Abstract
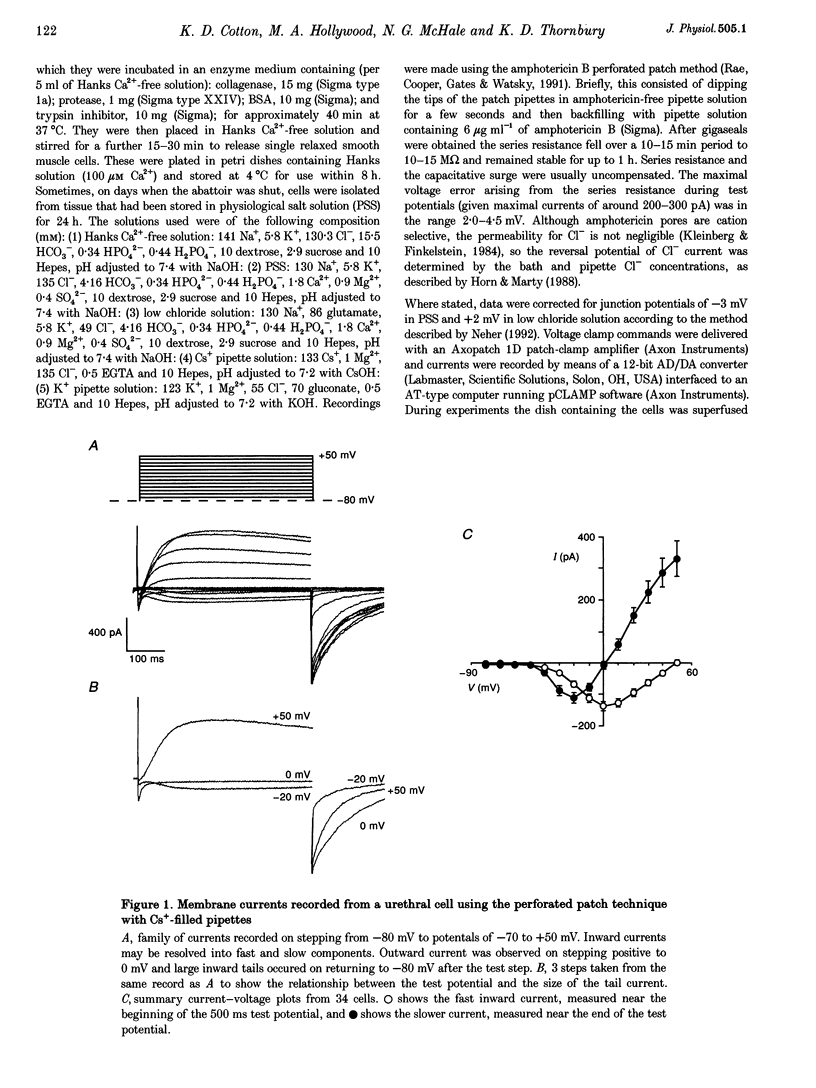
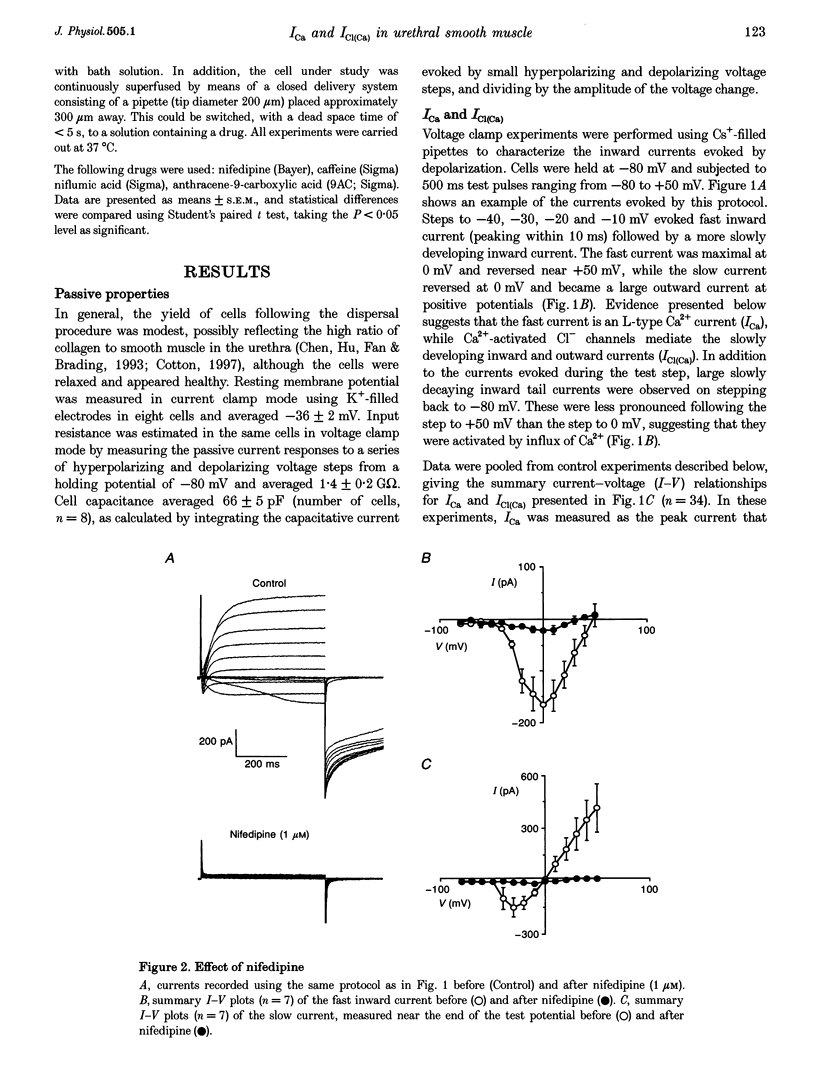
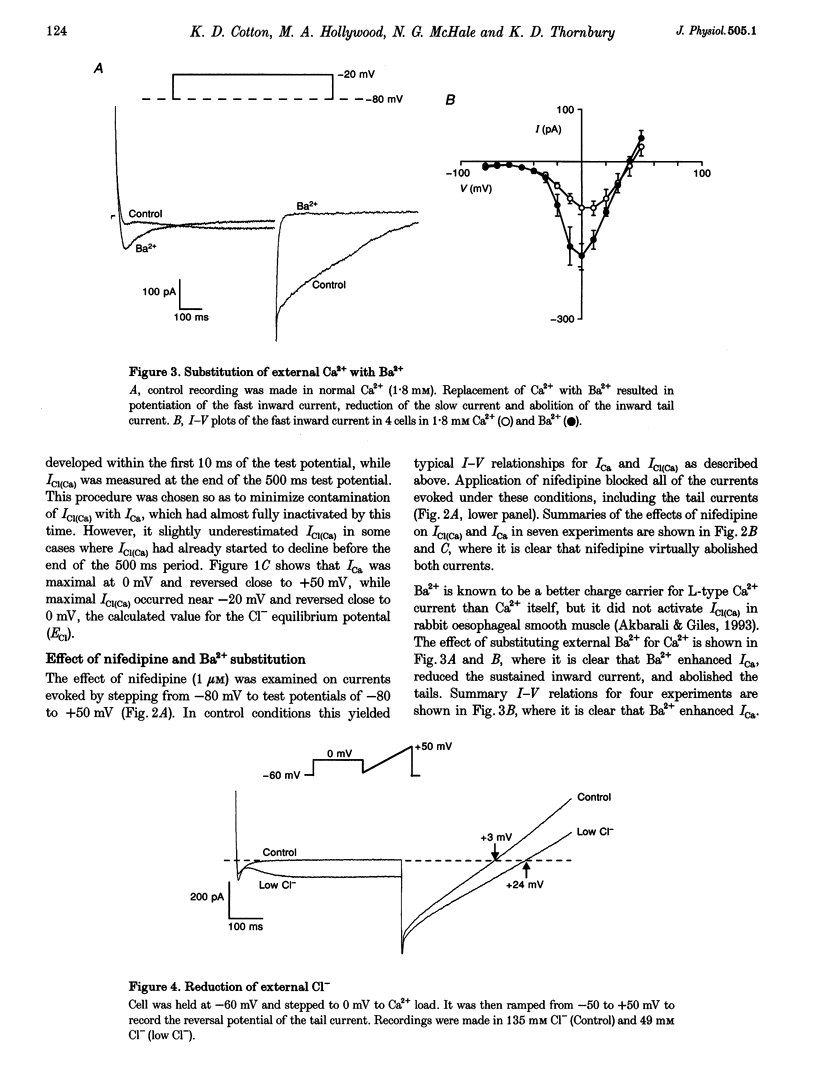
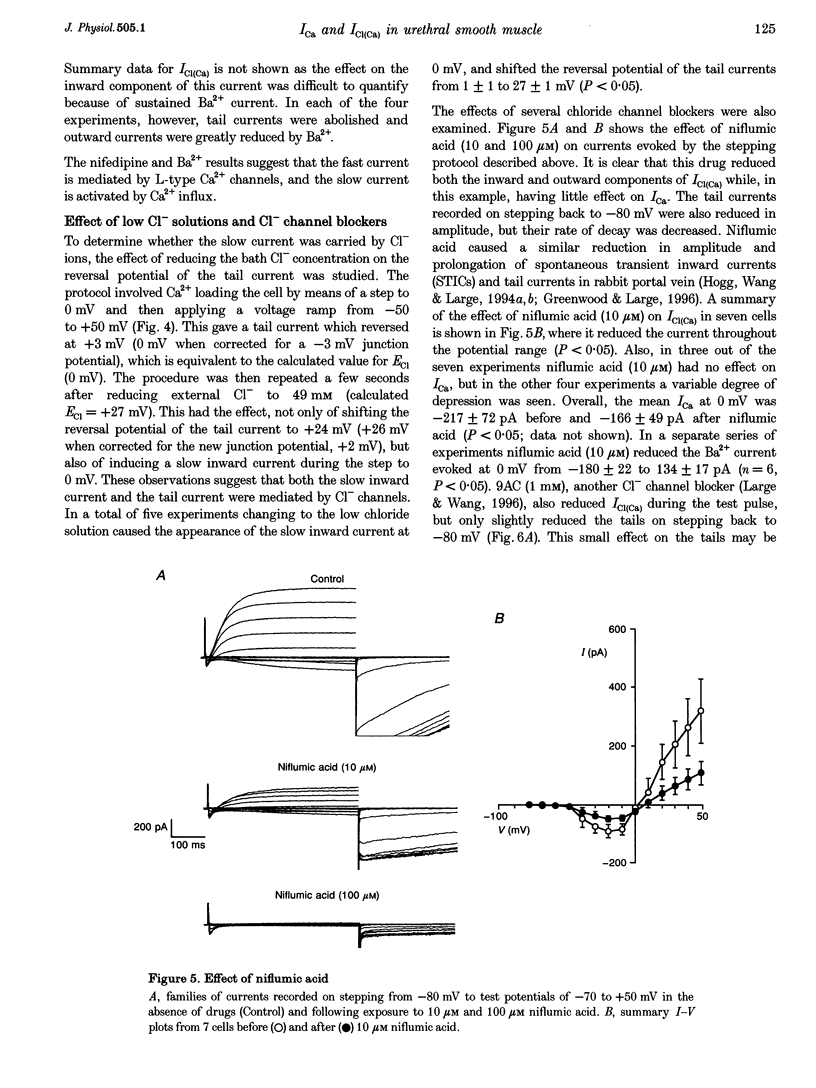
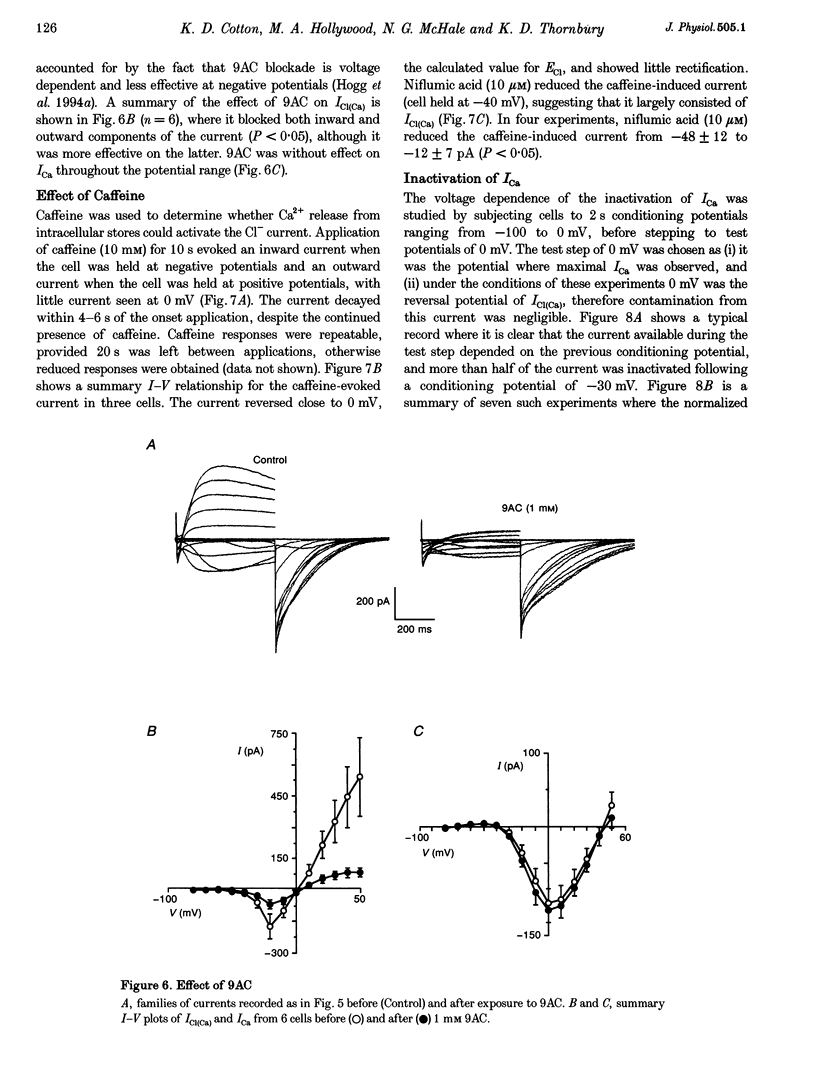
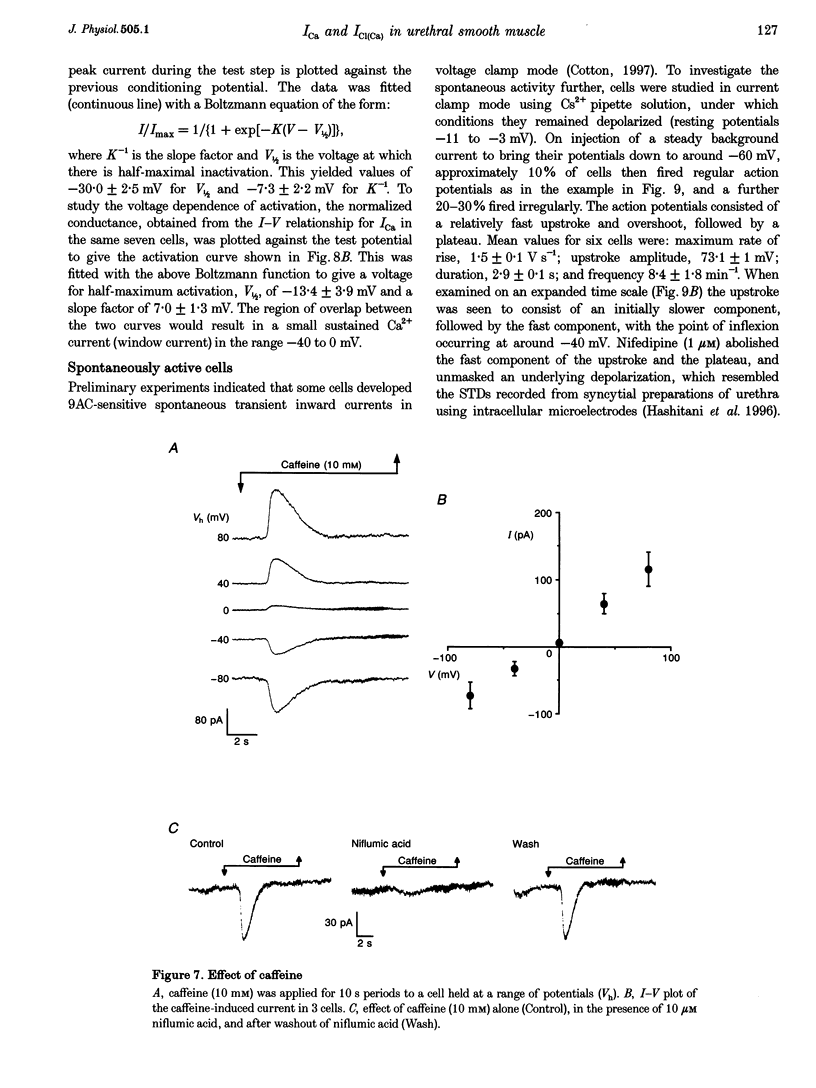
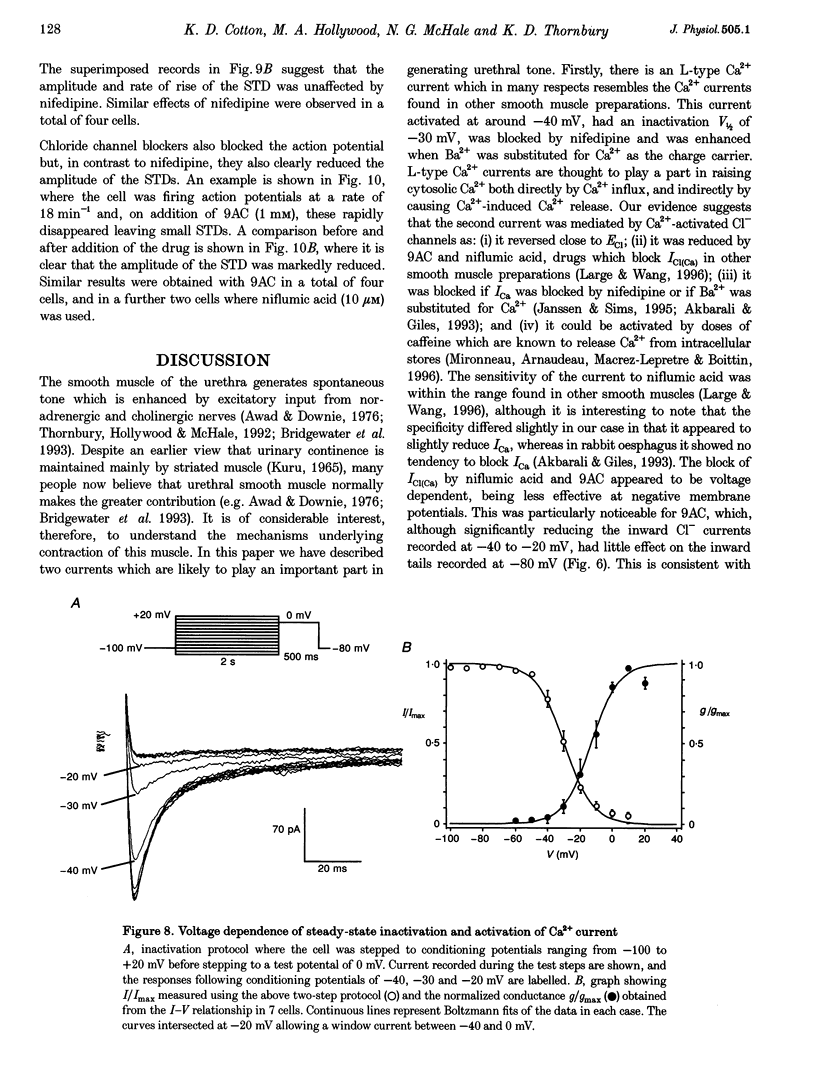
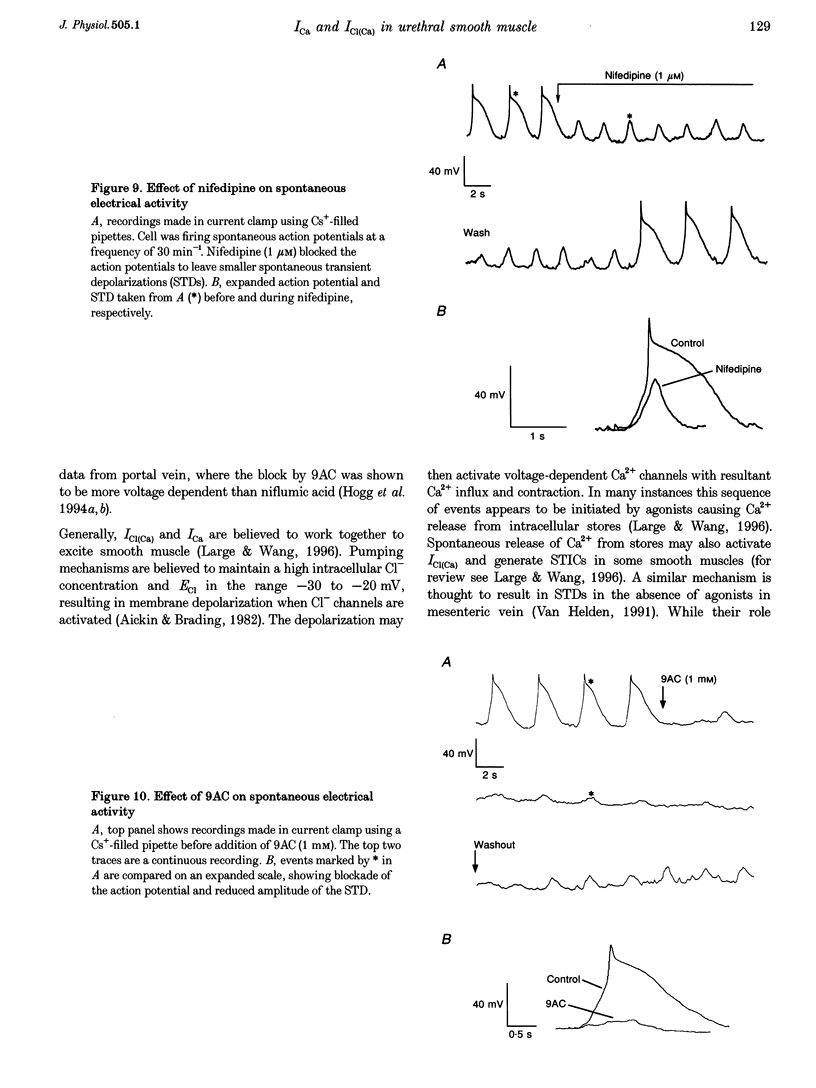
1. Isolated sheep urethral cells were studied using the perforated patch clamp technique (T = 37 degrees C). Depolarizing steps ranging from -40 to -10 mV evoked an inward current that peaked within 10 ms and a slower inward current. Stepping back to the holding potential of -80 mV evoked large inward tail currents. All three currents were abolished by nifedipine (1 microM). Substitution of external Ca2+ with Ba2+ resulted in potentiation of the fast inward current and blockade of the slow current and tails. 