Abstract
Background
Engineering the seed microenvironment with embedded bacteriophages and Plant Growth Promoting Rhizobacteria (PGPR) shows promise for enhancing germination, mitigating biotic and abiotic stressors, and improving resilience under challenging environmental conditions. This study aimed to enhance potato seed germination and control bacterial wilt caused by Ralstonia solanacearum and salinity by using novel technology to encapsulate, preserve, and deliver phage therapy and rhizobacteria.
Results
Silk fibroin and trehalose biomaterial combined with the phage P-PSG11 and Pseudomonas lalkuanensis were applied to potato seeds. A pot experiment was conducted to investigate pathogen suppression, salt tolerance, and plant growth enhancement. The combination of silk and trehalose effectively preserved both phage and bacteria for ≥ 8 weeks, maintaining both phage titers and bacterial colony counts. Seeds coated with the P-PSG11 and P. lalkuanensis mixture exhibited the highest germination rate at 93.5%, followed by P. lalkuanensis at 86.3%. In vivo evaluations showed significant increases in root length (72.7%, 61.0%, and 22.5%), plant height (71.5%, 65.1%, and 8.2%), and dry matter (129.1%, 125.7%, and 13.1%) for the P-PSG11 and P. lalkuanensis mixture, P. lalkuanensis, and P-PSG11, respectively. The incidence of wilt was significantly reduced by 88.2% and 81.2%, and salinity was mitigated by 83.3% and 79.2% for the P-PSG11 and P. lalkuanensis mixture and P. lalkuanensis treatment, respectively, compared to the control (p < 0.001). The viability of preserved P-PSG11 and P. lalkuanensis was confirmed after one year using phage titers and bacterial colonies.
Conclusion
This innovative approach enhanced plant growth, promoted seed germination, controlled wilt disease, and mitigated soil salinity.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-024-03657-y.
Keywords: Seed’s microenvironment, Bacteriophages, PGPR, Encapsulating, Salinity mitigation
Introduction
With approximately 800 million individuals currently experiencing food insecurity and a projected global population of 9.7 billion by 2050, there is an urgent need to increase food production significantly in the coming decades [1]. Changing climate patterns and the spread of transboundary diseases necessitate rapid crop adaptation to various stresses [2, 3]. Precision agriculture has been developed to respond to these challenges, utilizing advanced technologies to optimize food production. This approach aims to maximize crop yields while minimizing the use of resources such as water and agrochemicals, thus reducing the environmental impact [4–6]. Consequently, agriculture is transitioning towards sustainability and technological integration.
Seeds possess the highest added value among agricultural products, serving as a fundamental food source and as the cornerstone of agricultural practices [7]. In recent years, seed enhancement technologies have emerged, aimed at improving seed performance through tailored conditioning and specialized regimes [8]. Seed coatings have been devised to regulate seed surface properties, enrich soil with nutrients in specific locations, and modulate seed water uptake [7, 9]. However, the primary emphasis has been on studying payloads intended to improve seed germination based on soil properties and seed type rather than on the materials utilized for encapsulating and delivering these payloads.
This approach has restricted the development of seed coatings capable of encapsulating beneficial and fragile compounds, particularly plant growth-promoting rhizobacteria (PGPR) and bacteriophages. PGPR could enhance nutrient availability and phytohormone levels during plant-root interaction, while simultaneously reducing the environmental impact of synthetic fertilizers, salinity, and pesticides [10–12]. PGPR enhances soil fertility through nitrogen fixation, nutrient solubilization, and the production of growth regulators and antibiotics [13, 14]. When applied as inoculants, these biofertilizers multiply in soil and improve crop productivity through enhanced nutrient cycling [15]. Incorporating microbial inocula into artificial seed coatings can result in diminished microbial viability, potentially compromising the long-term storage capacity of coated seeds [16]. The artificial seed coating creates a challenging microenvironment for PGPR due to osmotic and desiccation stresses [17, 18]. Pseudomonas lalkuanensis, a recently characterized plant growth-promoting rhizobacterium (PGPR) from agricultural soil, exhibits strong antagonistic activity against plant pathogens while enhancing plant growth [19, 20]. The bacterium produces various antimicrobial compounds, including hydrogen cyanide (HCN), ammonia, siderophores, hydrolytic enzymes, and volatile organic compounds, making it a promising biocontrol agent due to its natural adaptation to the crop rhizosphere and mitigate salinity stress [21, 22]. Moreover, protective compounds intended to benefit the seed may unintentionally compromise the survival of symbiotic bacteria due to their biological activity [23, 24]. At the same time, the spread of microbial diseases through seeds is a major concern in agriculture, with the potential to cause significant yield losses [25–30].
Ralstonia solanacearum is a devastating soil-borne plant pathogen that causes bacterial wilt disease in more than 200 plant species, including economically important crops such as potato, tomato, eggplant, pepper, tobacco, and banana [31, 32]. Its broad host range, global distribution, and high virulence makes it one of the most destructive bacterial plant pathogens worldwide [33, 34]. In pursuing sustainable food production, bacteriophages, natural viruses that target specific bacteria, are emerging as promising biocontrol tools. To combat agricultural diseases, scientists are pioneering new ways to deliver phages, including spraying them directly onto leaves [35], treating irrigation water [36], applying treatments to seed tubers [37], coating seeds, and developing protective shields for leaves [38]. However, several studies have highlighted seed transmission as a major route for plant pathogen transmission [29, 39–41]. Despite this, only a few studies in recent years have focused on effective plant biocontrol through seed coating [42, 43]. Developing efficient phage coatings requires a comprehensive understanding of seed surface binding mechanisms, enrichment strategies, and phage stability; however, this aspect has surprisingly received little systematic attention. Silk protein, a structural protein traditionally used in textiles, has been repurposed as a natural technical material with applications in regenerative medicine, drug delivery, implantable optoelectronics, and food coatings [44, 45].
In this study, we intended to develop a biomaterial-based approach to engineer seed coatings loaded with PGPR to enhance germination and alleviate soil salinity and bacteriophage to control bacterial pathogens. A biomaterial was formulated using silk protein extracted from Bombyx mori (B. mori) cocoons and trehalose for cellular protection. The blend was combined with a PGPR Pseudomonas lalkuanensis A101 and/or a bacteriophage (P-PSG11) targeting Ralstonia solanacearum, and applied onto seed surfaces, adapting the existing seed coating method dip coating. To the best of our knowledge, this is the first study reporting long-term stabilization of bacteria and phages in silk films applied to seeds for enhanced biocontrol effectiveness and stability in soil after application for sustainable agriculture.
