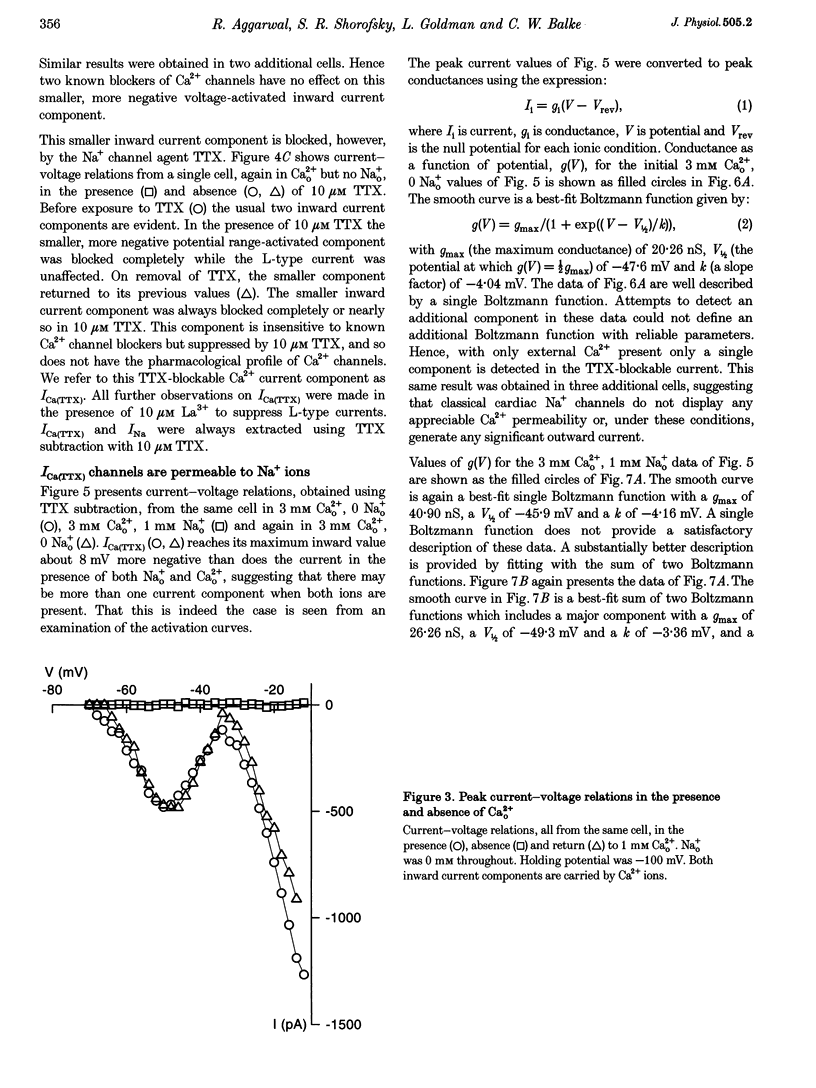
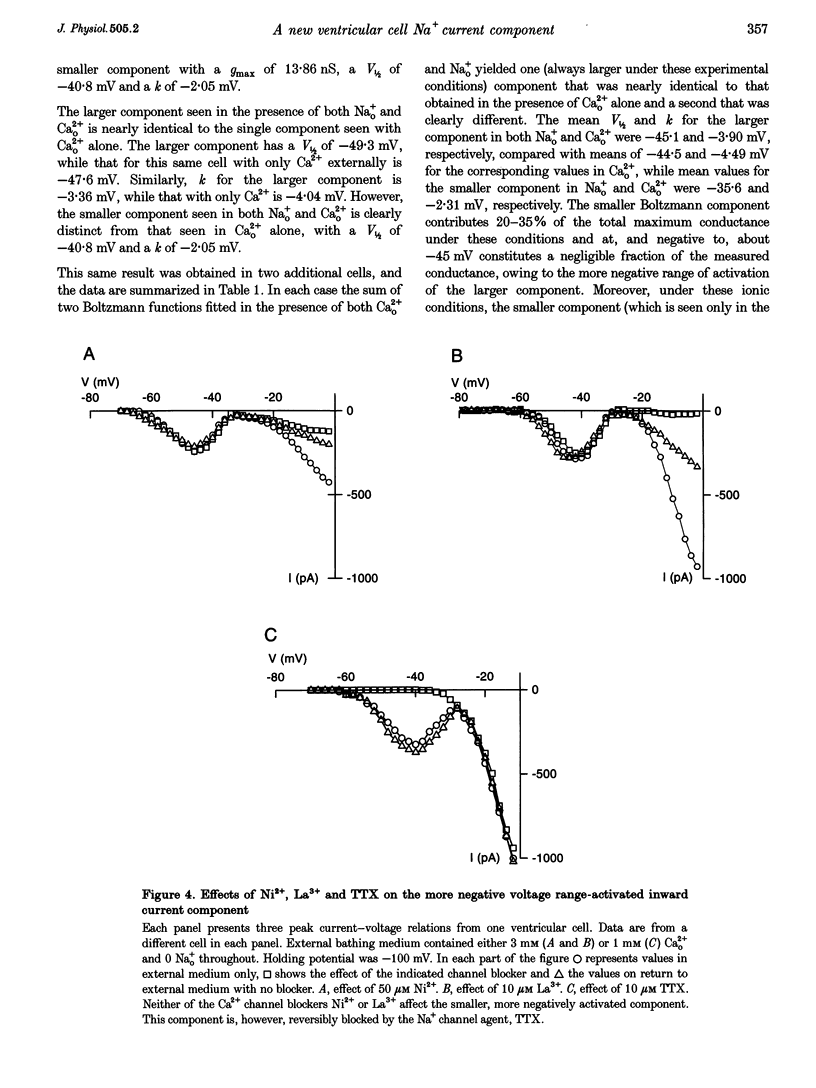
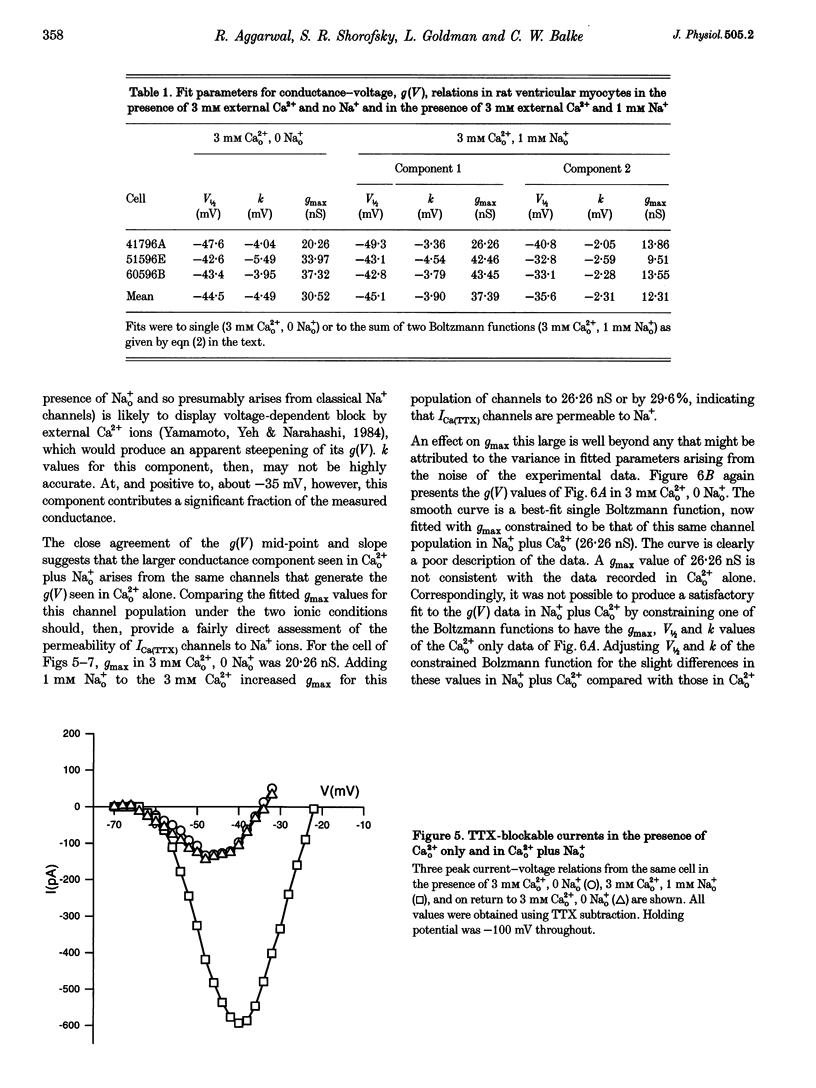
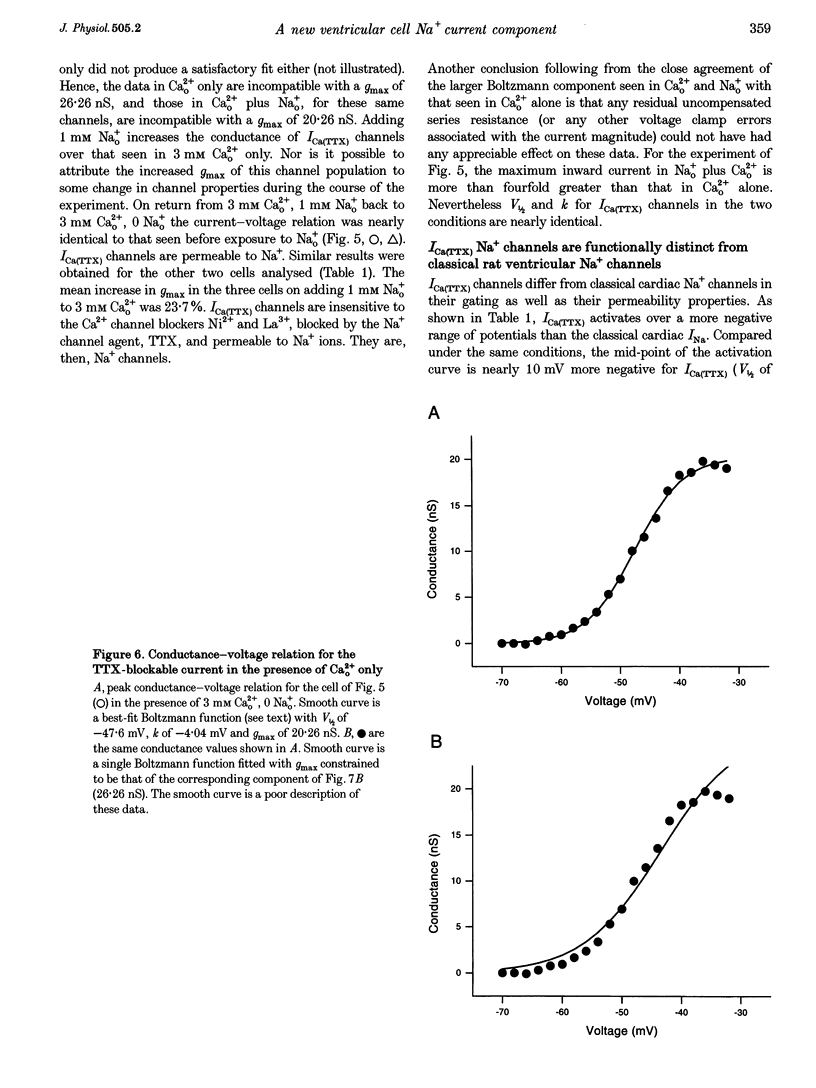
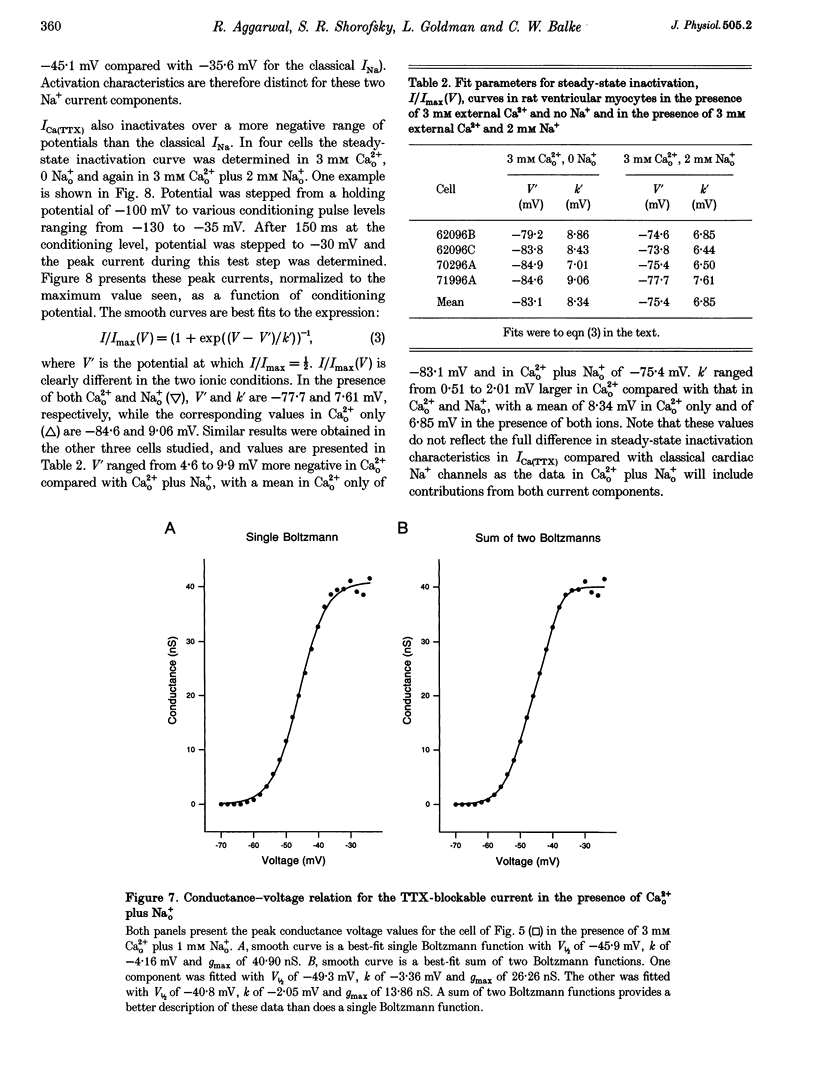
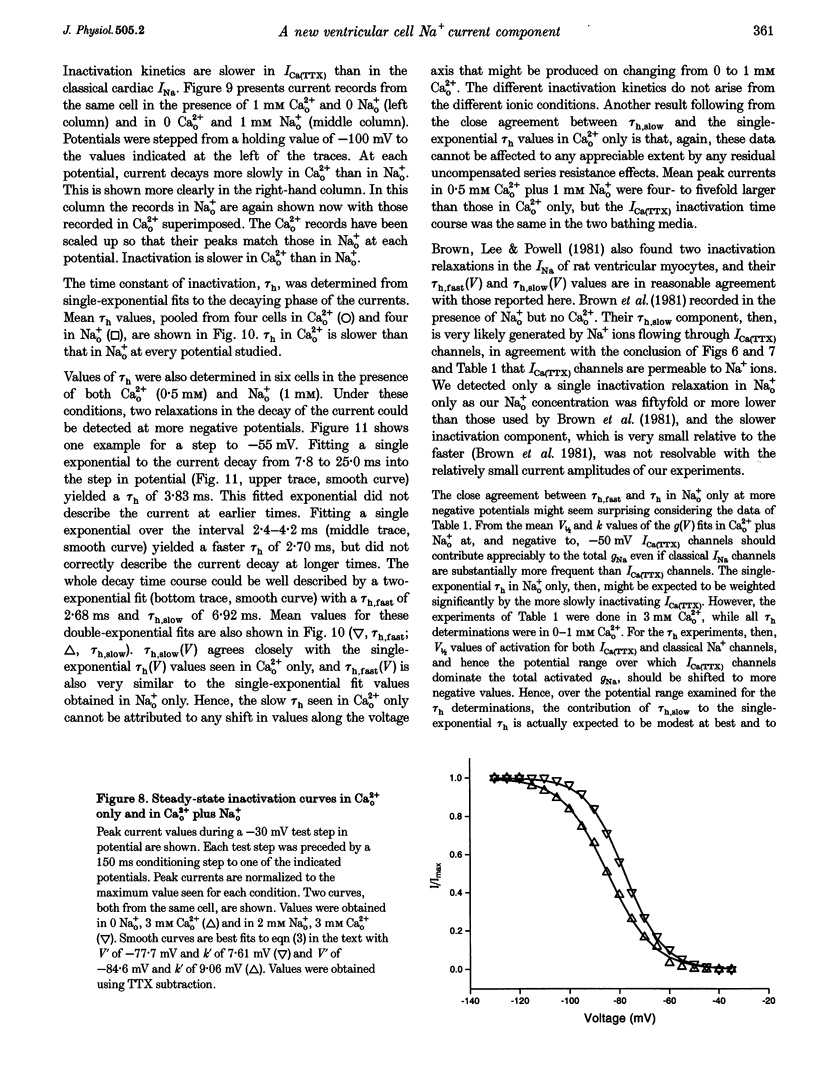
1. Whole-cell patch clamp currents from freshly isolated adult rat ventricular cells, recorded in external Ca2+ (Ca2+o) but no external Na+ (Na+o), displayed two inward current components: a smaller component that activated over more negative potentials and a larger component (L-type Ca2+ current) that activated at more positive potentials. The smaller component was not generated by Ca2+ channels. It was insensitive to 50 microM Ni2+ and 10 microM La3+ but suppressed by 10 microM tetrodotoxin (TTX). We refer to this component as ICa(TTX). 2. The conductance-voltage, g(V), relation in Ca2+o only was well described by a single Boltzmann function (half-maximum potential, V1/2, of -44.5; slope factor, k, of -4.49 mV, means of 3 cells). g(V) in Ca2+o plus Na+o was better described as the sum of two Boltzmann functions, one nearly identical to that in Ca2+o only (mean V1/2 of -45.1 and k of -3.90 mV), and one clearly distinct (mean V1/2 of -35.6 and k of -2.31 mV). Mean maximum conductance for ICa(TTX) channels increased 23.7% on adding 1 mM Na+o to 3 mM Ca2+o. ICa(TTX) channels are permeable to Na+ ions, insensitive to Ni2+ and La3+ and blocked by TTX. They are Na+ channels. 