Abstract
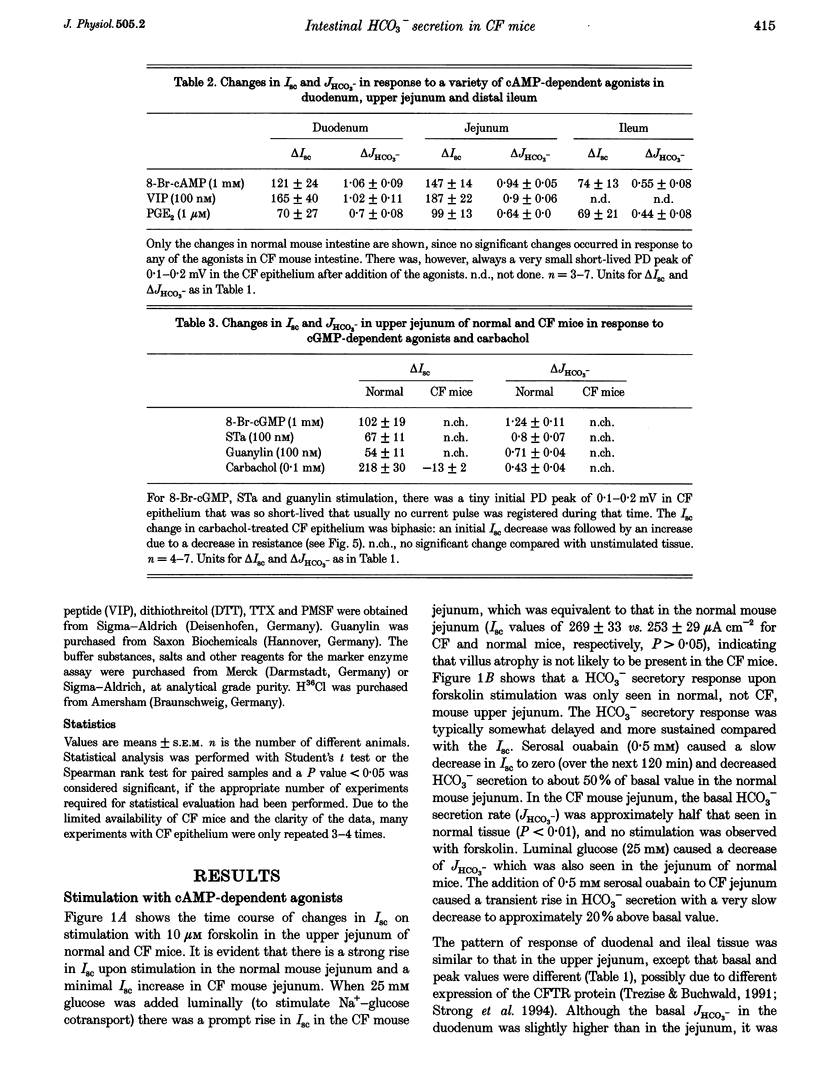
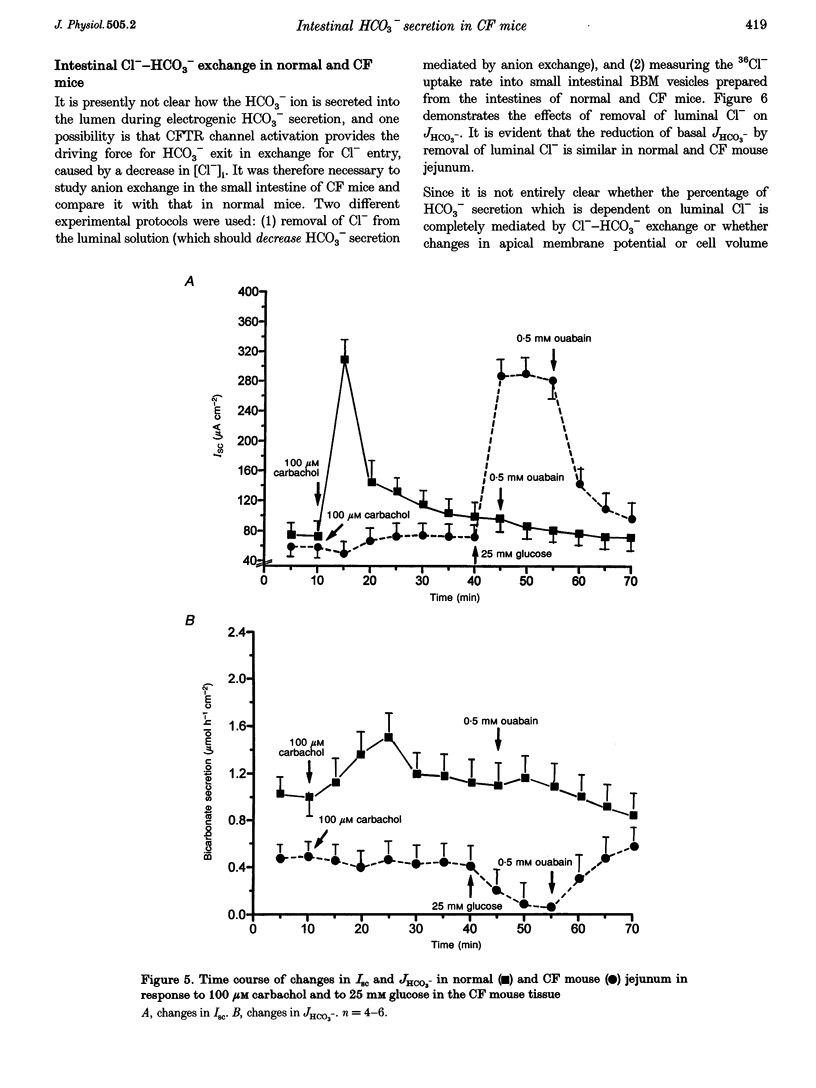
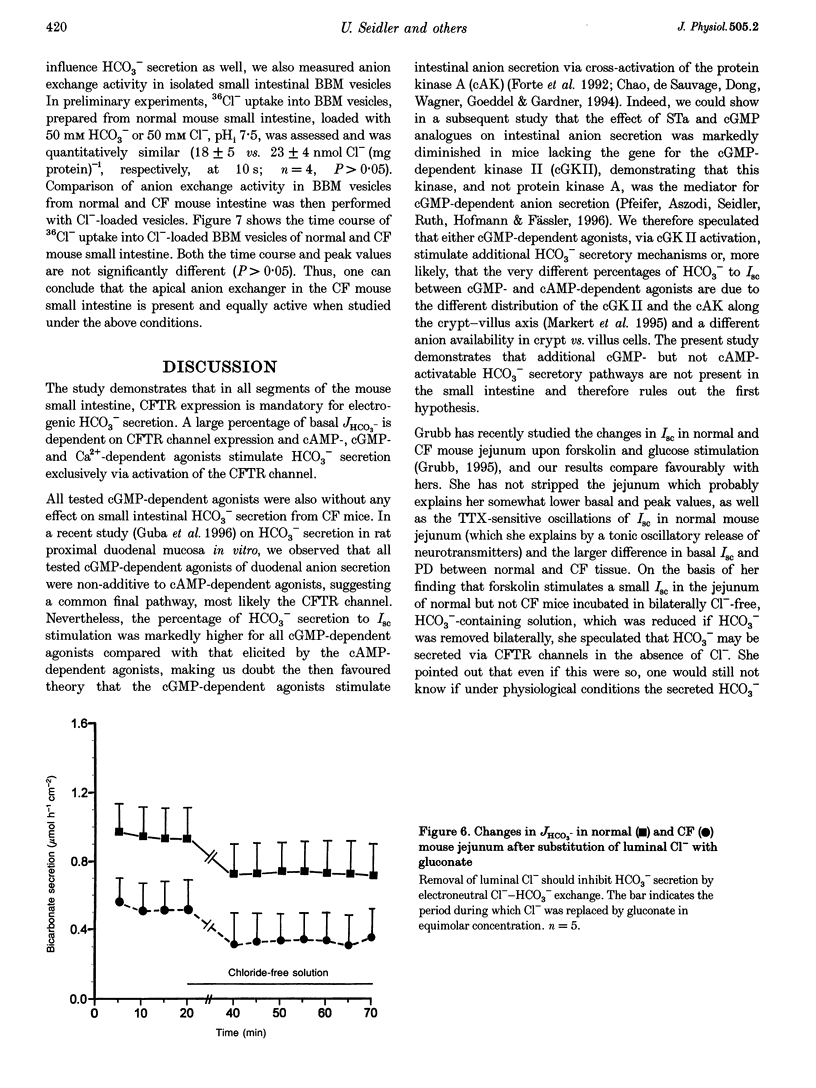
1. Most segments of the gastrointestinal tract secrete HCO3-, but the molecular nature of the secretory mechanisms has not been identified. We had previously speculated that the regulator for intestinal electrogenic HCO3- secretion is the cystic fibrosis transmembrane regulator (CFTR) channel. To prove this hypothesis, we have now measured HCO3- secretion by pH-stat titration, and recorded the electrical parameters of in vitro duodenum, jejunum and ileum of mice deficient in the gene for the CFTR protein ('CF-mice') and their normal littermates. 2. Basal HCO3- secretory rates were reduced in all small intestinal segments of CF mice. Forskolin, PGE2, 8-bromo-cAMP and VIP (cAMP-dependent agonists), heat-stable enterotoxin of Escherichia coli (STa), guanylin and 8-bromo-cGMP (cGMP-dependent agonists) and carbachol (Ca2+ dependent) stimulated both the short-circuit current (Isc) and the HCO3- secretory rate (JHCO3-) in all intestinal segments in normal mice, whereas none of these agonists had any effect on JHCO3- in the intestine of CF mice. 3. To investigate whether Cl(-)-HCO3- exchangers, which have been implicated in mediating the response to some of these agonists in the intestine, were similarly active in the small intestine of normal and CF mice, we studied Cl- gradient-driven 36Cl- uptake into brush-border membrane (BBM) vesicles isolated from normal and CF mouse small intestine. Both the time course and the peak value for 4,4'-diisothiocyanostilbene-2',2-disulphonic acid (DIDS)-inhibited 36Cl- uptake was similar in normal and CF mice BBM vesicles. 4. In summary, the results demonstrate that the presence of the CFTR channel is necessary for agonist-induced stimulation of electrogenic HCO3- secretion in all segments of the small intestine, and all three intracellular signal transduction pathways stimulate HCO3- secretion exclusively via activation of the CFTR channel.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Flemström G., Garner A., Kivilaakso E. Gastroduodenal mucosal protection. Physiol Rev. 1993 Oct;73(4):823–857. doi: 10.1152/physrev.1993.73.4.823. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Welsh M. J. Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6003–6007. doi: 10.1073/pnas.88.14.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker R., Groot J. A. cAMP-mediated effects of ouabain and theophylline on paracellular ion selectivity. Am J Physiol. 1984 Feb;246(2 Pt 1):G213–G217. doi: 10.1152/ajpgi.1984.246.2.G213. [DOI] [PubMed] [Google Scholar]