Materials and methods
Materials fabrication and protein purification
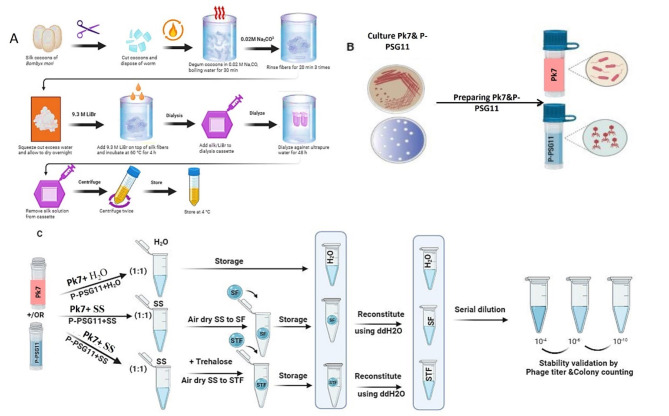
With slight modifications, silk protein was purified as previously described [29]. Briefly, silk fibroin was extracted from B. mori worm by boiling dime-sized pieces in 0.02 M sodium carbonate (Na2CO3) for ∼ 40–50 min. The degummed fibers were collected, rinsed three times in ultrapure water, and dried overnight in a fume hood. The dried fibers were dissolved in a 9.3 M lithium bromide (LiBr) solution (20% wt/vol) at 60 °C for 4 h. A 12 mL portion of the solution was dialyzed against 1 L of ultrapure water for three days (water changed three times daily) to remove the LiBr. The solubilized silk fibroin was then centrifuged at ∼ 12,700 g to remove insoluble silk particles. The concentration of silk in the resultant solution was determined and used at ∼ 4% (wt/vol). This silk solution was used for further experiments or stored at 4 °C for up to 1 month (Fig. 1).
Fig. 1.
Schematic diagram for silk solution preparation and downstream applications. (A) Schematic of the silk fibroin extraction procedure: The process from raw material (cocoons) to the final aqueous-based solution takes 4 days. (B) Preparation procedure for biocontrol agents P-PSG11 phage and P. lalkuanensis A101 bacteria (C) In vitro verification of phage titer and colony counting of PGPR: Determine the stability of P-PSG11 titer and P. lalkuanensis colony using serial dilution
Assembly of fabrication material
P-PSG11 was previously isolated and identified by our research group for the control of R. solanacearum [46, 47]. Phage P-PSG11 was prepared in Tris-HCl phage buffer at pH 7.5 (50 mM Tris-base, 150 mM NaCl, 10 mM MgCl2.6H2O, and 2 mM CaCl2) and amplified as previously described [31–33]. Phage titer was determined by spotting 10 μl of serially diluted lysate onto double agar layers containing host bacteria.
P. lalkuanensis A101, isolated from the tomato rhizosphere in Ismailia, Egypt, was cultivated using Luria Bertani (LB) media. The identity of P. lalkuanensis was confirmed through 16 S rDNA sequencing, with accession number CP084625.1 (Fig. S1). We investigated whether the phage P-PSG11 could inhibit the growth of P. lalkuanensis when combined, aiming to enhance biocontrol effectiveness.
Silk film preparation
After preparing phage P-PSG11 and P. lalkuanensis A101, they were mixed with nuclease-free water (ddH2O), silk film (SF), and silk trehalose film (STF). Phage P-PSG11 and/or P. lalkuanensis A101 were combined with the silk solutions (silk film and silk trehalose film) in a 1:1 ratio (i.e., 10 μL phage/bacteria + 10 μL solution). The mixtures were then spread onto nuclease-free plastic sheets and air-dried in a biosafety cabinet for approximately 30–40 min, as illustrated in Fig. 1. The resulting films were carefully removed using tweezers and transferred into 1.5 mL nuclease-free tubes for further testing.
Phage and PGPR preservation
Silk film (SF) and silk trehalose film (STF) samples containing P-PSG11 phage and P. lalkuanensis A101 were stored for over 8 weeks. Their continued effectiveness against R. solanacearum was then assessed in vitro. To evaluate stability, 190 μL nuclease-free water was added to the tubes containing SF or STF. After that, the phage P -PSG11 titers and P. lalkuanensis A101 colonies in the solutions were determined. To determine the phage titers, the double-layer agar method [31] was used. Briefly, the solutions were serially diluted in Tris-HCl buffer (pH 7.5). Then, 500 μL of R. solanacearum (OD600) was mixed with 10 μL of the serially diluted solutions, vortexed at 160 rpm, and incubated at 28 °C for 15 min. This mixture was combined with 4 mL soft agar, poured onto CTG agar plates (1% Casamino acid hydroxylate, 1% tryptone and 1.5% w/v, agar), and incubated overnight at 28 °C. Phage titers were calculated by multiplying the plaque count by the dilution factor. All experiments were done in triplicate (Fig. 1).
To assess P. lalkuanensis A101 integrity, the serially diluted silk solutions were plated on soft agar plates containing R. solanacearum and incubated overnight at 28 °C for colony counting. The soft agar plates were prepared by pouring 500 μL of R. solanacearum in 4 mL soft agar onto CTG plates.
Encapsulation of potato seed
Potato (Solanum tuberosum) seeds were sterilized with 50% bleach for 3 min, rinsed thrice in H2O, and air-dried. P-PSG11 phage was prepared for coating, and P. lalkuanensis A101 was grown overnight to OD600 (1. 80 mL) and centrifuged at 4200 rpm. The pellet was resuspended in 8 mL of 6% (w/v) silk fibroin-trehalose (1:3) suspension containing phage P-PSG11. Seeds were dipped in this solution for 2 min, dried, and planted 24 h later. The coating process aimed to apply 108 CFU of P. lalkuanensis A101 and/or P-PSG11 phage per seed. Fifty seed models were coated with a silk film incorporating P. lalkuanensis A101 and phage P-PSG11, followed by air drying. These coated seed models were subsequently utilized in a series of experiments. In vitro studies were conducted to assess germination rates, while in vivo experiments evaluated plant growth development, pathogen suppression efficacy, and salinity stress tolerance.