3. ICa(TTX) channels are distinct from classical cardiac Na+ channels. They activate and inactivate over a more negative range of potentials and have a slower time constant of inactivation than the classical Na+ channels. They are also distinct from yet another rat ventricular Na+ current component characterized by a much higher TTX sensitivity and by a persistent, non-fast-inactivating fraction. That ICa(TTX) channels activate over a more negative range of potentials than classical cardiac Na+ channels suggests that they may be critical for triggering the ventricular action potential and so of importance for cardiac arrhythmias.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Takahashi K. Tetrodotoxin-sensitive calcium-conducting channels in the rat hippocampal CA1 region. J Physiol. 1992 May;450:529–546. doi: 10.1113/jphysiol.1992.sp019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balke C. W., Wier W. G. Modulation of L-type calcium channels by sodium ions. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4417–4421. doi: 10.1073/pnas.89.10.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Glial and neuronal forms of the voltage-dependent sodium channel: characteristics and cell-type distribution. Neuron. 1989 Apr;2(4):1375–1388. doi: 10.1016/0896-6273(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Benoit E., Corbier A., Dubois J. M. Evidence for two transient sodium currents in the frog node of Ranvier. J Physiol. 1985 Apr;361:339–360. doi: 10.1113/jphysiol.1985.sp015649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossu J. L., Feltz A. Patch-clamp study of the tetrodotoxin-resistant sodium current in group C sensory neurones. Neurosci Lett. 1984 Oct 12;51(2):241–246. doi: 10.1016/0304-3940(84)90558-5. