Abstract
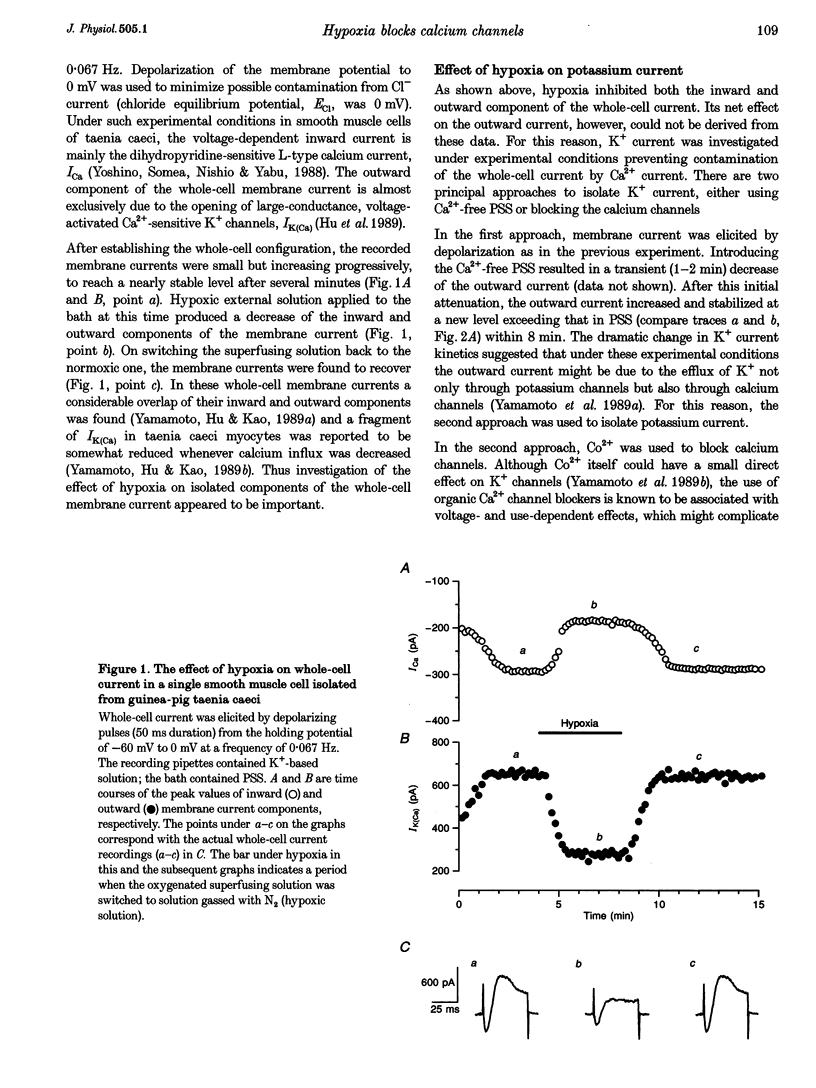
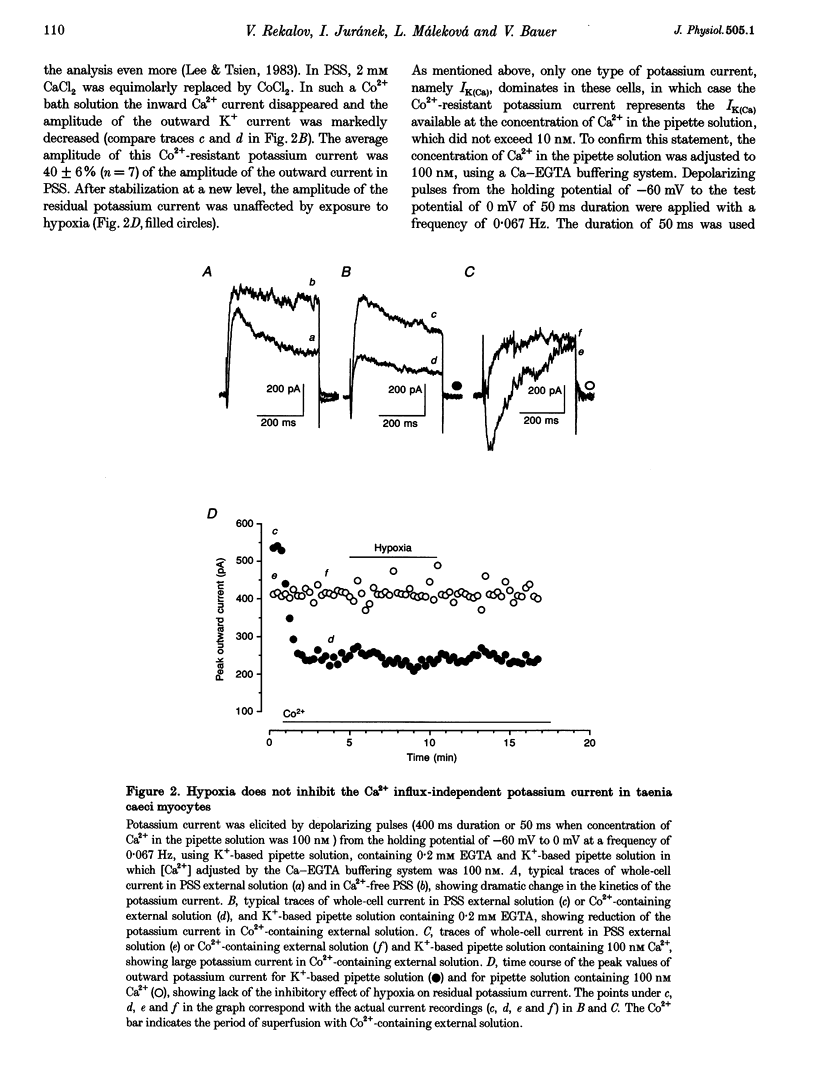
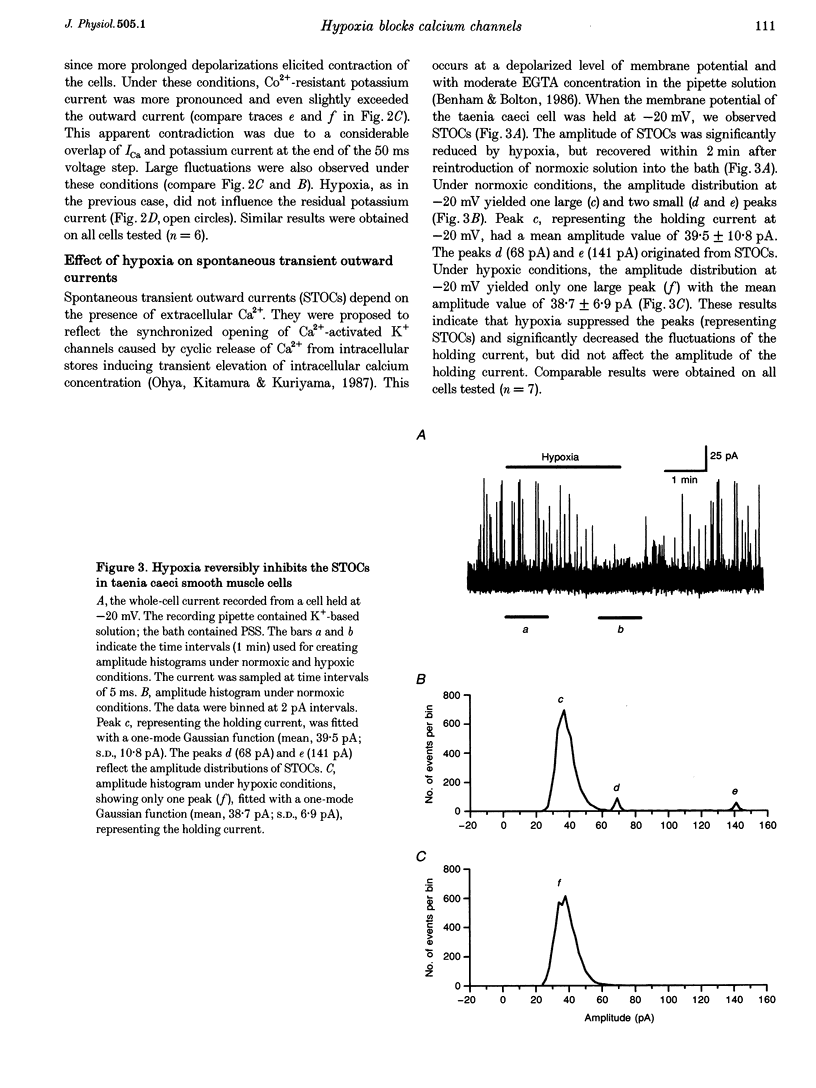
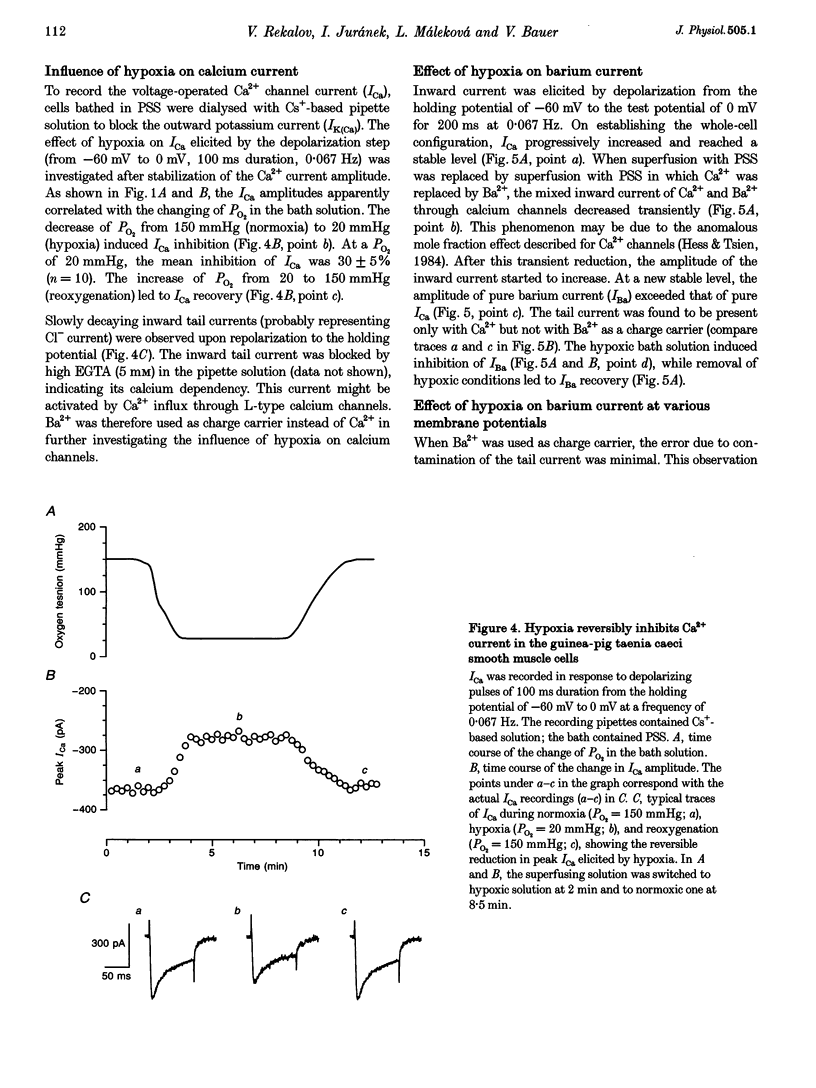
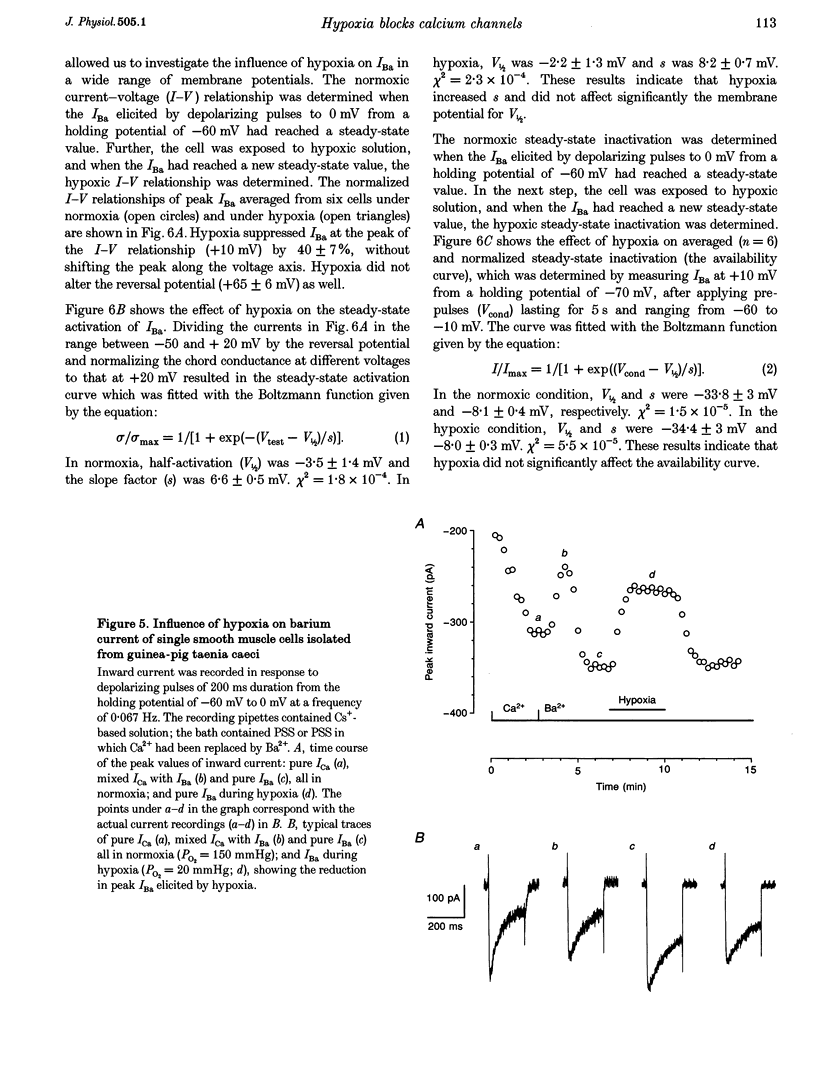
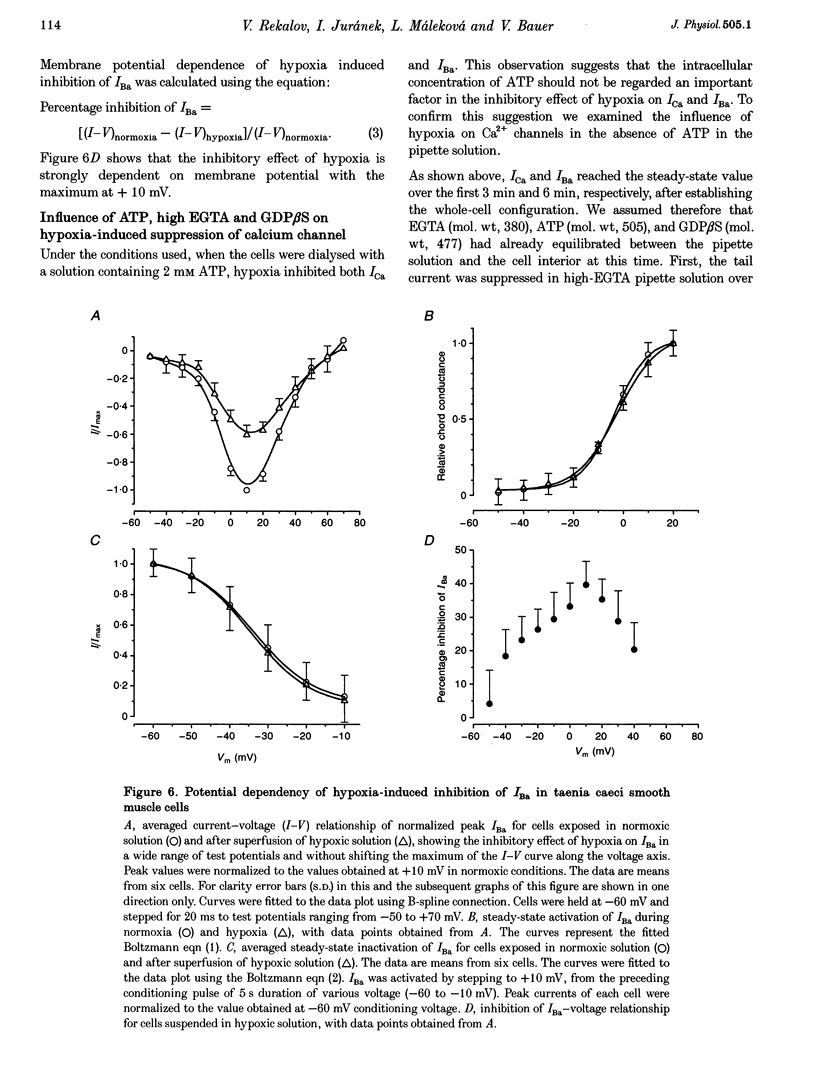
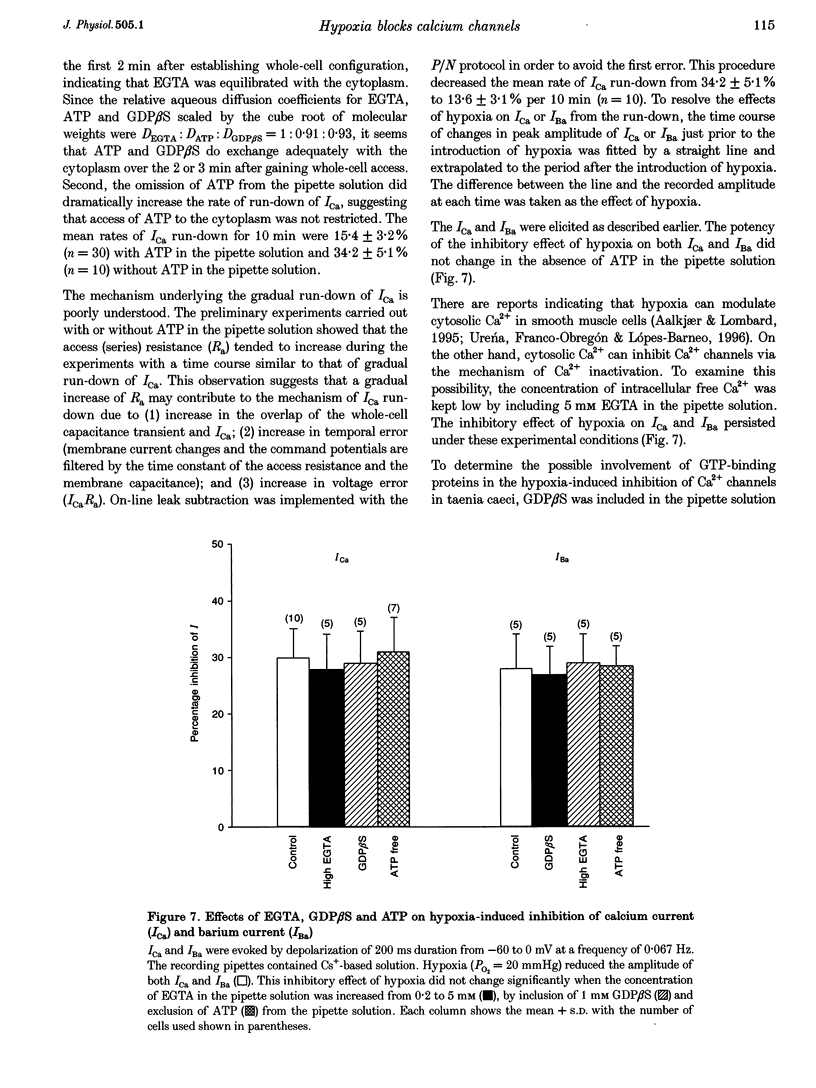
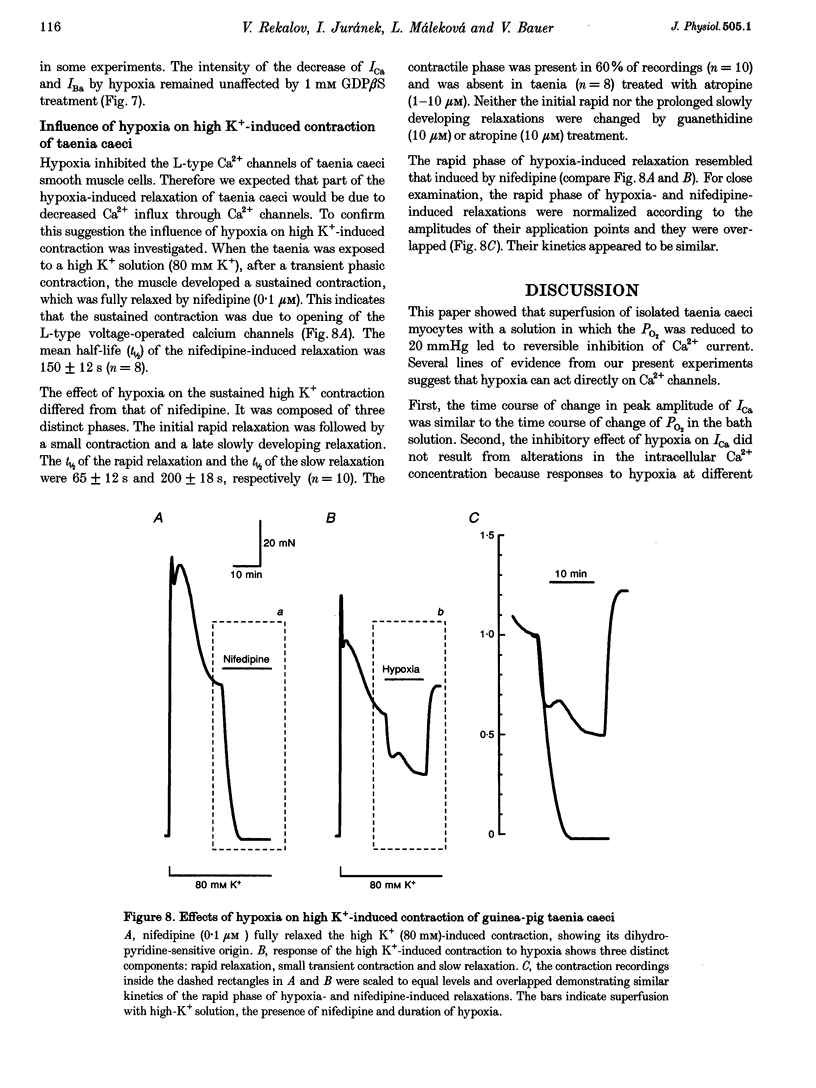
1. The effects of hypoxia on whole-cell current in single smooth muscle cells and on a high K(+)-induced contraction of strips of the guinea-pig taenia caeci were studied. 2. In physiological salt solution (PSS) and K(+)-based pipette solution, hypoxia (PO2 = 20 mmHg) reversibly inhibited both the inward Ca2+ current (ICa) and outward Ca(2+)-activated K+ current (IK(Ca)) components of the whole-cell current. 3. In PSS and Cs(+)-based pipette solution, hypoxia reversibly suppressed ICa by 30 +/- 5% at 0 mV. 4. When Ba2+ was used as a charge carrier, the IBa was suppressed by hypoxia in a potential-dependent manner, with the maximum of 40 +/- 7% at +10 mV. Alterations of