Abstract
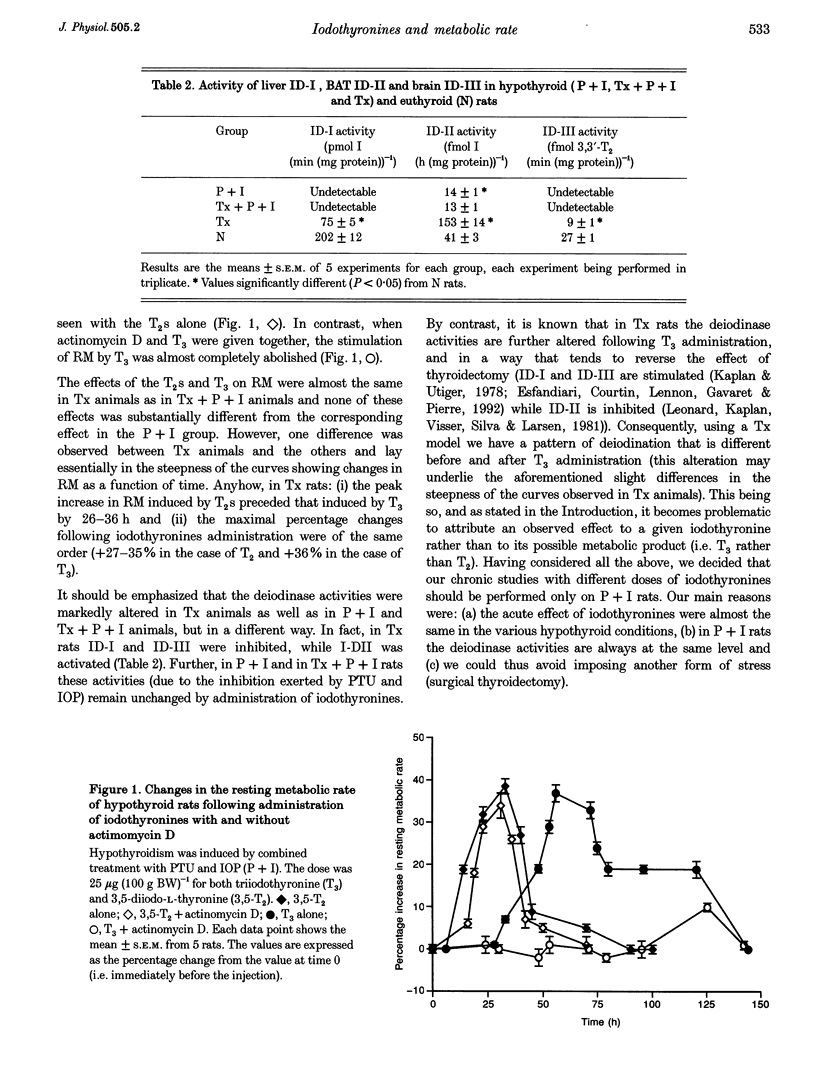
1. Although the first evidence of a relationship between the thyroid and metabolism was reported in 1895, the mechanism by which thyroid hormones influence resting metabolic rate in whole animals is still poorly understood. This paper reports an attempt to test whether diiodothyronines (T2s) and triiodothyronine (T3) have different roles in the control of resting metabolism (RM). 2. Changes in resting metabolic rate were measured in hypothyroid rats treated acutely (25 micrograms (100 g body weight)-1) either with one of the T2s or with T3. Injection of T3 induced an increase of about 35% in RM that started 25-30 h after the injection and lasted until 5-6 days after the injection, the maximal value being observed at 50-75 h. The injection of T2s evoked a temporally different pattern of response. The increases in RM started 6-12 h after the injection, had almost disappeared after 48 h, and the maximal stimulation was observed at 28-30 h. 3. When actinomycin D (an inhibitor of protein synthesis) and T3 were given together, the stimulation of RM was almost completely abolished. The simultaneous injection of actinomycin D and either of the T2s, on the other hand, did not cause any attenuation of the stimulation seen with the T2s alone. 4. Following chronic treatment (3 weeks) with either T3 or T2s there was a stimulation of organ growth only after the administration of T3. 5. Chronic administration of either T2s or T3 to hypothyroid rats significantly enhanced the oxidative capacity of each of the tissues considered. In the case of T2s the stimulation was almost the same whether it was expressed as an increase in specific activity or total tissue activity. In the case of T3 the increases were, in the main, secondary to the hypertrophic or hyperplastic effect. 6. These results indicate that T2s and T3 exert different effects on RM. The effects of T2s are rapid and possibly mediated by their direct interaction with mitochondria. Those of T3 are slower and more prolonged, and at least partly attributable to a modulation of the cellularity of tissues that are metabolically very active.