Seed-coated germination
Germination rates were assessed using five replicates of coated potato seeds (Solanum tuberosum No. Huashu2, sourced from Huazhong Agricultural University, Wuhan, China). Comparisons were made with uncoated seeds (5 seeds) and R. solanacearum - infected seeds (5 seeds). Seeds were placed on moistened filter paper in Petri dishes at 25 °C. Germinating seedlings were counted after 7, 14, and 21 days. The total germination percentage was calculated as the average of the five replications using the following formula:
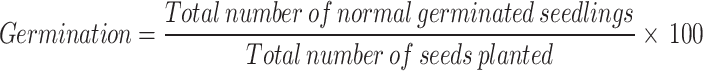 |
Root and shoot lengths were measured on 10 randomly selected seedlings during germination counts. Root length was measured from the collar to the primary root tip, while shoot length was measured from the collar to the primary shoot tip, and both were expressed as mean lengths in centimeters. Seedling Vigor Index was calculated according to the method described by Manonmani, et al., 2002 [48], using the following formula:
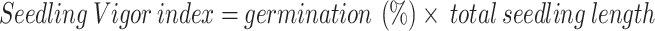 |
Scanning electron microscopy (SEM) images
Scanning electron microscopy (SEM) was used to examine cross-sections of silk (S) and silk-trehalose (ST) coatings applied by dip coating. Micrographs, all taken at the same magnification, revealed uniform film thicknesses of approximately 5 μm for both coating types. These cross-sectional images provided insights into the coatings’ internal structure, density, and the integration of trehalose within the silk matrix. This microstructural analysis is crucial for understanding the coatings’ potential effectiveness in preserving biocontrol agents and influencing seed germination.
Pot experiment
A pot experiment was conducted from June to August 2023 to evaluate the efficacy of P-PSG11 phage and P. lalkuanensis in promoting potato plant growth, managing bacterial wilt, and mitigating salinity stress. Environmental conditions ranged from 28 to 37 °C, with 58–85% relative humidity and 15–20 Klux light intensity. Potato seeds were coated with phage P-PSG11 and/or P. lalkuanensis. Sterile soil, prepared by air-drying, grinding, and 2 mm sieving, was placed in plastic pots (18 cm height, 26.5 cm diameter) at 5 kg per pot. The soil was analyzed for various properties, including pH, particle size distribution, and soluble cations and anions concentrations [49]. The soil characteristics used in this study are provided in Table S1. Pots were fertilized with superphosphate (1.0 g/pot, 15.5% P2O5, equivalent to 31.0 kg P2O5/ha) prior to sowing. Split applications of ammonium sulphate (0.60 g N/pot) and potassium sulphate (0.25 g K2O/pot) were administered 20 days after sowing. The experiment was conducted using a completely randomized design comprising nine distinct treatments, and each treatment was replicated five times. The treatments included: (1) a negative control as coated seeds without biocontrol agents; (2) the pathogen R. solanacearum PS-X4-1, isolated previously from the field and kept in our lab [47] ; (3) salinity stress induced by NaCl at 8 dS/m; (4) R. solanacearum combined with P. lalkuanensis; (5) R. solanacearum with phage P-PSG11; (6) R. solanacearum in combination with both P. lalkuanensis and phage P-PSG11; (7) NaCl stress with P. lalkuanensis; (8) NaCl stress with phage P-PSG11; and (9) NaCl stress combined with both P. lalkuanensis and phage P-PSG11. This design allowed for the examination of individual and combined effects of biotic (pathogen and beneficial microorganisms) and abiotic (salinity) stresses on the experimental subjects. Salinity treatments were prepared by mixing 12 g NaCl in 1.2 L water with 650 g soil. Plants were watered every third day, and plant heights and root lengths were measured at weeks 1 and 2 post-germination.
Long-term viability of stored phages and PGPR
Representative samples of STF were stored at room temperature for up to ≥ 1 year (406 days) to assess long-term stability. The detectability and viability of phage P-PSG11 and P. lalkuanensis A101 in these stored silk trehalose film samples were evaluated. Stability was verified using phage titration for P-PSG11 and colony counting for P. lalkuanensis A101. These methods quantified the concentration of viable agents, providing insights into their preservation within the STF matrix over an extended period at room temperature conditions.
Statistical analysis
All results are presented as mean values ± standard deviation (SD). Statistical analyses were conducted using one-way analysis of variance (ANOVA) with student’s t-test. Differences were considered statistically significant at the following levels: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. All statistical analyses were performed using GraphPad Prism software (version 8, GraphPad Software, San Diego, CA, USA).