concentrations of EGTA, GDB beta S or ATP in the pipette solution did not change the inhibitory effects of hypoxia on ICa and IBa. 5. In PSS with 2 mM CaCl2 replaced by CoCl2, hypoxia did not affect the Ca2+ influx-independent potassium current. 6. In cells voltage clamped at -20 mV hypoxia reversibly inhibited the spontaneous transient outward currents. 7. The response of high K(+)-contracted taenia caeci to hypoxia was composed of an initial rapid relaxation followed by a small transient contraction and slow relaxation. The transient contraction was blocked by atropine (1-10 microM), while relaxations were unaffected by atropine and guanethidine (10 microM). 8. The results show that hypoxia reversibly inhibits ICa and secondarily suppresses IK(Ca) due to decreased Ca2+ influx through Ca2+ channels. 9. It is suggested that inhibition of ICa was responsible for the rapid relaxation, whereas transient contraction may have been due to release of acetylcholine from nerve terminals upon hypoxia.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalkjaer C., Lombard J. H. Effect of hypoxia on force, intracellular pH and Ca2+ concentration in rat cerebral and mesenteric small arteries. J Physiol. 1995 Jan 15;482(Pt 2):409–419. doi: 10.1113/jphysiol.1995.sp020528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashoori F., Takai A., Tokuno H., Tomita T. Effects of glucose removal and readmission on potassium contracture in the guinea-pig taenia coli. J Physiol. 1984 Nov;356:33–48. doi: 10.1113/jphysiol.1984.sp015451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Bauer V., Rekalov V., Ito Y. Effects of phenylephrine on membrane currents in single smooth muscle cells of taenia caeci. Methods Find Exp Clin Pharmacol. 1994 Jun;16(5):337–346. [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose D., Bose R. Mechanics of guinea pig taenia coli smooth muscle during anoxia and rigor. Am J Physiol. 1975 Aug;229(2):324–328. doi: 10.1152/ajplegacy.1975.229.2.324. [DOI] [PubMed] [Google Scholar]