Full text
PDF



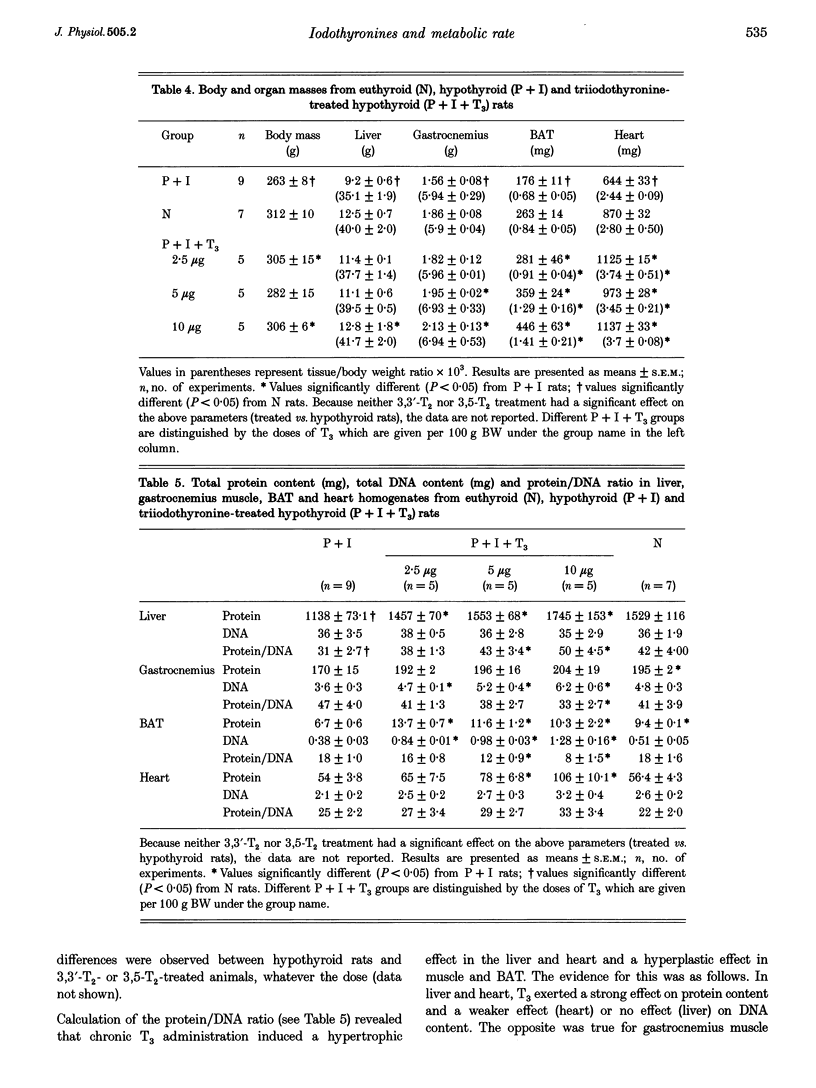



Selected References
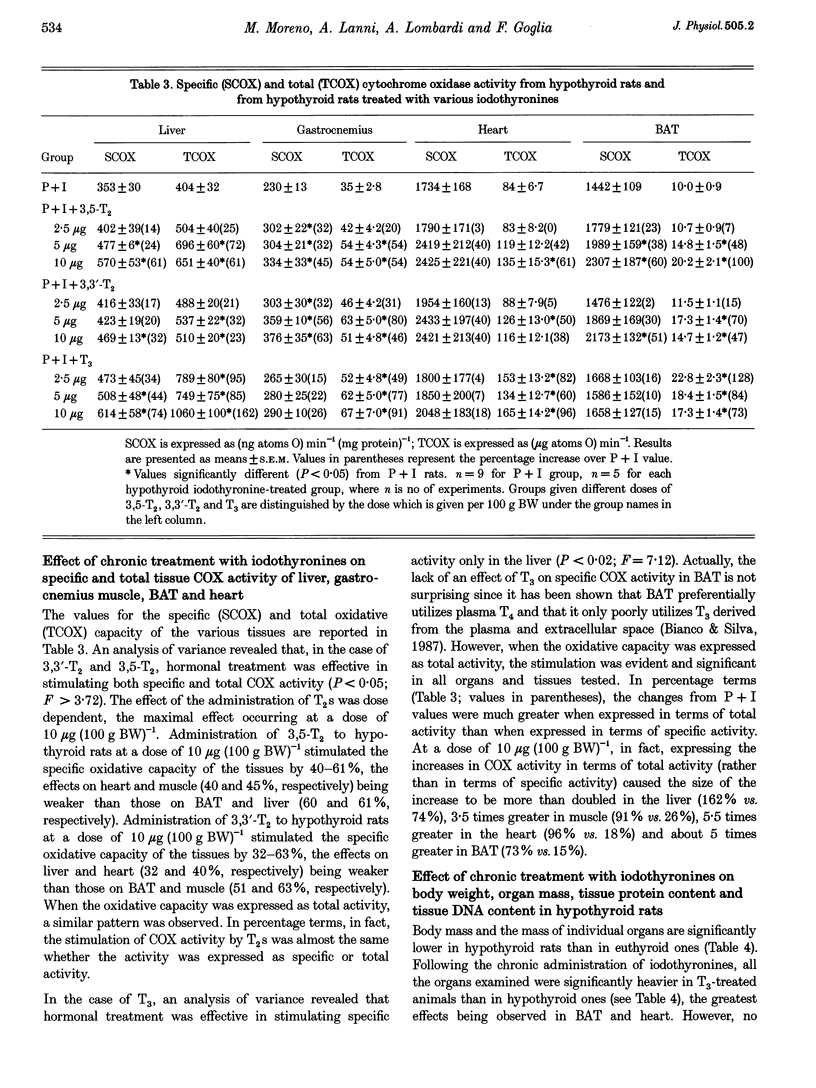
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson K. J., Burger A. G. A study of the relationship between thermogenesis and thyroid hormones. J Clin Endocrinol Metab. 1980 Jul;51(1):84–89. doi: 10.1210/jcem-51-1-84. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré H., Bailly L., Rouanet J. L. Increased oxidative capacity in skeletal muscles from cold-acclimated ducklings: a comparison with rats. Comp Biochem Physiol B. 1987;88(2):519–522. doi: 10.1016/0305-0491(87)90337-3. [DOI] [PubMed] [Google Scholar]
- Bianco A. C., Silva J. E. Optimal response of key enzymes and uncoupling protein to cold in BAT depends on local T3 generation. Am J Physiol. 1987 Sep;253(3 Pt 1):E255–E263. doi: 10.1152/ajpendo.1987.253.3.E255. [DOI] [PubMed] [Google Scholar]
- Dümmler K., Müller S., Seitz H. J. Regulation of adenine nucleotide translocase and glycerol 3-phosphate dehydrogenase expression by thyroid hormones in different rat tissues. Biochem J. 1996 Aug 1;317(Pt 3):913–918. doi: 10.1042/bj3170913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfandiari A., Courtin F., Lennon A. M., Gavaret J. M., Pierre M. Induction of type III deiodinase activity in astroglial cells by thyroid hormones. Endocrinology. 1992 Oct;131(4):1682–1688. doi: 10.1210/endo.131.4.1396314. [DOI] [PubMed] [Google Scholar]
- Francavilla S., Cordeschi G., Properzi G., Di Cicco L., Jannini E. A., Palmero S., Fugassa E., Loras B., D'Armiento M. Effect of thyroid hormone on the pre- and post-natal development of the rat testis. J Endocrinol. 1991 Apr;129(1):35–42. doi: 10.1677/joe.0.1290035. [DOI] [PubMed] [Google Scholar]
- GOLDBERG I. H., RABINOWITZ M. Actionmycin D inhibition of deoxyribonucleic acid-dependent synthesis of ribonucleic acid. Science. 1962 Apr 27;136(3513):315–316. doi: 10.1126/science.136.3513.315. [DOI] [PubMed] [Google Scholar]