2. Changing the chloride equilibrium potential (ECl) from 0 to +27 mV shifted the reversal potential of the tail currents from 1 +/- 1 to 27 +/- 1 mV (number of cells, n = 5). Chloride channel blockers, niflumic acid (10 microM) and anthracene-9-carboxylic acid (9AC, 1 mM), reduced the slow current and tails suggesting that these were Ca(2+)-activated Cl- currents, ICl(Ca). 4. Caffeine (10 mM) induced currents that reversed at ECl and were blocked by niflumic acid (10 microM). 5. In current clamp mode, some cells developed spontaneous transient depolarizations (STDs) and action potentials. Short exposure to nifedipine blocked the action potentials and unmasked STDs. In contrast, 9AC and niflumic acid reduced the amplitude of the STDs and blocked the action potentials. 6. In conclusion, these cells have both L-type ICa and ICl(Ca). The former appears to be responsible for the upstroke of the action potential, while the latter may act as a pacemaker current.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Brading A. F. Measurement of intracellular chloride in guinea-pig vas deferens by ion analysis, 36chloride efflux and micro-electrodes. J Physiol. 1982 May;326:139–154. doi: 10.1113/jphysiol.1982.sp014182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarali H. I., Giles W. R. Ca2+ and Ca(2+)-activated Cl- currents in rabbit oesophageal smooth muscle. J Physiol. 1993 Jan;460:117–133. doi: 10.1113/jphysiol.1993.sp019462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad S. A., Downie J. W. Relative contributions of smooth and striated muscles to the canine urethral pressure profile. Br J Urol. 1976 Oct;48(5):347–354. doi: 10.1111/j.1464-410x.1976.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Bridgewater M., MacNeil H. F., Brading A. F. Regulation of tone in pig urethral smooth muscle. J Urol. 1993 Jul;150(1):223–228. doi: 10.1016/s0022-5347(17)35451-4. [DOI] [PubMed] [Google Scholar]