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Sodium current in single rat heart muscle cells. J Physiol. 1981 Sep;318:479–500. doi: 10.1113/jphysiol.1981.sp013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachelin A. B., De Peyer J. E., Kokubun S., Reuter H. Sodium channels in cultured cardiac cells. J Physiol. 1983 Jul;340:389–401. doi: 10.1113/jphysiol.1983.sp014768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraboeuf E., Deroubaix E., Coulombe A. Effect of tetrodotoxin on action potentials of the conducting system in the dog heart. Am J Physiol. 1979 Apr;236(4):H561–H567. doi: 10.1152/ajpheart.1979.236.4.H561. [DOI] [PubMed] [Google Scholar]
- Eick R. T., Yeh J., Matsuki N. Two types of voltage dependent na channels suggested by differential sensitivity of single channels to tetrodotoxin. Biophys J. 1984 Jan;45(1):70–73. doi: 10.1016/S0006-3495(84)84113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felipe A., Knittle T. J., Doyle K. L., Tamkun M. M. Primary structure and differential expression during development and pregnancy of a novel voltage-gated sodium channel in the mouse. J Biol Chem. 1994 Dec 2;269(48):30125–30131. [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellens M. E., George A. L., Jr, Chen L. Q., Chahine M., Horn R., Barchi R. L., Kallen R. G. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):554–558. doi: 10.1073/pnas.89.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A. L., Jr, Knittle T. J., Tamkun M. M. Molecular cloning of an atypical voltage-gated sodium channel expressed in human heart and uterus: evidence for a distinct gene family. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4893–4897. doi: 10.1073/pnas.89.11.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilly W. F., Armstrong C. M. Threshold channels--a novel type of sodium channel in squid giant axon. 1984 May 31-Jun 6Nature. 309(5967):448–450. doi: 10.1038/309448a0. [DOI] [PubMed] [Google Scholar]
- Goldman L., Hahin R. Initial conditions and the kinetics of the sodium conductance in Myxicola giant axons. II. Relaxation experiments. J Gen Physiol. 1978 Dec;72(6):879–898. doi: 10.1085/jgp.72.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonoi T., Sherman S. J., Catterall W. A. Voltage clamp analysis of tetrodotoxin-sensitive and -insensitive sodium channels in rat muscle cells developing in vitro. J Neurosci. 1985 Sep;5(9):2559–2564. doi: 10.1523/JNEUROSCI.05-09-02559.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann S. H., Terlau H., Stühmer W., Imoto K., Numa S. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature. 1992 Apr 2;356(6368):441–443. doi: 10.1038/356441a0. [DOI] [PubMed] [Google Scholar]
- Ju Y. K., Saint D. A., Gage P. W. Inactivation-resistant channels underlying the persistent sodium current in rat ventricular myocytes. Proc Biol Sci. 1994 May 23;256(1346):163–168. doi: 10.1098/rspb.1994.0065. [DOI] [PubMed] [Google Scholar]
- Kallen R. G., Sheng Z. H., Yang J., Chen L. Q., Rogart R. B., Barchi R. L. Primary structure and expression of a sodium channel characteristic of denervated and immature rat skeletal muscle. Neuron. 1990 Feb;4(2):233–242. doi: 10.1016/0896-6273(90)90098-z. [DOI] [PubMed] [Google Scholar]
