Abstract
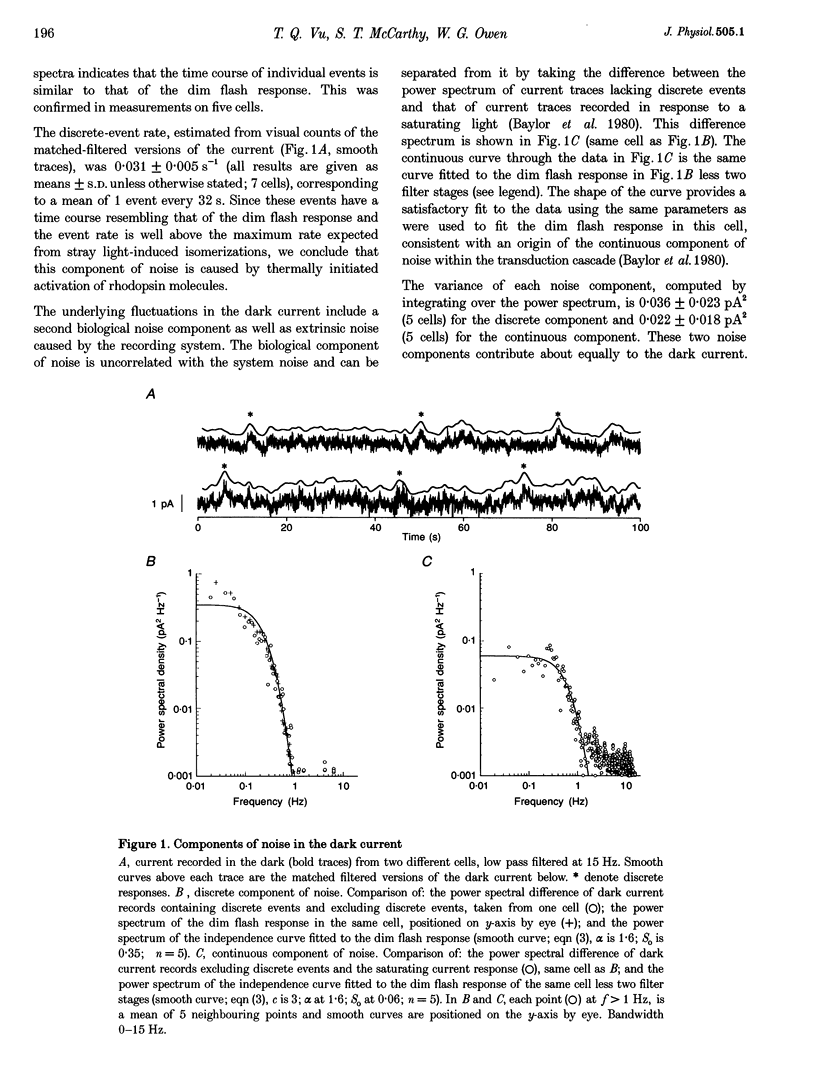
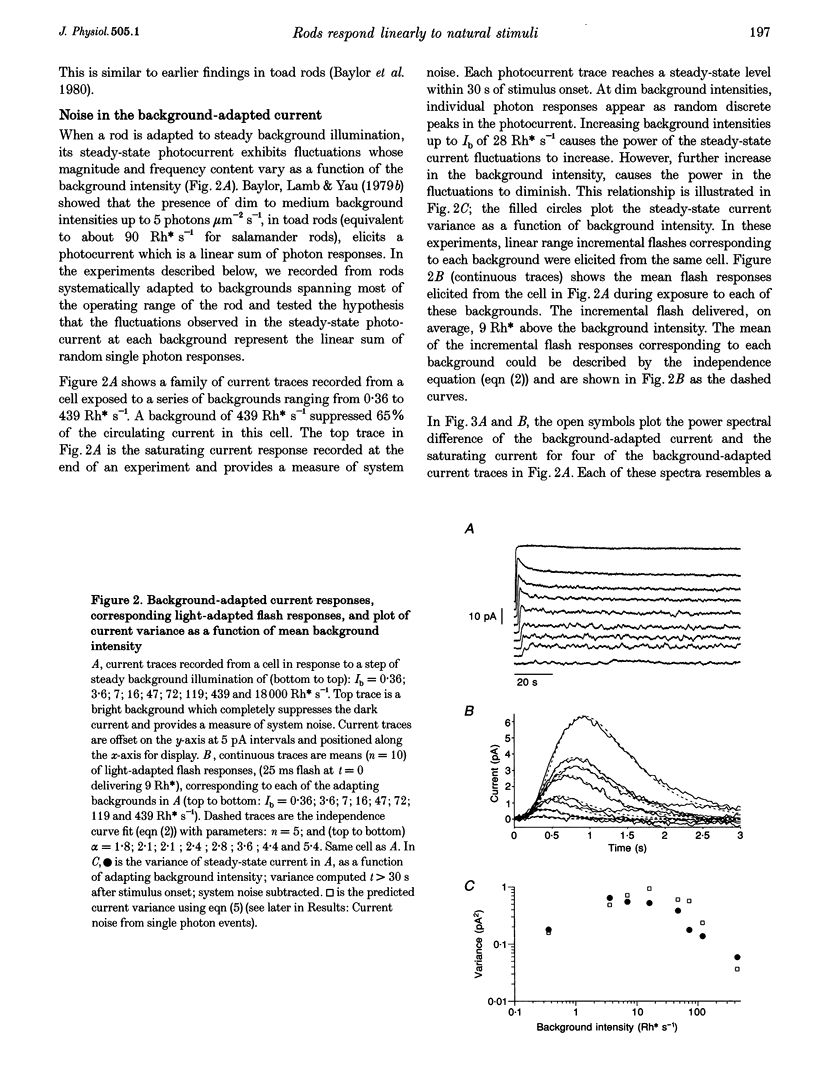
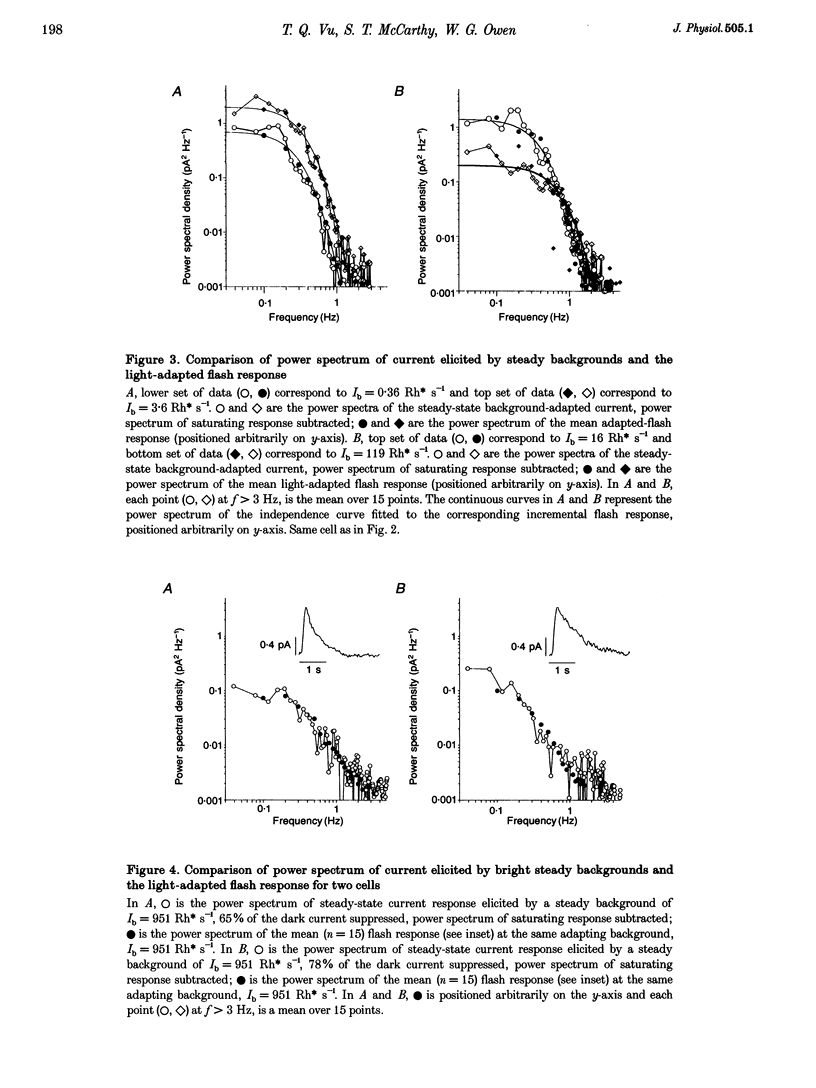
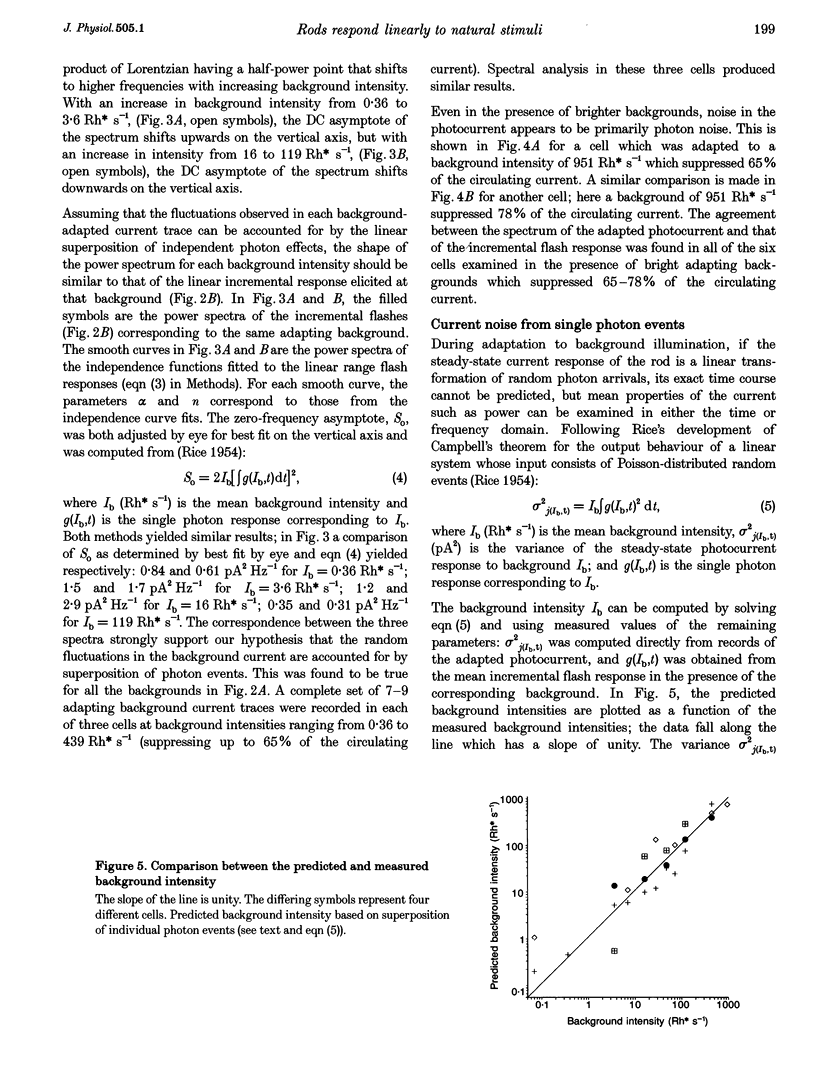
1. We examined signal, noise and response properties of salamander rod photoreceptors by measuring: (a) the circulating current of rods which were adapted to darkness and to a wide range of backgrounds; (b) contrasts of natural environments; (c) the effect of adaptation on the linear response range of rods; and (d) the behaviour of rods responding to dynamically modulated stimuli having a range of contrasts found in nature. 2. In the dark, the circulating current contained two noise components analogous to those described in toad. A discrete noise component consisted of events occurring at a rate of 1 event per 32 s (21 degrees C) and had a variance of 0.036 pA2. A continuous noise component contributed 0.022 pA2 to the dark current, roughly equal to the discrete noise variance. 3. Exposure to a wide range of steady backgrounds (suppressing up to 80% of the circulating current), elicited a sustained fluctuating photocurrent having a power spectrum which resembled those of single photon responses and was consistent with the linear summation of single photon events; this indicates that the primary source of noise in the current is caused by the light. 4. Eighty-nine per cent of the contrasts (C) measured in natural environments had magnitude of C < 50%, where C = magnitude of I - Imean/magnitude of Imean. The linear response range elicited by brief flashes expanded with brighter backgrounds, well-encompassing flash contrasts of 100%. 5. Dynamically modulated stimuli and incremental flashes having contrasts similar to those in natural scenes elicited small currents which deviated by a few picoamps about the mean and the transfer functions computed from each type of stimulus-response pair closely corresponded to one another. These results indicate that in natural environments, rods behave as linear small-signal transducers of light.
