Abstract
We report the first identifications of species and element used to produce Paleolithic bone needles. Archaeologists have used the tailored, fur-fringed garments of high latitude foragers as modern analogs for the clothes of Paleolithic foragers, arguing that the appearance of bone needles and fur bearer remains in archaeological sites c. 40,000 BP is indirect evidence for the advent of tailored garments at this time. These garments partially enabled modern human dispersal to northern latitudes and eventually enabled colonization of the Americas ca. 14,500 BP. Despite the importance of bone needles to explaining global modern human dispersal, archaeologists have never identified the materials used to produce them, thus limiting understanding of this important cultural innovation. We use Zooarchaeology by Mass Spectrometry (ZooMS) and Micro-CT scanning to establish that bone needles at the ca. 12,900 BP La Prele site (Wyoming, USA) were produced from the bones of canids, felids, and hares. We propose that these bones were used by the Early Paleoindian foragers at La Prele because they were scaled correctly for bone needle production and readily available within the campsite, having remained affixed to pelts sewn into complex garments. Combined with a review of comparable evidence from other North American Paleoindian sites, our results suggest that North American Early Paleoindians had direct access to fur-bearing predators, likely from trapping, and represent some of the most detailed evidence yet discovered for Paleoindian garments.
Introduction
Thermoregulation was a fundamental constraint on the pacing and character of global modern human dispersal. Modern humans are primarily physiologically adapted to the climates of sub-Saharan Africa where much of their evolutionary lineage exists [1–4], so expansion into northern latitudes required the adoption of technologies that buffered early modern humans against increasingly severe cold conditions [5–9]. Of the various technologies and behaviors enacted to cope with cold temperatures, complex, tailored garments are among the most important.
Tailored garments are an improvement over draped garments because they adhere closely to the skin [10] and closely stitched seams provide a water and windtight barrier against the elements [11]. Once equipped with such garments, modern humans had the capacity to expand their range to places from which they were previously excluded due to the threat of hypothermia or death from exposure.
Little direct evidence exists for these sorts of garments (but see [12]), but indirect evidence for them includes bone needles and the bones of fur bearers. The rationale for assuming bone needles are an archaeological proxy for tailored garments is simple. Sewers produce tailored garments with intricate, tightly stitched seams, which require needles to assemble rather than awls, which create more widely-spaced and coarsely perforated seams [13]. Bone needles emerge in Eurasia beginning ca. 40,000 BP [8, 14–17] and in North American Paleoindian sites between ca. 12,000 and 13,000 BP [18, 19].
The bones of fur bearers have received less attention as an indirect proxy for tailored garment production, and the link between the phenomena is more complicated, having its basis in foraging theory. Fur bearers such as canids, felids, leporids, and mustelids possess pelts with soft, tightly spaced hairs that trap a layer of stationary air near the skin’s surface [20]. These animals are typically ranked low in a diet breadth framework [21, 22] because they are small, difficult to hunt, and in the case of carnivores sparse on the landscape. Thus, their presence in archaeological assemblages is hard to explain from a purely dietary framework. In ethnographic settings, foragers often cull furbearers only for their pelts and only with the use of traps to limit search time [23, 24]. If their primary product is fur rather than meat, then furbearers might confound optimal foraging models as traditionally conceived based on kilocalorie value. Thus, archaeologists have explained their presence in Paleolithic contexts otherwise dominated by ungulate remains as a result of pelt acquisition [25–27].
Here, we present evidence for tailored garment production using fur bearers from the La Prele site (Fig 1). La Prele is an Early Paleoindian (ca. 12,900 cal BP) mammoth kill and campsite buried over 3 m deep on a tributary on the North Platte River near Douglas, WY associated with diagnostic artifacts of the Clovis and Folsom cultural complexes [28]. Ten seasons of excavations in four major blocks (A through D) have yielded tens of thousands of artifacts associated with a single occupation [29]. Among the wide diversity of artifacts recovered from the site thus far are 32 bone needle fragments. We use Zooarchaeology by Mass Spectrometry (ZooMS) and Micro-CT to argue that the Early Paleoindian foragers at La Prele had primary access to fur bearers, the bones from which they produced bone needles and the furs from which they incorporated into tailored garments.