- Kirsch G. E., Brown A. M. Kinetic properties of single sodium channels in rat heart and rat brain. J Gen Physiol. 1989 Jan;93(1):85–99. doi: 10.1085/jgp.93.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze D. L., Lacerda A. E., Wilson D. L., Brown A. M. Cardiac Na currents and the inactivating, reopening, and waiting properties of single cardiac Na channels. J Gen Physiol. 1985 Nov;86(5):691–719. doi: 10.1085/jgp.86.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire S., Piot C., Seguin J., Nargeot J., Richard S. Tetrodotoxin-sensitive Ca2+ and Ba2+ currents in human atrial cells. Receptors Channels. 1995;3(2):71–81. [PubMed] [Google Scholar]
- Meves H., Vogel W. Calcium inward currents in internally perfused giant axons. J Physiol. 1973 Nov;235(1):225–265. doi: 10.1113/jphysiol.1973.sp010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Characteristics of the second inward current in cells isolated from rat ventricular muscle. Proc R Soc Lond B Biol Sci. 1983 Oct 22;219(1217):447–469. doi: 10.1098/rspb.1983.0084. [DOI] [PubMed] [Google Scholar]
- Ogata N., Tatebayashi H. Ontogenic development of the TTX-sensitive and TTX-insensitive Na+ channels in neurons of the rat dorsal root ganglia. Brain Res Dev Brain Res. 1992 Jan 17;65(1):93–100. doi: 10.1016/0165-3806(92)90012-l. [DOI] [PubMed] [Google Scholar]