- Chen H. I., Hu P., Fan P. L., Brading A. F. Pharmacological evaluation of the alpha-adrenoceptors of the rabbit urethra. Eur Urol. 1993;24(1):144–147. doi: 10.1159/000474282. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer M. R., Lederer W. J., Cannell M. B. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol. 1996 Jan;270(1 Pt 1):C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- Cotton K. D., Hollywood M. A., McHale N. G., Thornbury K. D. Outward currents in smooth muscle cells isolated from sheep mesenteric lymphatics. J Physiol. 1997 Aug 15;503(Pt 1):1–11. doi: 10.1111/j.1469-7793.1997.001bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay F. S. Calcium sparks in vascular smooth muscle: relaxation regulators. Science. 1995 Oct 27;270(5236):588–589. doi: 10.1126/science.270.5236.588. [DOI] [PubMed] [Google Scholar]
- Greenwood I. A., Large W. A. Analysis of the time course of calcium-activated chloride "tail" currents in rabbit portal vein smooth muscle cells. Pflugers Arch. 1996 Oct;432(6):970–979. doi: 10.1007/s004240050224. [DOI] [PubMed] [Google Scholar]
- Hashitani H., Van Helden D. F., Suzuki H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br J Pharmacol. 1996 Aug;118(7):1627–1632. doi: 10.1111/j.1476-5381.1996.tb15584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg R. C., Wang Q., Large W. A. Effects of Cl channel blockers on Ca-activated chloride and potassium currents in smooth muscle cells from rabbit portal vein. Br J Pharmacol. 1994 Apr;111(4):1333–1341. doi: 10.1111/j.1476-5381.1994.tb14891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen L. J., Sims S. M. Ca(2+)-dependent Cl- current in canine tracheal smooth muscle cells. Am J Physiol. 1995 Jul;269(1 Pt 1):C163–C169. doi: 10.1152/ajpcell.1995.269.1.C163. [DOI] [PubMed] [Google Scholar]