Fig 1. Archaeological plan map of La Prele Blocks B and D showing the locations of recovered bone needle fragments.
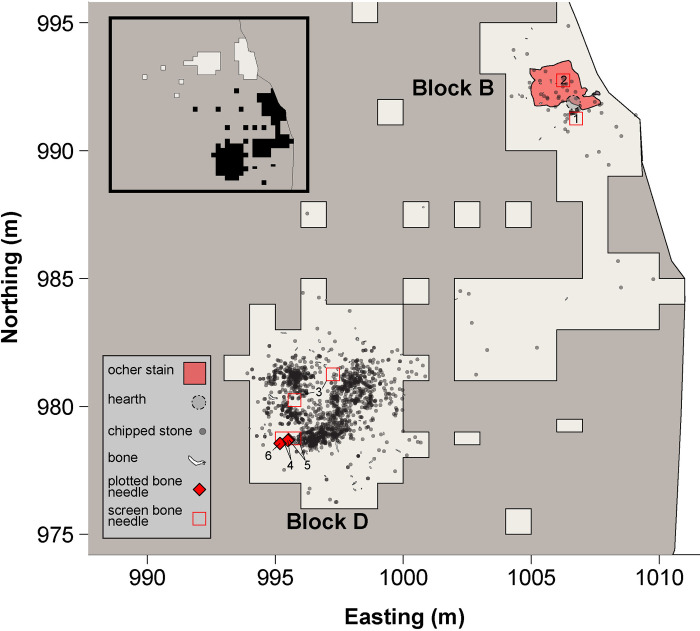
Inset map shows entire site; black shaded polygons depict area of interest. Numbered bone needle provenience locations refer to bone needle ID’s of Table 1.
Materials and methods
The artifacts used in this study were recovered from archaeological excavations by the University of Wyoming Department of Anthropology between 2015 and 2022 from the La Prele site, located on private land in Converse County, Wyoming. The artifacts are housed at the University of Wyoming in the lab of Todd Surovell. No permits were required for the described study, which complied with all relevant regulations.
We obtained high resolution images and metric data for bone needles and comparative specimens using a ZEISS Xradia 610 Versa using the following X-ray tube parameters: 60 kV, 108 μA and 6.5 W of power. Images were collected using either the 4x objective (bone needle fragments) or the 0.4 × objective (animal bones), and a final pixel size of 4–10 μm. A total of 4501 projection images were acquired using 360 degrees of sample rotation. Scout and Scan™ Reconstructor (v16.1) was used to generate the final tomograms. We obtained metric attributes using Dragonfly software (Comet Technologies, v2022.2). For each comparative specimen or bone needle fragment, we obtained two measurements (thickness and width) at five locations evenly spaced down the long axis of each specimen. We measured each specimen in five locations to characterize the range of variation present within each specimen, regardless of the specimen’s total size.
For three archaeological samples (FS 2222, 4678, and 14770), we used minimally destructive methods for the extraction of soluble collagen [30, 31] at the University of Manchester’s Ancient Biomolecules Laboratory. Collagen was solubilized in 0.3 M HCl and exchanged into 50 mM ammonium bicarbonate using a 10 kDa ultrafilter. After trypsin digestion, samples were analyzed with a Bruker Rapiflex MALDI TOF/TOF mass spectrometer. The remaining archaeological samples were processed using destructive methods [30, 32–34]. Small needle fragments (1 to 20 mg) were ground in a clean agate mortar and pestle. The bone powder was demineralized with 0.6 M HCl, and humic acids were extracted with 0.1 M NaOH. After gelatinization for 1 hour at 65°C in ammonium bicarbonate, samples were digested with porcine trypsin. Spectra were generated using a Sciex 5800 MALDI-TOF mass spectrometer in the Basile Lab at the University of Wyoming.