Results
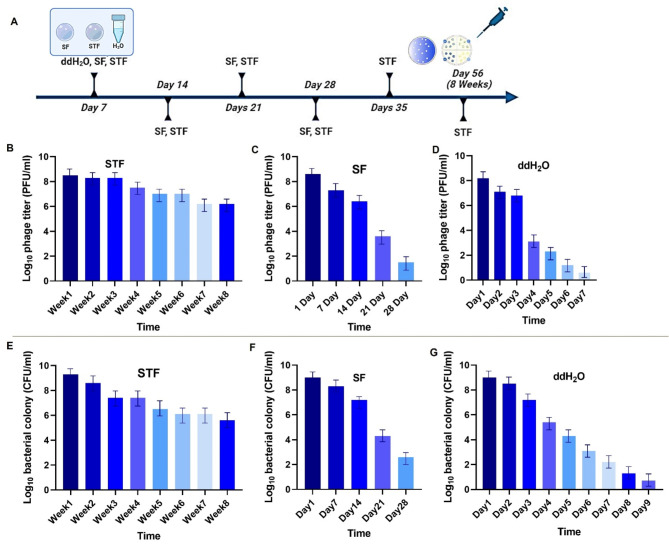
Stability of P. lalkuanensis A101 and P-PSG11 in silk films
Silk-based biomaterials (silk fibroin and a silk trehalose mixture) and double-distilled water (ddH2O) were used as preservation media for biocontrol agents rhizobacterium P. lalkuanensis A101 and bacteriophage P-PSG11 stored under room-temperature conditions (Fig. 2A). Upon mixing the silk biomaterials with either rhizobacterium P. lalkuanensis A101 or bacteriophage P-PSG11 the solutions were converted to films (i.e. silk fibroin film (SF) and silk trehalose film (STF)) using the drop-casting and spray drying method. For STF, a mixture ratio of 1:3 was selected due to its optimal solution viscosity and effective preservation of both P. lalkuanensis A101 and P-PSG11 (data not shown). Unless indicated specifically, the ST mixture ratio 1:3 was used in the following experiments. As seen in Fig. 2B-G, the viability of of rhizobacterium P. lalkuanensis A101 and bacteriophage P-PSG11 decreased gradually over time with that of STF showing the least drop in viability. Briefly, after 8 weeks of storage in the STF, the average weekly phage titers for P-PSG11 ranged between 10− 8 and 10− 9 plaque-forming units (PFU) per milliliter (Fig. 2B). Concurrently, colony counts for P. lalkuanensis A101 remained between 10− 7 and 10− 8 colony-forming units (CFU) per milliliter (Fig. 2E). In contrast, when using SF as a preservation medium, both agents remained stable for only 28 days. Storage in ddH2O at room temperature resulted in the shortest stability period of 7 days. These results highlight the superior efficacy of STF in preserving the bacteriophage and P. lalkuanensis A101 for extended periods without compromising viability. The stability achieved with the STF significantly outperformed the SF and ddH2O, demonstrating its potential as an effective preservation medium for these biological agents.
Fig. 2.
Stability of P-PSG11 and P. lalkuanensis A101 embedded in different films at room temperature: A) Timeline preservation in the silk trehalose film (STF), silk film (SF) and ddH2O. B, C, D) P-PSG11 phage titers were preserved in STF, SF, and ddH2O, respectively. E, F, G) P. lalkuanensis A101 colonies preserved in STF, SF, and ddH2O, respectively
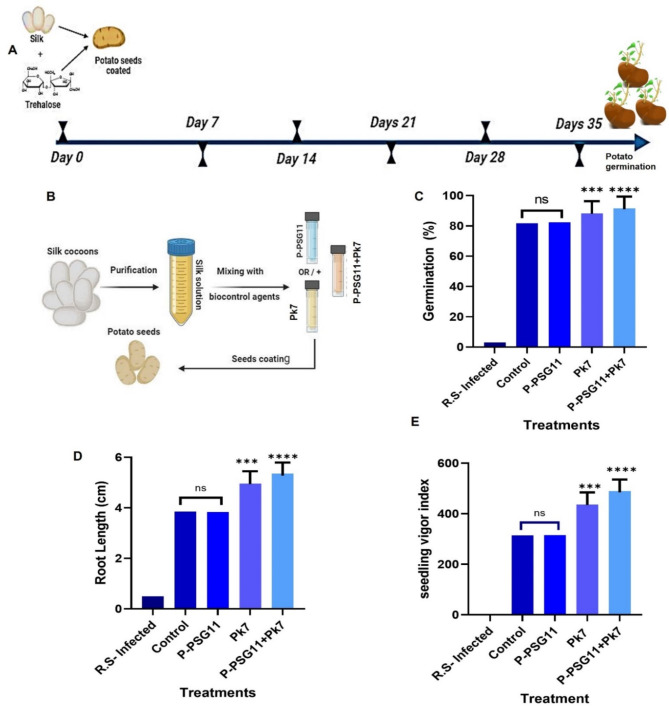
Germination boost of encapsulated potato seeds
Using a storage period of 4 weeks, potato seeds were subjected to different test conditions to assess their germination quality under controlled laboratory conditions (Fig. S3). The test conditions included infection with R. solanacearum (R.S -infected), treatment with water only (control), coating with a mixture of silk and trehalose (containing either P-PSG11 phage only (P-PSG11), P. lalkuanensis A101 only (A101), and a mixture of both (P-PSG11 + A101)) then airdried to form STF (Fig. 3A, B).
Fig. 3.
Evaluating germination of potato seeds under test conditions: (A) Timeline of potato germination in vitro. (B) Schematic representation of encapsulation. (C) Germination percentage of the potato seeds. (D) Potato root lengths under different treatments. (E) Seedling vigor index among all treatments. Statistical significance: ns, no significance; *** P < 0.001; **** P < 0.0001
As illustrated in Fig. 3C, seeds coated with a combination of bacteriophage P-PSG11 and P. lalkuanensis A101 in the STF demonstrated the most favorable germination outcome. After 4 weeks of storage, these seeds achieved the highest germination rate of 93.5% (P < 0.0001). Seeds coated with P. lalkuanensis A101 showed the second-highest germination rate at 86.3% (P < 0.001). In contrast, seeds primed with P-PSG11 phage and water exhibited a lower germination percentage of 81.8%. Seeds infected with R. solanacearum showed almost no germination (3%).
As shown in Fig. 3D, it was also found that seeds treated with the P-PSG11and P. lalkuanensis A101 combination produced the longest roots, measuring 5.4 cm (P < 0.0001), followed by P. lalkuanensis A101-treated seeds at 4.9 cm (P < 0.001). Seeds treated with P-PSG11, and untreated seeds had shorter root lengths of 2.85 cm, while infected seeds showed minimal root growth of 0.5 cm. Seed priming and coating treatments showed statistically significant differences in their effects on seedling vigor (Fig. 3). At the same time, the seedling vigor index, a measure of overall seedling health and potential, varied considerably among treatments (Fig. 3E). Seeds treated with the combination of bacteriophage P-PSG11 and P. lalkuanensis A101 exhibited the highest seedling vigor index of 489.5 (P < 0.0001). This was followed by seeds treated with P. lalkuanensis A101 alone, with a seedling vigor index of 436.8 (P < 0.001). In contrast, untreated control seeds and those treated with P-PSG11 showed similar but lower seedling vigor indices of 314.7 and 315, respectively. Seeds infected with R. solanacearum displayed a markedly lower seedling vigor index of just 1.50, indicating severe impairment of seedling development.