Full text
PDF


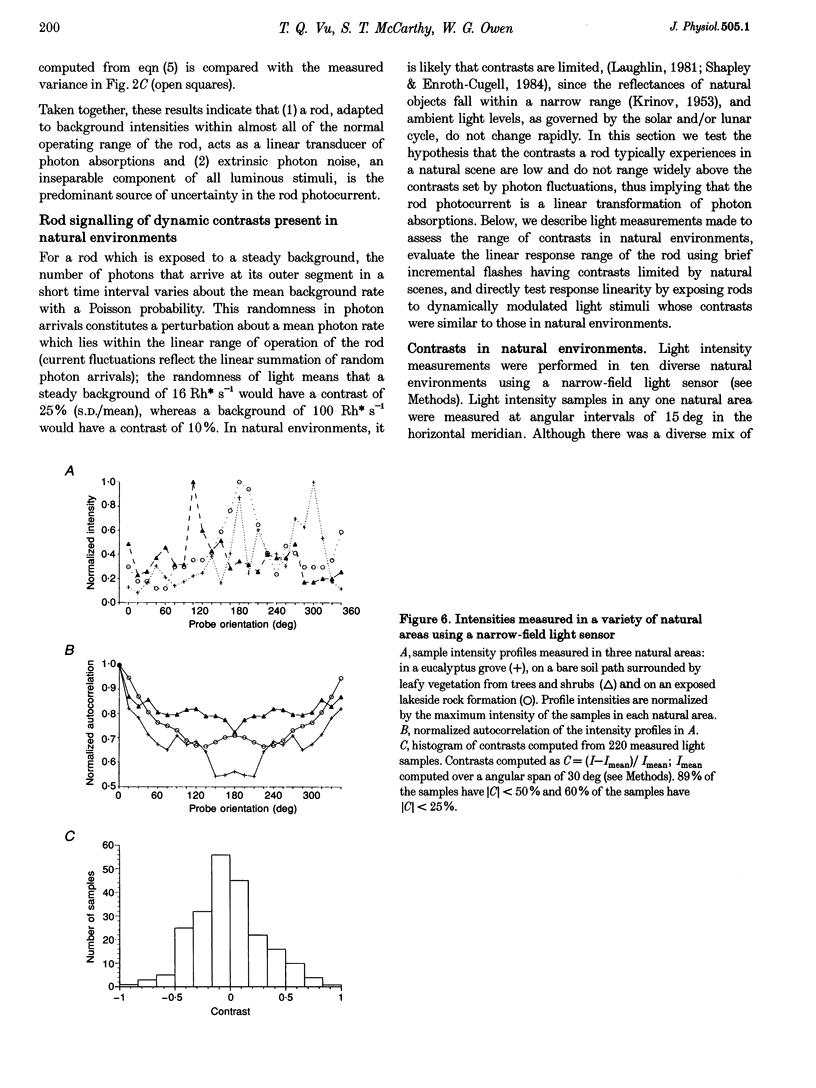
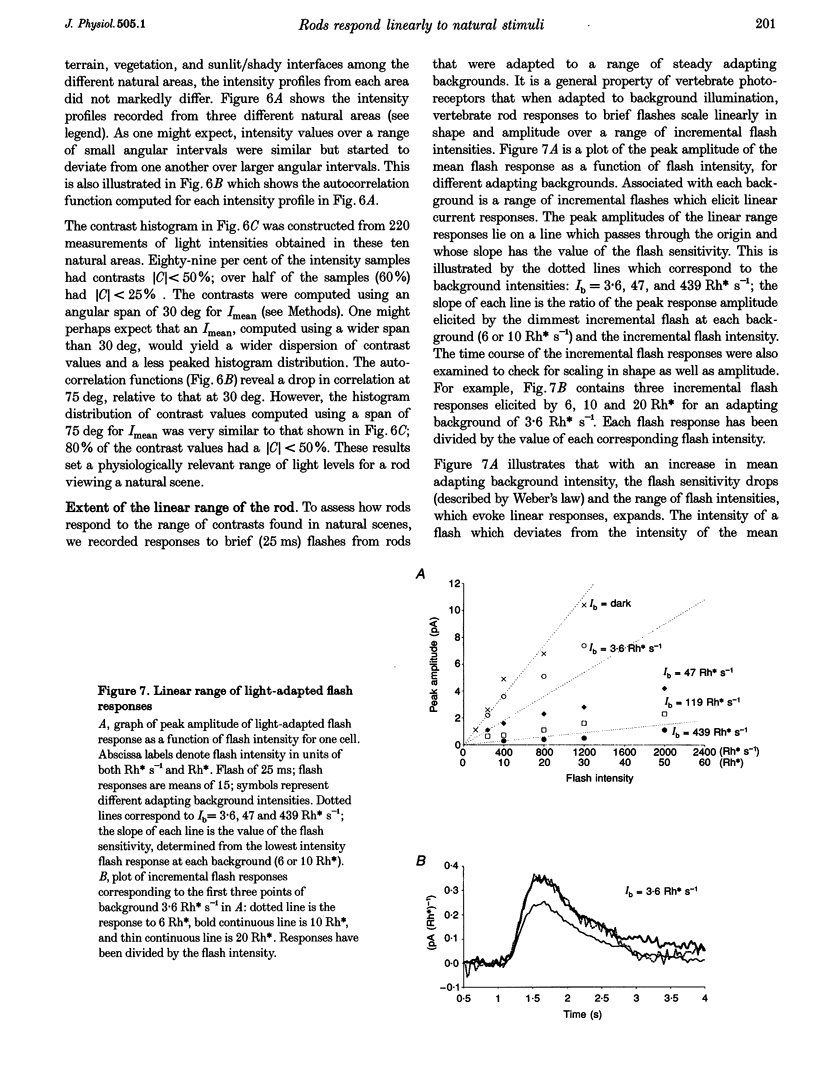
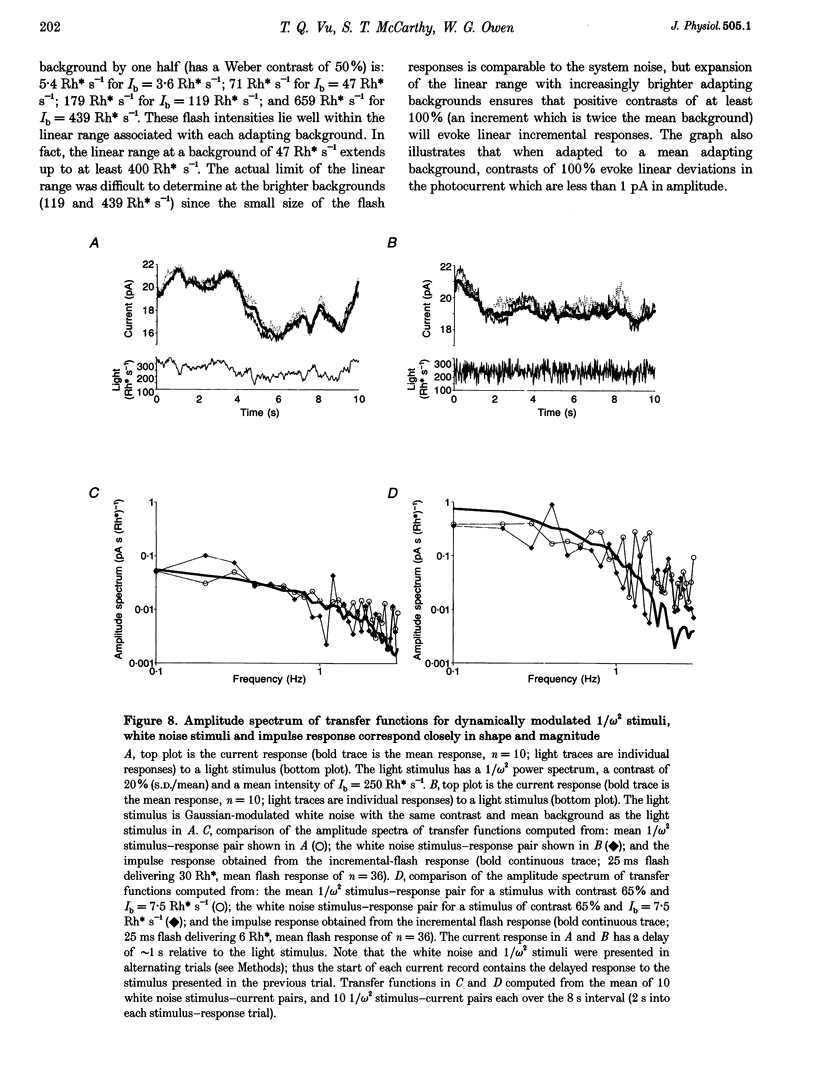


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodoia R. D., Detwiler P. B. Patch-clamp recordings of the light-sensitive dark noise in retinal rods from the lizard and frog. J Physiol. 1985 Oct;367:183–216. doi: 10.1113/jphysiol.1985.sp015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y., Atick J. J., Reid R. C. Efficient coding of natural scenes in the lateral geniculate nucleus: experimental test of a computational theory. J Neurosci. 1996 May 15;16(10):3351–3362. doi: 10.1523/JNEUROSCI.16-10-03351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L. Sensitivity of toad rods: Dependence on wave-length and background illumination. J Physiol. 