To identify taxa, we mostly relied on published marker peptides [32, 35, 36] in combination with MALDI-TOF spectra of tryptic digests run on species specifically included for this study. For the latter taxa, we recorded the presence or absence of known marker peptides in mass spectra (S1 File). Additional species analyzed for this study are Miracinonyx trumani (American cheetah-like cat), Puma concolor (mountain lion), Smilodon fatalis (saber-toothed cat), Vulpes velox (swift fox), Uroycyon cinereoargenteus (gray fox), Aeonocyon dirus (dire wolf), Lepus californicus (black-tailed jackrabbit), Lepus americanus (snowshoe hare), Lepus townsendii (white-tailed jackrabbit), Sylvilagus audubonii (desert cottontail), Brachylagus idahoensis (pygmy rabbit), and Ochotona princeps (American pika). Specimens of extant species were sampled from the modern zooarchaeology comparative collection at the University of Wyoming. We sampled a Miracinonyx trumani innominate (Cat no. 1750A) from Little Canyon Creek Cave (48WA323), Wyoming curated at the University of Wyoming Archaeological Repository. We sampled a Smilodon fatalis cranium (SUI 15–548) from Rancho La Brea and an Aeonocyon dirus radius (SUI-149737) from the West Noadaway River, Mills County Iowa. Both specimens are curated at the Paleontological Repository, University of Iowa, Iowa City. The A. dirus radius was collected by Jim McClarnon in May, 2019 in shallow water resting on the bed of the West Nodaway River, about 6 km north of the small town of Villisca, Montgomery County, Iowa, in the northeast quarter of Section 4, T71N-R36W, or 0.5 km north of the location where U.S. Highway 34 crosses the river. It was donated to the Paleontological Repository, Department of Earth and Environmental Sciences, University of Iowa (SUI), Iowa City, Iowa, on March 6, 2024 for permanent curation.
Using the nomenclature of Brown [37], the α2 793 marker peptide (D from Buckley et al. 2009) [32] was most reliable for identifying the family of origin of archaeological samples as shown in S1 File; peaks at 2129.1 m/z, 2131.1 m/z, and 2163.1 m/z were identified as Leporidae, Canidae, and Felidae, respectively. Additional identification details are provided below.
The presence of α2 978 (“peptide A”) peaks at 1221.6/1235.6 m/z, a α2 793 peak at 2129.1 m/z (D), and a α1 586 peaks at 2883.4/2899.4 m/z (F) positively identified a sample as a lagomorph. The additional presence of an α2 454 peak at 2808.3 m/z (E) distinguishes hares from rabbits and pika, and that peptide occurs in all three archaeological specimens identified as derived from lagomorph bone. Therefore, the needle and preform fragments manufactured from lagomorph bone were derived from a jackrabbit or hare.
Carnivores were identified based on the presence of the α1 586 peptide pair at 2853.4/2869.4 m/z in combination with the α2 757 peaks at 2983.5/2999.5 m/z. Canids could generally be identified by the presence of the α2 978 peaks at 1210.7/1226.7 m/z, although those peptides were not observed in gray fox (Urocyon cinereoargenteus) or dire wolf (Aenocyon dirus). Red fox (Vulpes vulpes) was identified by the presence of the α2 484 peptide at 1437.7 m/z. All needle specimens identified as having been manufactured from canid bone exhibit this peak and thus were positively identified as red fox.