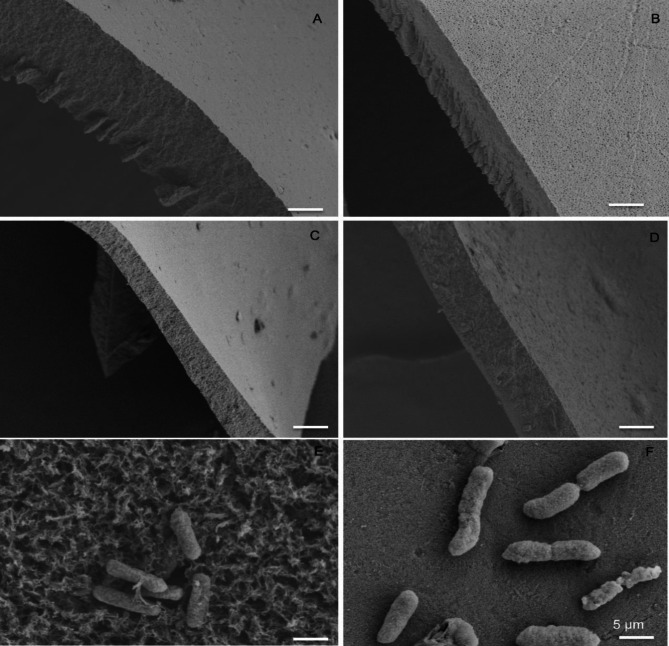
Scanning electron microscopy (SEM) validation
SEM was used to assess if trehalose had any effects on the films and if P. lalkuanensis A101 was encapsulated within the film. As shown in Fig. 4A and D, SEM images revealed that adding trehalose had no effect on the film formation, and the coating thickness was consistently in the range of 5 ± 2 μm. Furthermore, bacteria P. lalkuanensis A101 could be embedded within the film matrix (Fig. 4E), with a similar shape to the unembedded bacteria (Fig. 4F).
Fig. 4.
SEM micrographs of the silk films formed at the same solution mass by dip coating. Micrographs depicting the cross-section of the pure silk film (A), the ST (ratio 3:1) film (B), the ST (ratio 1:1) film (C), the ST (ratio 1:3) film (D), the ST film with A101 (E) and the unembedded bacteria A101 (F). Bar size: 5 μm
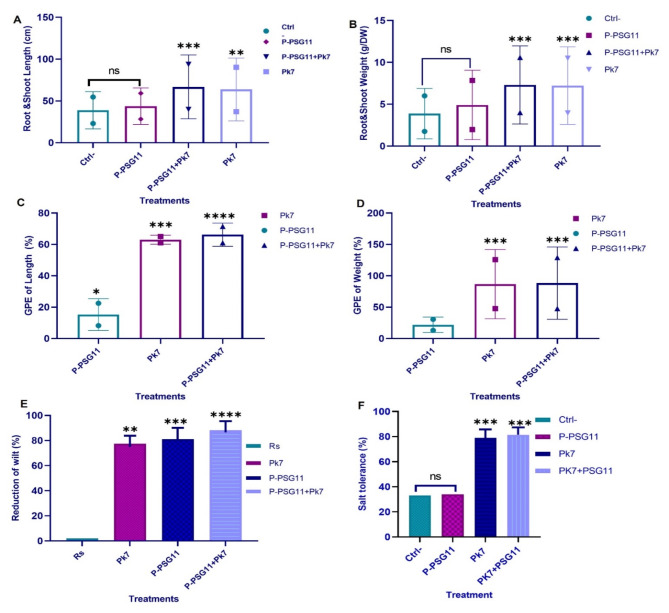
Pot experiments
Pot experiments were conducted to evaluate plant growth, antagonistic effects, and salt tolerance of the potato plants developed from seeds dip-coated with the STF (ratio 1:3) containing P. lalkuanensis A101.
and/or P-PSG11. Potato seeds under different treatments were cultivated for 35 days and compared to untreated control seeds (Fig. S4).
Plant growth enhancement
The application of STF containing P. lalkuanensis A101 and P-PSG11 as PGPR and biocontrol agents in potato seed coatings resulted in significant improvements (P < 0.001) in root elongation (Fig. 5A) and dry matter content of both roots and shoots (Fig. 5B). The growth promotion effect (GPE%) on root length was most pronounced with the P. lalkuanensis A101 and P-PSG11 combination (72.7%, P < 0.0001), followed by P. lalkuanensis A101 (61.0%), and P-PSG11 (22.5%), compared to untreated controls (Fig. 5A). Root dry weight increases were most substantial with the mixture of P-PSG11 and P. lalkuanensis A101 (129.1%), followed by P. lalkuanensis A101 (125.7%) and P-PSG11 (13.1%) compared to controls. Similarly, P-PSG11 and P. lalkuanensis A101 showed the highest increase in shoot dry weight, surpassing the control by 71.38% (Fig. 5B).
Fig. 5.
Evaluating plant growth promotion, disease suppression, and salt tolerance under pot experiment conditions. (A) The shoot and root length; (B) the biomass of potato plants developed from the seeds coated with the STF embedded with P-PSG11 + A101, P-PSG11, A101 or none of them (control) after 35 days of cultivation; (C) GPE % of potato seeds coated with the STF embedded with P-PSG11 + A101, P-PSG11, or A101 on plant length; (D) biomass compared to the control; E) the percentage of potato wilt incidence when planting the seeds coated with the STF embedded with P-PSG11 + A101, P-PSG11, A101 or none of them, in soil spiked with R. solanacearum; and F) the percentage of salt tolerance when planting the seeds coated with the STF embedded with P-PSG11 + A101, P-PSG11, A101 or none of them, in soil adding with NaCl. ANOVA analysis is used to analyze the significance between groups (ns, no significance; * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001)
Significant increases in plant length or height were also observed (Fig. 5C). Compared to the control, the GPE% was most pronounced with the P. lalkuanensis A101 and P-PSG11 combination (71.5%), followed by P. lalkuanensis A101 (65.1%), and P-PSG11 (8.2%). The P-PSG11 and P. lalkuanensis A101 combination exhibited the highest GPE% for plant fresh weight (111.3%), followed by P. lalkuanensis A101 (103.5%) and P-PSG11 (35.24%) (Fig. 5D). The P-PSG11 and P. lalkuanensis A101 combination consistently demonstrated the most significant GPE% across all measured parameters. This indicates its substantial impact on potato development when applied as a seed coating under the pot conditions.