- Bridgewater M., Cunnane T. C., Brading A. F. Characteristic features of inhibitory junction potentials evoked by single stimuli in the guinea-pig isolated taenia caeci. J Physiol. 1995 May 15;485(Pt 1):145–155. doi: 10.1113/jphysiol.1995.sp020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerk D. G., Goldstick T. K. Spatial variation of aortic wall oxygen diffusion coefficient from transient polarographic measurements. Ann Biomed Eng. 1992;20(6):629–646. doi: 10.1007/BF02368610. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Poyton R. O. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996 Jul;76(3):839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- Estapé D., Gòdia F., Solà C. Determination of glucose and ethanol effective diffusion coefficients in Ca-alginate gel. Enzyme Microb Technol. 1992 May;14(5):396–401. doi: 10.1016/0141-0229(92)90009-d. [DOI] [PubMed] [Google Scholar]
- Franco-Obregón A., López-Barneo J. Differential oxygen sensitivity of calcium channels in rabbit smooth muscle cells of conduit and resistance pulmonary arteries. J Physiol. 1996 Mar 1;491(Pt 2):511–518. doi: 10.1113/jphysiol.1996.sp021235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Obregón A., Ureña J., López-Barneo J. Oxygen-sensitive calcium channels in vascular smooth muscle and their possible role in hypoxic arterial relaxation. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4715–4719. doi: 10.1073/pnas.92.10.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hu S. L., Yamamoto Y., Kao C. Y. The Ca2+-activated K+ channel and its functional roles in smooth muscle cells of guinea pig taenia coli. J Gen Physiol. 1989 Nov;94(5):833–847. doi: 10.1085/jgp.94.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y., Paul R. J. Effects of hypoxia on high-energy phosphagen content, energy metabolism and isometric force in guinea-pig taenia caeci. J Physiol. 1990 May;424:41–56. doi: 10.1113/jphysiol.1990.sp018054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y., Takagi K., Urakawa N. Tension maintenance, calcium content and energy production of the taenia of the guinea-pig caecum under hypoxia. J Physiol. 