For one sample (FS 4678), a felid identification could be made by presence of the α2 978 peptide at 1207.6/1223.6 m/z and the absence of the α2 793 peptide at 2131.1 m/z. Two additional felid identifications were made using the presence of the α2 793 marker. That peptide allowed discrimination between subfamily Felinae and subfamilies Pantherinae and Machairodontinae. The former show a peak at 2163.1 m/z and the latter at 2147.1 m/z. All feline needle specimens show the 2163.1 m/z marker peptide, and thus can be identified as having been derived from one of four possible taxa: bobcat (Lynx rufus), lynx (Lynx canadensis), mountain lion (Puma concolor), or American cheetah (Miracinonyx trumani). Mustelidae and Procyonidae can be eliminated as candidate taxa based on the absence of the 1219.6/1235.6 α2 978 peptide pair in all needle spectra.
Results
We recovered 32 bone needle fragments from La Prele from 11 field specimen (FS) numbers representing 10 distinct provenience locations, including 17 from Block B and 15 from Block D (Fig 1; Table 1). Ten fragments from Block D are what we interpret as two bone needle preform fragments. The preform fragments are relatively wide (mean = 1.96 mm, SD = 0.48 mm, range = 1.06–2.91 mm) and square compared to finished bone needles but are visibly worked. Consistent with other Paleoindian bone needles [18], needle fragments are an average of 1.5 mm wide (mean = 1.45 mm; SD = .33 mm; range = 0.54–2.13 mm). All bone needle fragments are associated with dense accumulations of artifacts, likely the interiors of houses [38], and are typically discarded near concentrations of burned artifacts indicative of hearth-centered activity areas.
Table 1. Summary of bone needle artifacts from La Prele.
| FS | ID | N | E | Z | block | type | width mean, SD, range (μ) | thickness mean, SD, range (μ) | count | ZooMS ID (genus) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2222* | 1 | 991.250 | 1006.750 | 97.300 | B | N | 1560 +/- 43; 1502–1627 | 1347 +/- 78; 1216–1459 | 3 | Vulpes |
| 4678* | 2 | 992.750 | 1006.250 | 97.350 | B | N | 1980 +/- 54; 1911–2057 | 1399 +/- 271; 872–1684 | 3 | Lynx, Puma, or Miracinonyx |
| 4957* | 2 | 992.750 | 1006.250 | 97.300 | B | N | 1751 +/- 103; 1563–1888 | 1666 +/- 117; 1468–1833 | 5 | Lynx, Puma, or Miracinonyx |
| 5854* | 2 | 992.750 | 1006.250 | 97.300 | B | N | 1222 +/-190; 876–1458 | 1051 +/-225; 539–1397 | 6 | Lynx, Puma, or Miracinonyx |
| 12378* | 3 | 980.250 | 995.750 | 97.400 | D | N | 2036 +/- 79; 1929–2129 | 1226 +/- 35; 1172–1265 | 1 | Lepus |
| 11543* | 3 | 981.250 | 997.250 | 97.500 | D | N | 1601 +/-41; 1538–1584 | 1312 +/- 31; 1283–1352 | 2 | no result |
| 14617 | 4 | 978.699 | 995.529 | 97.442 | D | P | 1632 +/-240; 1099–2164 | 882 +/- 309; 188–1394 | 6 | Lepus |
| 14770 | 5 | 978.673 | 995.489 | 97.431 | D | P | 1971 +/- 174; 1678–2157 | 1022 +/- 82; 819–1106 | 1 | Vulpes |
| 14726* | 5 | 978.750 | 995.750 | 97.400 | D | N | Small tip, not scanned | 1 | Vulpes | |
| 14477 | 6 | 978.554 | 995.182 | 97.451 | D | P | 2408 +/- 230; 2042–2911 | 1650 +/- 320; 1315–2407 | 1 | Lepus |
| 14511* | 4 | 978.750 | 995.250 | 97.450 | D | P | 2075 +/- 535; 1056–2701 | 1147 +/- 271; 592–1421 | 3 | Lepus |
*screen artifacts.