- Pappone P. A. Voltage-clamp experiments in normal and denervated mammalian skeletal muscle fibres. J Physiol. 1980 Sep;306:377–410. doi: 10.1113/jphysiol.1980.sp013403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J. B., Ortiz M. Slow currents through single sodium channels of the adult rat heart. J Gen Physiol. 1985 Jul;86(1):89–104. doi: 10.1085/jgp.86.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogart R. B., Cribbs L. L., Muglia L. K., Kephart D. D., Kaiser M. W. Molecular cloning of a putative tetrodotoxin-resistant rat heart Na+ channel isoform. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8170–8174. doi: 10.1073/pnas.86.20.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M. L., Narahashi T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J Neurosci. 1992 Jun;12(6):2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint D. A., Ju Y. K., Gage P. W. A persistent sodium current in rat ventricular myocytes. J Physiol. 1992;453:219–231. doi: 10.1113/jphysiol.1992.sp019225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y., Wasserstrom J. A., Furukawa T., Jia H., Arentzen C. E., Hartz R. S., Singer D. H. Characterization of the sodium current in single human atrial myocytes. Circ Res. 1992 Sep;71(3):535–546. doi: 10.1161/01.res.71.3.535. [DOI] [PubMed] [Google Scholar]
- Scanley B. E., Fozzard H. A. Low conductance sodium channels in canine cardiac Purkinje cells. Biophys J. 1987 Sep;52(3):489–495. doi: 10.1016/S0006-3495(87)83237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sills M. N., Xu Y. C., Baracchini E., Goodman R. H., Cooperman S. S., Mandel G., Chien K. R. Expression of diverse Na+ channel messenger RNAs in rat myocardium. Evidence for a cardiac-specific Na+ channel. J Clin Invest. 