Bioprotection against R. solanacearum
The STF containing P-PSG11 and P. lalkuanensis A101 mixture, P-PSG11 only, and P. lalkuanensis A101 only significantly reduced wilt incidence by 88.20%, 81.15%, and 77.45%, respectively (P < 0.0001) (Fig. 5E) when planting the seeds into the soil spiked with R. solanacearum. These biocontrol agents improved plant survival from 8.45% (without antagonist) to over 88% after seed coating application under pot experiment conditions. Even when R. solanacearum was introduced 35 days post the seed planting, the P-PSG11 and P. lalkuanensis A101 combination still showed some effects in reducing wilt symptoms.
Salinity mitigation
Potato seeds coated with the P-PSG11 and/or P. lalkuanensis A101 embedded ST film were grown in saline (8 dS/m, by adding NaCl to the topsoil) and non-saline soils for four weeks.
Seeds coated with STF containing either P. lalkuanensis A101 only or a P-PSG11 and P. lalkuanensis A101 mixture showed significantly higher germination rates (∼ 80%) under saline conditions compared to uncoated seeds, which had a germination rate of ∼ 35% (Fig. 5F). In contrast, the seeds coated with P-PSG11 had no difference from the uncoated seeds. Furthermore, throughout the observation period, seedlings from the seeds coated with P. lalkuanensis A101 or a mixture of P-PSG11 and P. lalkuanensis A101 exhibited greater height and more developed root systems than the control seedlings. These results demonstrated that the salt tolerance is mainly due to P. lalkuanensis A101.
Determining the integrity of long-term stored phage and PGPR
One STF embedded with P-PSG11 + A101 was prepared on April 1, 2023, stored at room temperature for over one year, and evaluated for activity on May 10, 2024 (406 days). As shown in Fig. 6, biocontrol agents (phage 10− 6 PFU and bacteria 10− 5 CFU) preserved in the STF maintained their viability, indicating that trehalose effectively protected the phage and the bacteria. In contrast, silk films without trehalose did not provide the same level of protection. This finding demonstrates that the STF effectively preserves these agents well, ensuring more than one year of activity.
Fig. 6.
Evaluation of the long-term stability and activity of P-PSG11 phage and A101 bacteria preserved in the ST film. The activity of the phage P-PSG11 and the bacteria A101 preserved in STF was maintained, as evidenced by growth detected using (A) bacterial colony and (B) phage titer to assay after storage at room temperature for one year
Discussion
Phage therapy and PGPR have been instrumental in enhancing plant growth and managing various phytopathogens. Bacteriophages, being highly specific to their target pathogens and environmentally friendly, present a low risk to non-target organisms [46]. P. lalkuanensis, as a PGPR, shows potential for both biocontrol and enhancement of plant growth. Once plant growth is optimized and diseases are controlled, these agents require careful storage or transportation at low temperatures. Typically, they must be kept at low temperatures (-20 ℃ for short durations or -80 ℃ for extended periods), particularly for phages and PGPR. Failure to maintain proper storage conditions can lead to degradation, resulting in inconsistent or invalid test results [50, 51]. This degradation could potentially impact the effectiveness of crop treatments [52, 53]. Drawing inspiration from tardigrades’ resilience and Bombyx Mori’s silk-producing abilities, we developed a method to engineer the seed microenvironment.
In the current study, we investigated the potential of using extracted silk solution to create films [54] as an alternative to preserve P. lalkuanensis A101 and phage P-PSG11. A method was developed to preserve phages and PGPR at room temperatures (≥ 25 ℃) without needing cold chain storage and transportation. Our bioinspired approach combines a disaccharide, known for its role in anhydrobiosis, with a structural protein that offers mechanical strength, easy fabrication, adhesion, flexibility, and controlled degradation [18]. Both the bacteriophage P-PSG11 and P. lalkuanensis A101 utilized in this study survived encapsulation within the biomaterial coating, were preserved over time, and were successfully released into the soil. Seeds coated with STF yielded plants that grew faster and stronger in saline soil and effectively controlled R. solanacearum.
In our preservation study, P. lalkuanensis A101 and P-PSG11 preserved in SF and STF exhibited prolonged stability at room temperature compared to preservation in ddH2O. The STF demonstrated the highest stability, followed by the SF. Specifically, the STF remained stable for 8 weeks at room temperature (25 ℃ − 28 ℃), while the SF maintained stability for 28 days. These findings suggest that STF could potentially be utilized for the long-term stabilization of bacteria and phage beyond the 8-week timeframe. Due to limited availability, we could only retain one sample to assess the STF’s ability to preserve P. lalkuanensis A101 and P-PSG11 phage over one year. Despite this limitation, our initial results indicate that the technique may be promising for preserving and transporting beneficial microorganisms for agricultural and research purposes [55, 56].
In evaluating the germination of coated seeds, dip coating was chosen due to its cost-effectiveness, scalability, and simplicity, making it accessible to all farmers across various resource settings [57]. Among the materials investigated, the silk-trehalose mixture with a 1:3 ratio was selected for its superior mechanical properties, solution viscosity, and preservation efficacy for both P. lalkuanensis A101 and phage P-PSG11. The coating process was tailored to apply ∼ 108 CFU of P. lalkuanensis A101 bacteria per seed, aligning with the standards typically mandated by policymakers for PGPR applications [58]. This concentration ensures an adequate initial population of beneficial bacteria to colonize the rhizosphere and promote plant growth. Results (Fig. 5E) showed that the seed coating composed of P. lalkuanensis A101, phage P-PSG11, and silk-trehalose in a 1:3 ratio was particularly evident in biocontrol against R. solanacearum and resistance to high salinity (8 dS/m). This coating significantly enhanced seed germination and produced more robust seedlings under these stress conditions.