1984 Feb;347:149–159. doi: 10.1113/jphysiol.1984.sp015058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaki H., Suzuki T., Urakawa N., Ishida Y., Shibata S. High K+,Na+-deficient solution inhibits tension, O2 consumption, and ATP synthesis in smooth muscle. Jpn J Pharmacol. 1982 Aug;32(4):727–733. doi: 10.1254/jjp.32.727. [DOI] [PubMed] [Google Scholar]
- Knull H. R., Bose D. Reversibility of mechanical and biochemical changes in smooth muscle due to anoxia and substrate depletion. Am J Physiol. 1975 Aug;229(2):329–333. doi: 10.1152/ajplegacy.1975.229.2.329. [DOI] [PubMed] [Google Scholar]
- Krisanda J. M., Paul R. J. Energetics of isometric contraction in porcine carotid artery. Am J Physiol. 1984 May;246(5 Pt 1):C510–C519. doi: 10.1152/ajpcell.1984.246.5.C510. [DOI] [PubMed] [Google Scholar]
- Nasu T., Yui K., Nakagawa H., Ishida Y. Role of glycolysis in the tension development under anoxia in guinea pig taenia coli. Jpn J Pharmacol. 1982 Feb;32(1):65–71. doi: 10.1254/jjp.32.65. [DOI] [PubMed] [Google Scholar]