- Berschneider H. M., Knowles M. R., Azizkhan R. G., Boucher R. C., Tobey N. A., Orlando R. C., Powell D. W. Altered intestinal chloride transport in cystic fibrosis. FASEB J. 1988 Jul;2(10):2625–2629. doi: 10.1096/fasebj.2.10.2838365. [DOI] [PubMed] [Google Scholar]
- Brown D. R., Parsons A. M., O'Grady S. M. Substance P produces sodium and bicarbonate secretion in porcine jejunal mucosa through an action on enteric neurons. J Pharmacol Exp Ther. 1992 Jun;261(3):1206–1212. [PubMed] [Google Scholar]
- Chao A. C., de Sauvage F. J., Dong Y. J., Wagner J. A., Goeddel D. V., Gardner P. Activation of intestinal CFTR Cl- channel by heat-stable enterotoxin and guanylin via cAMP-dependent protein kinase. EMBO J. 1994 Mar 1;13(5):1065–1072. doi: 10.1002/j.1460-2075.1994.tb06355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L. L., Grubb B. R., Yankaskas J. R., Cotton C. U., McKenzie A., Boucher R. C. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in Cftr(-/-) mice. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):479–483. doi: 10.1073/pnas.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., MacVinish L. J., Hickman M. E., Ratcliff R., Colledge W. H., Evans M. J. Ion-transporting activity in the murine colonic epithelium of normal animals and animals with cystic fibrosis. Pflugers Arch. 1994 Oct;428(5-6):508–515. doi: 10.1007/BF00374572. [DOI] [PubMed] [Google Scholar]
- Dagher P. C., Rho J. I., Charney A. N. Mechanism of bicarbonate secretion in rat (Rattus rattus) colon. Comp Biochem Physiol Comp Physiol. 1993 May;105(1):43–48. doi: 10.1016/0300-9629(93)90171-y. [DOI] [PubMed] [Google Scholar]
- Davis G. R., Morawski S. G., Santa Ana C. A., Fordtran J. S. Evaluation of chloride/bicarbonate. Exchange in the human colon in vivo. J Clin Invest. 1983 Feb;71(2):201–207. doi: 10.1172/JCI110760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Pandol S. J. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest. 1986 Feb;77(2):348–354. doi: 10.1172/JCI112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemström G., Garner A., Nylander O., Hurst B. C., Heylings J. R. Surface epithelial HCO3(-) transport by mammalian duodenum in vivo. Am J Physiol. 1982 Nov;243(5):G348–G358. doi: 10.1152/ajpgi.1982.243.5.G348. [DOI] [PubMed] [Google Scholar]