In conclusion, our approach has demonstrated significant efficacy in controlling Ralstonia solanacearum and alleviating soil salinity stress by coating potato seeds with the STF embedded with P-PSG11 and A101, compared to uncoated (control) potato seeds. This innovative seed coating method shows promise for revolutionizing agricultural practices by enhancing crop resilience and sustainability. Despite the promising results, this study was limited by sample size, focusing on a single crop (potatoes) and a specific set of stressors (R. solanacearum and soil salinity). Future developments in microbial inoculants will prioritize the creation of precise and scalable delivery mechanisms for beneficial microbes and the development of multifunctional microbe solutions tailored to various crops. Characterizing the properties of the silk films produced, such as mechanical strength, moisture content, and degradation rates, would further help determine how the seed coating should be used in real applications. These advancements can potentially address critical challenges in water, energy, and food security (WEFS), particularly those related to climate change, soil degradation, and population growth.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was financially supported by the Wuhan Institute of Virology, Chinese Academy of Sciences, China (ZDRW-ZS-2016-4). The authors are grateful to Jun Hua, Meng Wei, Fang Fang, Mysara Griesh, Mohamed Ali Farag Taha, and Fazal Mehmood Khan for their support and assistance in the lab during the study’s conduct.
Author contributions
Conceptualization, S.M., and H.W.; methodology, S.M., software, S.M., and R.N.; validation, S.M., and H.W.; formal analysis, S.M., and R.N., investigation, S.M.; H.Y.; resources, S.M. and H.W.; data curation, S.M., and H.Y.; writing—original draft preparation, S.M.; writing—review and editing, S.M., H.W.; visualization, S.M. and H.W.; supervision, H.W.; project administration, S.M. and H.W.; funding acquisition, H.W. All authors have read, reviewed and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Biosafety Laboratory, Wuhan Institute of Virology, Chinese Academy of Sciences, China (ZDRW-ZS-2016-4). The authors are grateful to the CAS-TWAS program for funding S. M. A. M. during her PhD studies at the University of Chinese Academy of Science (Beijing, China) and Wuhan Institute of Virology (Wuhan, China).
Data availability
All data generated or analyzed during this study are included in this article and its supplementary files. The protocol for silk fabrication, which provides detailed information about seed coating, can be found in the supplementary PDF. The raw data of Pseudomonas lalkuanensis A101 is available at NCBI BioProject: PRJNA1183823 (https://www.ncbi.nlm.nih.gov/search/all/?term=PRJNA1183823).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Organization WH. The state of food security and nutrition in the world 2019: safeguarding against economic slowdowns and downturns. Food & Agriculture Org. 2019;2009.
- 2.Pretty J. J.P.T.o.t.R.S.B.B.S., Agricultural sustainability: concepts, principles and evidence. 2008;363(1491):447–465. [DOI] [PMC free article] [PubMed]
- 3.Jamal MR et al. Challenges and adaptations for resilient rice production under changing environments in Bangladesh. 2023;12(6):1217.
- 4.Godfray, H. Charles J., et al. "Food security: the challenge of feeding 9 billion people." science 327.5967 (2010): 812-818. [DOI] [PubMed]
- 5.Gebbers R, Adamchuk VIJS. Precision agriculture and food security. 2010;327(5967):828–31. [DOI] [PubMed] [Google Scholar]
- 6.Gomiero TJS. Soil degradation, land scarcity and food security: Reviewing a complex challenge. 2016;8(3):281.
- 7.Pedrini, Simone, et al. "Seed coating: science or marketing spin?." Trends in plant science 22.2 (2017): 106-116. [DOI] [PubMed]
- 8.Taylor AG. Seed storage, germination, quality, and enhancements, in The physiology of vegetable crops. CABI Wallingford UK. 2020:1–30.
- 9.Deaker R, et al. Legume seed inoculation technology—a review. 2004;36(8):1275–88. [Google Scholar]
- 10.Lugtenberg B. F.J.A.r.o.m. Kamilova. Plant-growth-promoting rhizobacteria. 2009;63:541–56. [DOI] [PubMed] [Google Scholar]
- 11.Vessey JKJP, soil. Plant growth promoting rhizobacteria as biofertilizers. 2003;255:571–586.
- 12.Bulgarelli D, et al. Structure and functions of the bacterial microbiota of plants. 2013;64:807–38. [DOI] [PubMed] [Google Scholar]
- 13.Sinha RK et al. Embarking on a second green revolution for sustainable agriculture by vermiculture biotechnology using earthworms: reviving the dreams of Sir Charles Darwin. 2010;2(7):113.
- 14.Bhardwaj D et al. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. 2014;13:1–10. [DOI] [PMC free article] [PubMed]
- 15.Singh JS et al. Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. 2011;140(3–4):339–353.
- 16.O’Callaghan MJAm. and biotechnology, Microbial inoculation of seed for improved crop performance: issues and opportunities. 2016;100(13):5729–5746. [DOI] [PMC free article] [PubMed]
- 17.Zvinavashe AT et al. Engineering the plant microenvironment to facilitate plant-growth-promoting microbe association. 2021;69(45):13270–13285. [DOI] [PubMed]
- 18.Zvinavashe AT et al. A bioinspired approach to engineer seed microenvironment to boost germination and mitigate soil salinity. 2019;116(51):25555–25561. [DOI] [PMC free article] [PubMed]
- 19.Singh D et al. Harnessing nature’s defenders: unveiling the potential of microbial consortia for plant defense induction against Alternaria blight in cumin. 2024:1–24. [DOI] [PubMed]
- 20.Liang, Dong, et al. "Complete Genome Sequence and Function Gene Identify of Prometryne-Degrading Strain Pseudomonas sp. DY-1." Microorganisms 9.6 (2021): 1261. [DOI] [PMC free article] [PubMed]
- 21.Abdelaziz AM et al. Biocontrol of soil borne diseases by plant growth promoting rhizobacteria. 2023;48(2):105–127.
- 22.Murthy KN, et al. Biocontrol potential of plant growth-promoting rhizobacteria (PGPR) against Ralstonia solanacearum: current and future prospects, in Biocontrol Agents and secondary metabolites. Elsevier; 2021. pp. 153–80.
- 23.Soltanighias T, Vaid RK, R.C.C.t.M PJM. Agricultural microbial genetic resources: application and preservation at microbial resource centers. 2018:141–173.
- 24.Ghoniem AA et al. Enhanced resistance of Vigna unguiculata to Fusarium oxysporum via Rubia cordifolia extract and growth-promoting endophytic Bacillus amyloliquefaciens DW6. 2024:1–25.