- Goglia F., Lanni A., Horst C., Moreno M., Thoma R. In vitro binding of 3,5-di-iodo-L-thyronine to rat liver mitochondria. J Mol Endocrinol. 1994 Dec;13(3):275–282. doi: 10.1677/jme.0.0130275. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Jump D. B., Narayan P., Towle H., Oppenheimer J. H. Rapid effects of triiodothyronine on hepatic gene expression. Hybridization analysis of tissue-specific triiodothyronine regulation of mRNAS14. J Biol Chem. 1984 Mar 10;259(5):2789–2797. [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in liver and kidney homogenates from hyperthyroid and hypothyroid rats. Endocrinology. 1978 Jul;103(1):156–161. doi: 10.1210/endo-103-1-156. [DOI] [PubMed] [Google Scholar]
- Köhrle J. Thyroid hormone deiodination in target tissues--a regulatory role for the trace element selenium? Exp Clin Endocrinol. 1994;102(2):63–89. doi: 10.1055/s-0029-1211267. [DOI] [PubMed] [Google Scholar]
- Lanni A., Moreno M., Cioffi M., Goglia F. Effect of 3,3'-di-iodothyronine and 3,5-di-iodothyronine on rat liver mitochondria. J Endocrinol. 1993 Jan;136(1):59–64. doi: 10.1677/joe.0.1360059. [DOI] [PubMed] [Google Scholar]
- Lanni A., Moreno M., Cioffi M., Goglia F. Effect of 3,3'-diiodothyronine and 3,5-diiodothyronine on rat liver oxidative capacity. Mol Cell Endocrinol. 1992 Aug;86(3):143–148. doi: 10.1016/0303-7207(92)90138-v. [DOI] [PubMed] [Google Scholar]
- Lanni A., Moreno M., Horst C., Lombardi A., Goglia F. Specific binding sites for 3,3'-diiodo-L-thyronine (3,3'-T2) in rat liver mitochondria. FEBS Lett. 1994 Sep 5;351(2):237–240. doi: 10.1016/0014-5793(94)00840-x. [DOI] [PubMed] [Google Scholar]
- Lanni A., Moreno M., Lombardi A., Goglia F. Calorigenic effect of diiodothyronines in the rat. J Physiol. 1996 Aug 1;494(Pt 3):831–837. doi: 10.1113/jphysiol.1996.sp021536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni A., Moreno M., Lombardi A., Goglia F. Rapid stimulation in vitro of rat liver cytochrome oxidase activity by 3,5-diiodo-L-thyronine and by 3,3'-diiodo-L-thyronine. Mol Cell Endocrinol. 