We attempted ZooMS on 11 bone needle fragments, 1 from each of the 11 bone needle FS numbers. We successfully obtained spectra from 10 specimens, including 4 from Block B and 6 from Block D. From these, we identified three families of mammalian fauna, including Canidae, Felidae, and Leporidae (Fig 2; S1 File). Block B contains needle fragments produced from canid and felid bone and Block D contains needle and preform fragments produced from canid and leporid bone.
Fig 2. MALDI-TOF spectra for three needle fragments in comparison to reference spectra for closely related taxa (red fox, bobcat, and black-tailed jackrabbit).
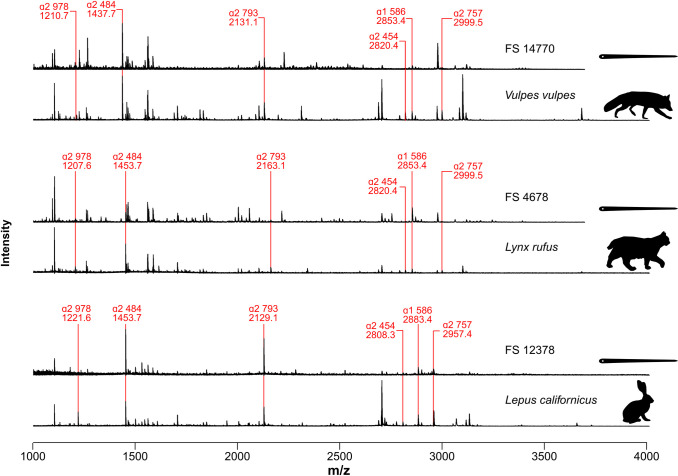
Needle FS 14770 (upper) is identified as Vulpes vulpes. Needle FS 4678 (middle) is identified as subfamily Felinae. Needle FS 12378 (lower) is identified as genus Lepus.
Possible canid species in Pleistocene Wyoming include the coyote (Canis latrans), gray wolf (Canis lupus), gray fox (Urocyon cinereoargenteus), red fox (Vulpes vulpes), swift fox (Vulpes velox), the extinct Pleistocene Dire wolf (Aenocyon dirus), and possibly domesticated dogs (Canis familiaris) maintained by the La Prele site’s inhabitants [39]. All canid bone needle fragments are positively identified as red fox (Vulpes vulpes) based on the presence of the α2 484 marker peptide peak at 1437.7 m/z [32].
Possible felid species in Pleistocene Wyoming include the Canada lynx (Lynx canadensis), bobcat (Lynx rufus), mountain lion (Puma concolor), saber-toothed cat (Smilodon fatalis), American cheetah-like cat (Miracinonyx trumani), Scimitar cat (Homotherium sp.), American lion (Panthera atrox), and Pleistocene North American jaguar (Panthera onca augusta). We have not compiled reference spectra for the Scimitar cat (Homotherium). Block B needle fragments are derived from the subfamily Felinae, meaning Lynx sp., Puma concolor, or Miracinonyx trumani.
Leporids in Wyoming include species of both hares (Lepus sp.) and rabbits (Sylvilagus sp.). Extant Wyoming hares are likely the same as those present during the Pleistocene and include the white-tailed jackrabbit (Lepus townsendii), the black-tailed jackrabbit (Lepus californicus), and the snowshoe hare (Lepus americanus). Rabbits include the desert cottontail (Sylvilagus audobonii), mountain cottontail (Sylvilagus nuttallii), and likely the pygmy rabbit (Brachylagus idahoensis), though the latter’s Pleistocene distribution in Wyoming is poorly understood. All four Block D leporid bone needles are hare (Lepus sp.), two of which we identified as bone needle preforms. All preform fragments are hare, suggesting that the La Prele site inhabitants of Block D were processing hare bones during needle production when the site was abandoned.
We estimate that La Prele has yielded a minimum number of four bone needles and two bone needle preforms based on a combination of their morphological characteristics, spatial distributions, and ZooMS results (Fig 3). We think Block B contains at least two bone needles. One Block B bone needle (ID-1) produced from Vulpes bone is represented by three fragments from a single 50 cm x 50 cm quadrant (quad) and 5 cm level. The other Block B bone needle (ID-2) produced from Lynx/Puma/Miracinonyx bone is represented by 14 fragments from a single quad dispersed across two levels.