- 25.Morris CE et al. Surprising niche for the plant pathogen Pseudomonas syringae. 2007;7(1):84–92. [DOI] [PubMed]
- 26.Darrasse, Armelle, et al. "Transmission of plant-pathogenic bacteria by nonhost seeds without induction of an associated defense reaction at emergence." Applied and Environmental Microbiology 76.20 (2010): 6787-6796. [DOI] [PMC free article] [PubMed]
- 27.Mansfield J et al. Top 10 plant pathogenic bacteria in molecular plant pathology. 2012;13(6):614–629. [DOI] [PMC free article] [PubMed]
- 28.Burdman S, Walcott RJMpp. Acidovorax citrulli: generating basic and applied knowledge to tackle a global threat to the cucurbit industry. 2012;13(8):805–815. [DOI] [PMC free article] [PubMed]
- 29.Johnston-Monje D, Gutiérrez JP, L.A.B.J. .F.i.M. Lopez-Lavalle. Seed-transmitted bacteria Fungi Dominate Juvenile Plant Microbiomes. 2021;12:737616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giovanardi, Davide, et al. "Impact of bacterial spot outbreaks on the phytosanitary quality of tomato and pepper seeds." Plant Pathology 67.5 (2018): 1168-1176.
- 31.Choudhary, Devendra Kumar, et al. "Ralstonia solanacearum: A wide spread and global bacterial plant wilt pathogen." Journal of Pharmacognosy and Phytochemistry 7.2 (2018): 85-90.
- 32.Álvarez B et al. On the life of Ralstonia solanacearum, a destructive bacterial plant pathogen. 2010;1:267–279.
- 33.Nion YA, Toyota KJM, environments. Recent Trends Control Methods Bacterial wilt Dis Caused Ralstonia solanacearum. 2015;30(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wubshet Z. Economic importance and management of ginger bacterial wilt caused by Ralstonia solanacearum. J I J o R S I S. 2018;4(2):1–11. [Google Scholar]
- 35.Balogh B, et al. Control citrus Canker citrus Bacterial spot Bacteriophages. 2008;92(7):1048–52. [DOI] [PubMed] [Google Scholar]
- 36.Álvarez B, López MM, Biosca EGJFiM. Biocontrol Major Plant Pathogen Ralstonia solanacearum Irrig Water host Plants Novel Waterborne Lytic Bacteriophages. 2019;10:492073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKenna, F., et al. "Novel in vivo use of a polyvalent Streptomyces phage to disinfest Streptomyces scabies‐infected seed potatoes." Plant pathology 50.6 (2001): 666-675.
- 38.Balogh, B., et al. "Improved efficacy of newly formulated bacteriophages for management of bacterial spot on tomato." Plant disease 87.8 (2003): 949-954. [DOI] [PubMed]
- 39.Shade A, Jacques M-A. and M.J.C.o.i.m. Barret. Ecological patterns of seed microbiome diversity, transmission, and assembly. 2017;37:15–22. [DOI] [PubMed]
- 40.Darrasse A et al. Niches and routes of transmission of Xanthomonas citri pv. fuscans to bean seeds. 2018;422:115–128.
- 41.Vishunavat K, Prabakar K, Anand TJMD. Seed health: testing and management. 2023:335.
- 42.Rocha I et al. Seed coating: a tool for delivering beneficial microbes to agricultural crops. 2019;10:1357. [DOI] [PMC free article] [PubMed]
- 43.Kavusi, Elaheh, et al. "Delivery of beneficial microbes via seed coating for medicinal and aromatic plant production: a critical review." Journal of Plant Growth Regulation 42.2 (2023): 575-597.
- 44.Holland C et al. The biomedical use of silk: past, present, future. 2019;8(1):1800465. [DOI] [PubMed]
- 45.Zhou Z, et al. Engineering the future of silk materials through advanced manufacturing. 2018;30(33):1706983. [DOI] [PubMed] [Google Scholar]
- 46.Mousa, Samar, et al. "Microbial profiling of potato-associated rhizosphere bacteria under bacteriophage therapy." Antibiotics 11.8 (2022): 1117. [DOI] [PMC free article] [PubMed]
- 47.Wei C et al. Developing a bacteriophage cocktail for biocontrol of potato bacterial wilt. Virologica Sinica 32 (2017): 476–84. [DOI] [PMC free article] [PubMed]
- 48.Manonmani, V., and K. Vanangamudi. "Effect of seed source and size on seed germination and seedling vigour of sandal (Santalum album)." Journal of Tropical Forest Science 14.1 (2002): 150-155.
- 49.Sparks DL, et al. Methods of soil analysis, part 3: Chemical methods. Volume 14. Wiley; 2020.
- 50.Zhou Z, et al. Engineering the future of silk materials through advanced manufacturing. Adv Mater. 2018;30(33):1706983. [DOI] [PubMed] [Google Scholar]
- 51.O’Callaghan M. Microbial inoculation of seed for improved crop performance: issues and opportunities. Appl Microbiol Biotechnol. 2016;100(13):5729–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guziewicz NA, et al. Mechanisms of monoclonal antibody stabilization and release from silk biomaterials. 2013;34(31):7766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He, Jiuyang, et al. "Stabilization of RNA encapsulated in silk." ACS Biomaterials Science & Engineering 4.5 (2018): 1708-1715. [DOI] [PubMed]
- 54.Rockwood DN, et al. Materials fabrication from Bombyx mori silk fibroin. 2011;6(10):1612–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaidi A, et al. Role of plant growth promoting rhizobacteria in sustainable production of vegetables: current perspective. Sci Hort. 2015;193:231–9. [Google Scholar]
- 56.Sharma SB, et al. Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus. 2013;2:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malusá E, Sas-Paszt L, Ciesielska J. Technologies for beneficial microorganisms inocula used as biofertilizers. The scientific world journal, 2012;2012. [DOI] [PMC free article] [PubMed]
- 58.Malusa E, Vassilev N. A contribution to set a legal framework for biofertilisers. Appl Microbiol Biotechnol. 2014;98:6599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary files. The protocol for silk fabrication, which provides detailed information about seed coating, can be found in the supplementary PDF. The raw data of Pseudomonas lalkuanensis A101 is available at NCBI BioProject: PRJNA1183823 (https://www.ncbi.nlm.nih.gov/search/all/?term=PRJNA1183823).