1994 Feb;99(1):89–94. doi: 10.1016/0303-7207(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Lazar M. A. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993 Apr;14(2):184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Kaplan M. M., Visser T. J., Silva J. E., Larsen P. R. Cerebral cortex responds rapidly to thyroid hormones. Science. 1981 Oct 30;214(4520):571–573. doi: 10.1126/science.7291997. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Mellen S. A., Larsen P. R. Thyroxine 5'-deiodinase activity in brown adipose tissue. Endocrinology. 1983 Mar;112(3):1153–1155. doi: 10.1210/endo-112-3-1153. [DOI] [PubMed] [Google Scholar]
- Luciakova K., Nelson B. D. Transcript levels for nuclear-encoded mammalian mitochondrial respiratory-chain components are regulated by thyroid hormone in an uncoordinated fashion. Eur J Biochem. 1992 Jul 1;207(1):247–251. doi: 10.1111/j.1432-1033.1992.tb17044.x. [DOI] [PubMed] [Google Scholar]
- O'Reilly I., Murphy M. P. Studies on the rapid stimulation of mitochondrial respiration by thyroid hormones. Acta Endocrinol (Copenh) 1992 Dec;127(6):542–546. doi: 10.1530/acta.0.1270542. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Lane J. T., Thompson M. P. Functional relationship of thyroid hormone-induced lipogenesis, lipolysis, and thermogenesis in the rat. J Clin Invest. 1991 Jan;87(1):125–132. doi: 10.1172/JCI114961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Mariash C. N., Kinlaw W. B., Wong N. C., Freake H. C. Advances in our understanding of thyroid hormone action at the cellular level. Endocr Rev. 1987 Aug;8(3):288–308. doi: 10.1210/edrv-8-3-288. [DOI] [PubMed] [Google Scholar]
- Scarpulla R. C., Kilar M. C., Scarpulla K. M. Coordinate induction of multiple cytochrome c mRNAs in response to thyroid hormone. J Biol Chem. 1986 Apr 5;261(10):4660–4662. [PubMed] [Google Scholar]
- Schoenmakers C. H., Pigmans I. G., Visser T. J. Investigation of type I and type III iodothyronine deiodinases in rat tissues using N-bromoacetyl-iodothyronine affinity labels. Mol Cell Endocrinol. 1995 Feb;107(2):173–180. doi: 10.1016/0303-7207(94)03440-5. [DOI] [PubMed] [Google Scholar]