- Flemström G., Heylings J. R., Garner A. Gastric and duodenal HCO3- transport in vitro: effects of hormones and local transmitters. Am J Physiol. 1982 Feb;242(2):G100–G110. doi: 10.1152/ajpgi.1982.242.2.G100. [DOI] [PubMed] [Google Scholar]
- Forbush B., 3rd Assay of Na,K-ATPase in plasma membrane preparations: increasing the permeability of membrane vesicles using sodium dodecyl sulfate buffered with bovine serum albumin. Anal Biochem. 1983 Jan;128(1):159–163. doi: 10.1016/0003-2697(83)90356-1. [DOI] [PubMed] [Google Scholar]
- Forte L. R., Thorne P. K., Eber S. L., Krause W. J., Freeman R. H., Francis S. H., Corbin J. D. Stimulation of intestinal Cl- transport by heat-stable enterotoxin: activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1992 Sep;263(3 Pt 1):C607–C615. doi: 10.1152/ajpcell.1992.263.3.C607. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Pollard C. E., Harris A., Coleman L., Greenwell J. R., Argent B. E. Anion selectivity and block of the small-conductance chloride channel on pancreatic duct cells. Am J Physiol. 1990 Nov;259(5 Pt 1):C752–C761. doi: 10.1152/ajpcell.1990.259.5.C752. [DOI] [PubMed] [Google Scholar]
- Grubb B. R. Ion transport across the jejunum in normal and cystic fibrosis mice. Am J Physiol. 1995 Mar;268(3 Pt 1):G505–G513. doi: 10.1152/ajpgi.1995.268.3.G505. [DOI] [PubMed] [Google Scholar]
- Guba M., Kuhn M., Forssmann W. G., Classen M., Gregor M., Seidler U. Guanylin strongly stimulates rat duodenal HCO3- secretion: proposed mechanism and comparison with other secretagogues. Gastroenterology. 1996 Dec;111(6):1558–1568. doi: 10.1016/s0016-5085(96)70018-5. [DOI] [PubMed] [Google Scholar]
- Hanna S. D., Mircheff A. K., Wright E. M. Alkaline phosphatase of basal lateral and brush border plasma membranes from intestinal epithelium. J Supramol Struct. 1979;11(4):451–466. doi: 10.1002/jss.400110404. [DOI] [PubMed] [Google Scholar]
- Heylings J. R., Feldman M. Basal and PGE2-stimulated duodenal bicarbonate secretion in the rat in vivo. Am J Physiol. 1988 Oct;255(4 Pt 1):G470–G475. doi: 10.1152/ajpgi.1988.255.4.G470. [DOI] [PubMed] [Google Scholar]