- Noack T., Deitmer P., Lammel E. Characterization of membrane currents in single smooth muscle cells from the guinea-pig gastric antrum. J Physiol. 1992;451:387–417. doi: 10.1113/jphysiol.1992.sp019170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata R., Inoue Y., Nakano H., Ito Y., Kitamura K. Oestradiol-induced relaxation of rabbit basilar artery by inhibition of voltage-dependent Ca channels through GTP-binding protein. Br J Pharmacol. 1996 Jan;117(2):351–359. doi: 10.1111/j.1476-5381.1996.tb15198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Cellular calcium regulates outward currents in rabbit intestinal smooth muscle cell. Am J Physiol. 1987 Apr;252(4 Pt 1):C401–C410. doi: 10.1152/ajpcell.1987.252.4.C401. [DOI] [PubMed] [Google Scholar]
- Ureña J., Franco-Obregón A., López-Barneo J. Contrasting effects of hypoxia on cytosolic Ca2+ spikes in conduit and resistance myocytes of the rabbit pulmonary artery. J Physiol. 1996 Oct 1;496(Pt 1):103–109. doi: 10.1113/jphysiol.1996.sp021668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth R. M. Vasoconstrictor and vasodilator effects of hypoxia. Trends Pharmacol Sci. 1994 Feb;15(2):47–53. doi: 10.1016/0165-6147(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Hu S. L., Kao C. Y. Inward current in single smooth muscle cells of the guinea pig taenia coli. J Gen Physiol. 1989 Mar;93(3):521–550. doi: 10.1085/jgp.93.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Hu S. L., Kao C. Y. Outward current in single smooth muscle cells of the guinea pig taenia coli. J Gen Physiol. 1989 Mar;93(3):551–564. doi: 10.1085/jgp.93.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino M., Someya T., Nishio A., Yabu H. Whole-cell and unitary Ca channel currents in mammalian intestinal smooth muscle cells: evidence for the existence of two types of Ca channels. Pflugers Arch. 1988 Feb;411(2):229–231. doi: 10.1007/BF00582322. [DOI] [PubMed] [Google Scholar]