Fig 3. Bone needle and needle preform reconstructions and Micro-CT scans of comparative faunal specimens.
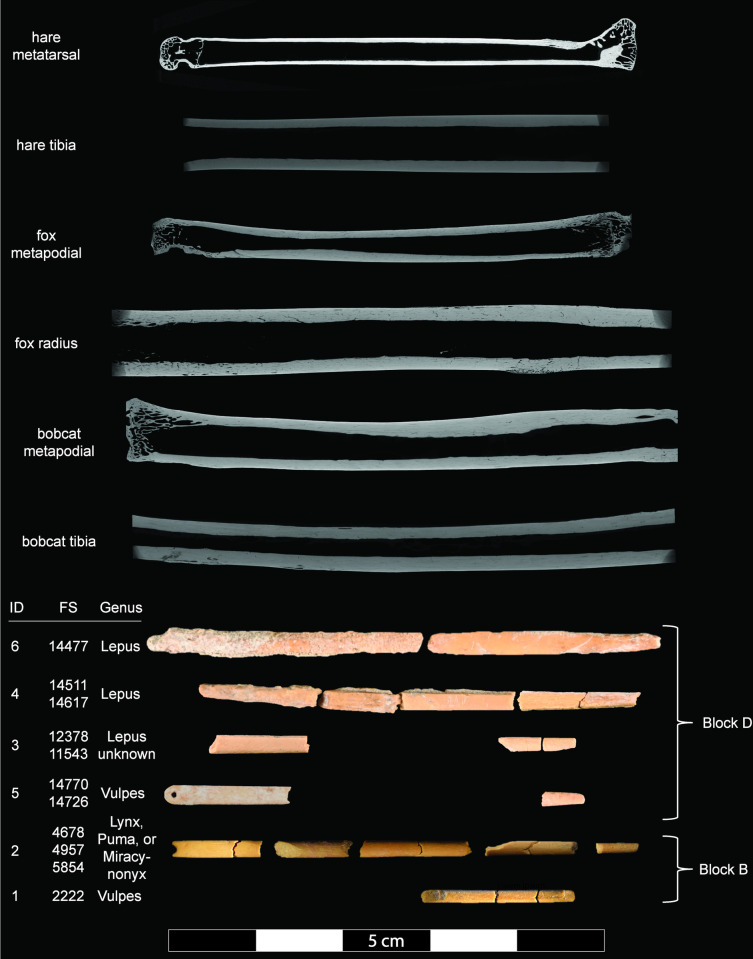
We estimate that Block D contains at least two bone needle preforms and two finished bone needles. We discovered fragments of two preforms made of Lepus sp. bone (IDs 4 and 6) from the same quad and level, one complete preform plotted in situ (ID-6) and the other recovered in three pieces from the screen (ID-4). We suspect that one piece-plotted artifact represented by two conjoining fragments (FS 14617) may be a non-conjoining fragment of needle ID-4 because it could have been recovered from as close as 3 cm to it in the adjacent quad, appears similar in size and shape, and is also made of Lepus sp. bone.
We recovered 2 Vulpes needle fragments (ID-5) in the same location as the Lepus preforms (IDs 4 and 6). One fragment is a plotted bone needle eye (FS 14770) and the other is a bone needle tip recovered from the screen matrix of the adjacent quad (FS 14726). Given their context, these artifacts could have been as close as 1 cm apart, so we believe they are fragments of the same needle.
Finally, a needle midsection made of hare bone (FS 12378) and a needle fragment from near the distal tip that did not yield a ZooMS spectrum (FS 11543) are close enough in metric dimensions to be from the same artifact (ID-3), though they are located around 1.8 m apart. Thus, we conservatively believe they are part of the same artifact, acknowledging that they may be fragments of different artifacts.