- Sekura R. D., Sato K., Cahnmann H. J., Robbins J., Jakoby W. B. Sulfate transfer to thyroid hormones and their analogs by hepatic aryl sulfotransferases. Endocrinology. 1981 Feb;108(2):454–456. doi: 10.1210/endo-108-2-454. [DOI] [PubMed] [Google Scholar]
- Sestoft L. Metabolic aspects of the calorigenic effect of thyroid hormone in mammals. Clin Endocrinol (Oxf) 1980 Nov;13(5):489–506. doi: 10.1111/j.1365-2265.1980.tb03415.x. [DOI] [PubMed] [Google Scholar]
- Soboll S. Thyroid hormone action on mitochondrial energy transfer. Biochim Biophys Acta. 1993 Aug 16;1144(1):1–16. doi: 10.1016/0005-2728(93)90024-a. [DOI] [PubMed] [Google Scholar]
- TATA J. R., ERNSTER L., LINDBERG O., ARRHENIUS E., PEDERSEN S., HEDMAN R. The action of thyroid hormones at the cell level. Biochem J. 1963 Mar;86:408–428. doi: 10.1042/bj0860408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TATA J. R., ERNSTER L., LINDBERG O. Control of basal metabolic rate by thyroid hormones and cellular function. Nature. 1962 Mar 17;193:1058–1060. doi: 10.1038/1931058a0. [DOI] [PubMed] [Google Scholar]
- TATA J. R. Inhibition of the biological action of thyroid hormones by actinomycin D and puromycin. Nature. 1963 Mar 23;197:1167–1168. doi: 10.1038/1971167a0. [DOI] [PubMed] [Google Scholar]
- Visser T. J., Docter R., Hennemann G. Radioimmunoassay of reverse tri-iodothyronine. J Endocrinol. 1977 May;73(2):395–396. doi: 10.1677/joe.0.0730395. [DOI] [PubMed] [Google Scholar]
- Visser T. J., Kaptein E., Terpstra O. T., Krenning E. P. Deiodination of thyroid hormone by human liver. J Clin Endocrinol Metab. 1988 Jul;67(1):17–24. doi: 10.1210/jcem-67-1-17. [DOI] [PubMed] [Google Scholar]
- Visser T. J., Krieger-Quist L. M., Docter R., Hennemann G. Radioimmunoassay of 3,3'-di-iodothyronine in unextracted serum: the effect of endogenous tri-iodothyronine. J Endocrinol. 1978 Dec;79(3):357–362. doi: 10.1677/joe.0.0790357. [DOI] [PubMed] [Google Scholar]