1989 Jul;84(1):331–336. doi: 10.1172/JCI114158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A., Tasaki I., Singer I., Lerman L. Effects of tetrodotoxin on excitability of squid giant axons in sodium-free media. Science. 1967 Jan 6;155(3758):95–97. doi: 10.1126/science.155.3758.95. [DOI] [PubMed] [Google Scholar]
- Weiss R. E., Horn R. Functional differences between two classes of sodium channels in developing rat skeletal muscle. Science. 1986 Jul 18;233(4761):361–364. doi: 10.1126/science.2425432. [DOI] [PubMed] [Google Scholar]
- Weiss R. E., Sidell N. Sodium currents during differentiation in a human neuroblastoma cell line. J Gen Physiol. 1991 Mar;97(3):521–539. doi: 10.1085/jgp.97.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt-Gallitelli M. F., Isenberg G. Extra- and intracellular lanthanum: modified calcium distribution, inward currents and contractility in guinea pig ventricular preparations. Pflugers Arch. 1985 Dec;405(4):310–322. doi: 10.1007/BF00595683. [DOI] [PubMed] [Google Scholar]
- Yamamoto D., Yeh J. Z., Narahashi T. Voltage-dependent calcium block of normal and tetramethrin-modified single sodium channels. Biophys J. 1984 Jan;45(1):337–344. doi: 10.1016/S0006-3495(84)84159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter YuI, Starmer C. F., Starobin J., Grant A. O. Late Na channels in cardiac cells: the physiological role of background Na channels. Biophys J. 1994 Jul;67(1):153–160. doi: 10.1016/S0006-3495(94)80464-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