Micro-CT scans of bone needle fragments indicate production from cortical bone. Although none of the bone needles from La Prele are complete due to use-related [18] and post-depositional fracturing, rare examples of mostly complete bone needles of comparable age from the Agate Basin [40] and Lindenmeier [41] sites are 5 to 7 cm long and 1.5 to 2.5 mm thick. The metapodials and long bones of canids, felids, and leporids are among the only bones present in their respective skeletons that meet these criteria (Fig 4), others being too curved (e.g., ribs), thin (e.g., scapulae), short (e.g., phalanges), or inappropriately shaped (e.g., vertebrae, innominate, or crania). Metapodial cortical bone thickness is especially similar to bone needle thickness. One would need only to score metapodials lengthwise with a flake or graving tool, split them, and then pare down their width through carving or abrading to achieve appropriate dimensions for bone needles. Thus, we think the most likely skeletal elements used to produce the La Prele bone needles were the metapodials of canids and felids and specifically the longer metatarsals of hares.
Fig 4. Metric comparisons between bone needle artifacts and comparative faunal specimens for La Prele.
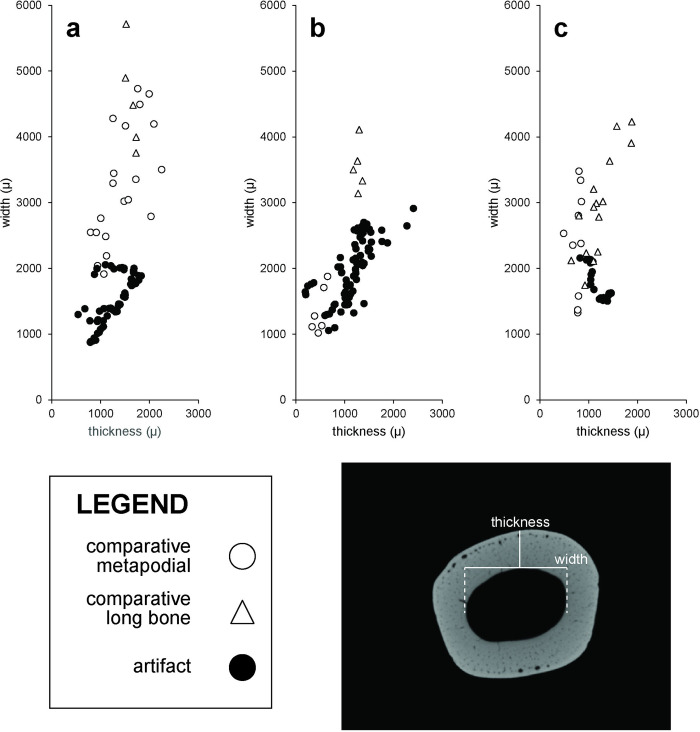
a) felid needles and Lynx rufus, b) Lepus needles/needle preforms and Lepus americanus, and c) Vulpes needles and Vulpes vulpes.
Discussion
Bone needles are a well-established archaeological phenomenon associated with the North American Younger Dryas chronozone (ca. 12,900–11,600 cal BP) [18, 19], when foragers are assumed to have produced tailored garments in response to the return of a near glacial climate. For instance, as few as four Folsom complex (ca. 12,900 to 12,350 cal BP) [42] campsites have been excavated in a way likely to yield bone needles (i.e., water screened through 1/16th inch mesh), yet at least five have produced them (including La Prele) [6]. Archaeologists have found bone needles in the campsites and burials of other Younger Dryas-aged cultures throughout North America as well [19]. Given the right preservation conditions and recovery techniques, bone needles are seemingly ubiquitous in Younger Dryas-aged campsites in North America.