- KURU M. NERVOUS CONTROL OF MICTURITION. Physiol Rev. 1965 Jul;45:425–494. doi: 10.1152/physrev.1965.45.3.425. [DOI] [PubMed] [Google Scholar]
- Kleinberg M. E., Finkelstein A. Single-length and double-length channels formed by nystatin in lipid bilayer membranes. J Membr Biol. 1984;80(3):257–269. doi: 10.1007/BF01868444. [DOI] [PubMed] [Google Scholar]
- Large W. A., Wang Q. Characteristics and physiological role of the Ca(2+)-activated Cl- conductance in smooth muscle. Am J Physiol. 1996 Aug;271(2 Pt 1):C435–C454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Cheng H., Rubart M., Santana L. F., Bonev A. D., Knot H. J., Lederer W. J. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995 Oct 27;270(5236):633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Teramoto N., Brading A. F. Activation by levcromakalim and metabolic inhibition of glibenclamide-sensitive K channels in smooth muscle cells of pig proximal urethra. Br J Pharmacol. 1996 Jun;118(3):635–642. doi: 10.1111/j.1476-5381.1996.tb15448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto N., Brading A. F. Nicorandil activates glibenclamide-sensitive K+ channels in smooth muscle cells of pig proximal urethra. J Pharmacol Exp Ther. 1997 Jan;280(1):483–491. [PubMed] [Google Scholar]
- Thornbury K. D., Hollywood M. A., McHale N. G. Mediation by nitric oxide of neurogenic relaxation of the urinary bladder neck muscle in sheep. J Physiol. 1992;451:133–144. doi: 10.1113/jphysiol.1992.sp019157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Helden D. F. Spontaneous and noradrenaline-induced transient depolarizations in the smooth muscle of guinea-pig mesenteric vein. J Physiol. 1991 Jun;437:511–541. doi: 10.1113/jphysiol.1991.sp018609. [DOI] [PMC free article] [PubMed] [Google Scholar]