1976 Sep;261(1):71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P., Attwell D. Kinetics of light-sensitive channels in vertebrate photoreceptors. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):379–388. doi: 10.1098/rspb.1985.0007. [DOI] [PubMed] [Google Scholar]
- Hárosi F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975 Sep;66(3):357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juusola M., Kouvalainen E., Järvilehto M., Weckström M. Contrast gain, signal-to-noise ratio, and linearity in light-adapted blowfly photoreceptors. J Gen Physiol. 1994 Sep;104(3):593–621. doi: 10.1085/jgp.104.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G. Comparison of the light-sensitive and cyclic GMP-sensitive conductances of the rod photoreceptor: noise characteristics. J Neurosci. 1986 Sep;6(9):2521–2526. doi: 10.1523/JNEUROSCI.06-09-02521.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter R. B. Sinusoidal and delta function responses of visual cells of the Limulus eye. J Gen Physiol. 1966 Jan;49(3):565–593. doi: 10.1085/jgp.49.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F., Baylor D. A. Molecular origin of continuous dark noise in rod photoreceptors. Biophys J. 1996 Nov;71(5):2553–2572. doi: 10.1016/S0006-3495(96)79448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruyter van Steveninck R. R., Lewen G. D., Strong S. P., Koberle R., Bialek W. Reproducibility and variability in neural spike trains. Science. 1997 Mar 21;275(5307):1805–1808. doi: 10.1126/science.275.5307.1805. [DOI] [PubMed] [Google Scholar]