- Hubel K. A. Bicarbonate secretion in rat ileum and its dependence on intraluminal chloride. Am J Physiol. 1967 Dec;213(6):1409–1413. doi: 10.1152/ajplegacy.1967.213.6.1409. [DOI] [PubMed] [Google Scholar]
- Isenberg J. I., Ljungström M., Säfsten B., Flemström G. Proximal duodenal enterocyte transport: evidence for Na(+)-H+ and Cl(-)-HCO3- exchange and NaHCO3 cotransport. Am J Physiol. 1993 Oct;265(4 Pt 1):G677–G685. doi: 10.1152/ajpgi.1993.265.4.G677. [DOI] [PubMed] [Google Scholar]
- Knickelbein R., Aronson P. S., Atherton W., Dobbins J. W. Sodium and chloride transport across rabbit ileal brush border. I. Evidence for Na-H exchange. Am J Physiol. 1983 Oct;245(4):G504–G510. doi: 10.1152/ajpgi.1983.245.4.G504. [DOI] [PubMed] [Google Scholar]
- Knickelbein R., Aronson P. S., Schron C. M., Seifter J., Dobbins J. W. Sodium and chloride transport across rabbit ileal brush border. II. Evidence for Cl-HCO3 exchange and mechanism of coupling. Am J Physiol. 1985 Aug;249(2 Pt 1):G236–G245. doi: 10.1152/ajpgi.1985.249.2.G236. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K., Gerlach L., Fröbe U., Greger R. Bicarbonate permeability of epithelial chloride channels. Pflugers Arch. 1991 Feb;417(6):616–621. doi: 10.1007/BF00372960. [DOI] [PubMed] [Google Scholar]
- Markert T., Vaandrager A. B., Gambaryan S., Pöhler D., Häusler C., Walter U., De Jonge H. R., Jarchau T., Lohmann S. M. Endogenous expression of type II cGMP-dependent protein kinase mRNA and protein in rat intestine. Implications for cystic fibrosis transmembrane conductance regulator. J Clin Invest. 1995 Aug;96(2):822–830. doi: 10.1172/JCI118128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minhas B. S., Sullivan S. K., Field M. Bicarbonate secretion in rabbit ileum: electrogenicity, ion dependence, and effects of cyclic nucleotides. Gastroenterology. 1993 Dec;105(6):1617–1629. doi: 10.1016/0016-5085(93)91056-n. [DOI] [PubMed] [Google Scholar]
- Novak I., Greger R. Properties of the luminal membrane of isolated perfused rat pancreatic ducts. Effect of cyclic AMP and blockers of chloride transport. Pflugers Arch. 1988 May;411(5):546–553. doi: 10.1007/BF00582376. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Pfeifer A., Aszódi A., Seidler U., Ruth P., Hofmann F., Fässler R. Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science. 1996 Dec 20;274(5295):2082–2086. doi: 10.1126/science.274.5295.2082. [DOI] [PubMed] [Google Scholar]
- Poulsen J. H., Fischer H., Illek B., Machen T. E. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R., Evans M. J., Cuthbert A. W., MacVinish L. J., Foster D., Anderson J. R., Colledge W. H. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nat Genet. 1993 May;4(1):35–41. doi: 10.1038/ng0593-35. [DOI] [PubMed] [Google Scholar]
- Strong T. V., Boehm K., Collins F. S. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J Clin Invest. 1994 Jan;93(1):347–354. doi: 10.1172/JCI116966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. J., Baxter P. S., Hardcastle J., Hardcastle P. T. Failure to induce secretion in jejunal biopsies from children with cystic fibrosis. Gut. 1988 Jul;29(7):957–962. doi: 10.1136/gut.29.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezise A. E., Buchwald M. In vivo cell-specific expression of the cystic fibrosis transmembrane conductance regulator. Nature. 1991 Oct 3;353(6343):434–437. doi: 10.1038/353434a0. [DOI] [PubMed] [Google Scholar]
- Turnberg L. A., Bieberdorf F. A., Morawski S. G., Fordtran J. S. Interrelationships of chloride, bicarbonate, sodium, and hydrogen transport in the human ileum. J Clin Invest. 1970 Mar;49(3):557–567. doi: 10.1172/JCI106266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaandrager A. B., Bajnath R., Groot J. A., Bot A. G., De Jonge H. R. Ca2+ and cAMP activate different chloride efflux pathways in HT-29.cl19A colonic epithelial cell line. Am J Physiol. 1991 Dec;261(6 Pt 1):G958–G965. doi: 10.1152/ajpgi.1991.261.6.G958. [DOI] [PubMed] [Google Scholar]
- Valverde M. A., O'Brien J. A., Sepúlveda F. V., Ratcliff R., Evans M. J., Colledge W. H. Inactivation of the murine cftr gene abolishes cAMP-mediated but not Ca(2+)-mediated secretagogue-induced volume decrease in small-intestinal crypts. Pflugers Arch. 1993 Dec;425(5-6):434–438. doi: 10.1007/BF00374869. [DOI] [PubMed] [Google Scholar]
- Yao B., Hogan D. L., Bukhave K., Koss M. A., Isenberg J. I. Bicarbonate transport by rabbit duodenum in vitro: effect of vasoactive intestinal polypeptide, prostaglandin E2, and cyclic adenosine monophosphate. Gastroenterology. 1993 Mar;104(3):732–740. doi: 10.1016/0016-5085(93)91008-6. [DOI] [PubMed] [Google Scholar]