Additionally, a relatively large number of Early Paleoindian (ca. 13,200–12,000 cal BP) sites contain the bones of fur-bearing carnivores and lagomorphs. In a compilation of 36 Clovis (ca. 13,200–12,700 cal BP) faunal assemblages, six (17%) contain canid remains, one of which also contains felid (Smilodon), though the Smilodon is likely not associated with human activity [43]. In a compilation of 17 Folsom campsites that contain preserved bone, six (35%) contain the bones of fur-bearing carnivores, including gray wolf (Canis lupus), coyote (Canis latrans), swift fox (Vulpes velox), red fox (Vulpes vulpes), domestic dog (Canis familiaris), and bobcat (Lynx rufus) [6]. Lagomorphs are even more common in Early Paleoindian sites, though their association with human activity is not always certain due to their natural abundance on the landscape and tendency to burrow into archaeological deposits. Around 31% of Clovis [43] and 73% of Folsom [44] faunal assemblages contain lagomorph bones, including La Prele [45], and they are overwhelmingly Lepus sp. when identifiable. In fact, lagomorphs are the second most common animal in Folsom sites next to bison. Evidently, Early Paleoindians often obtained fur-bearing animals, and this tendency appears more common in Younger Dryas-aged sites (i.e., Folsom-aged) when the use of pelts in tailored garments would have been more common.
Results from La Prele are consistent with this larger pattern of bone needles and fur-bearing mammals being associated with the Early Paleoindian period. At La Prele, the production of bone needles from fur-bearing mammal bone most likely occurred because both bone needles and fur-bearer pelts were used to produce tailored garments. We suspect that fur-bearer foot bones were available to sewers at La Prele due to the way in which fur-bearer pelts are commonly removed from animals, which typically leaves foot bones attached [26, 27, 40]. In need of a needle, sewers at La Prele could have simply removed a metapodial from a nearby pelt, either sewn into a garment or awaiting to be, split it, and then abraded the bone to a width of 1 to 3 mm for use.
By extension, these results are compelling evidence that the earliest North Americans routinely trapped game. Most fur-bearing carnivores possess small return rates during encounter-based hunting due to their small size, low population densities, and wary avoidance behaviors [24, 46], as do lagomorphs outside of large communal drives [47] for which no evidence exists in North America prior to the Early Holocene [48, 49]. However, those poor returns are mitigated through trapping, which diminishes the search time required to pursue these types of game. In a recent review of all well-established return rates in the ethnographic literature, Morin and colleagues [24] document relatively few fur bearers, and those present were invariably trapped or hunted in game drives rather than pursued in a solo hunting scenario. Given that ethnographic cases of solo, encounter-based hunting for fur bearers appear to be rare, we must assume that such animals were rarely if ever obtained in ways other than trapping or driving (in the case of lagomorphs). Moreover, spears with large points seem like impractical weapons for hunting small carnivores and hares. While game trapping is widely accepted for the Eurasian Paleolithic [50], it is not typically acknowledged for the North American Paleoindian record. Given that trapping technologies were maintained through North American colonization, we find it most likely that Early Paleoindians operated traplines, at least seasonally, to obtain fur-bearing animals alongside the hunts used to procure large game whose bones dominate Early Paleoindian archaeological sites [43]. A further expectation of trapping is an overrepresentation of males among fur-bearing carnivores in archaeological sites, which are more likely to be trapped given behavioral differences [51]. Sex determination of fur bearing carnivores in Paleoindian assemblages would thus be an interesting avenue for future research.
Conclusions
Our study is the first to identify the species and likely elements from which Paleoindians produced eyed bone needles. Bone needle production techniques have until this point been largely theoretical, based on incised bone objects argued to represent bone needle preforms [52, 53] and experimental needle production [54, 55]. We were able to clarify some aspects of bone needle production techniques through the combined use of ZooMS and Micro-CT scanning, the first applications of both of these technologies to the study of Paleoindian bone needles (but not beads [56]).
Our results are strong evidence for tailored garment production using bone needles and fur-bearing animal pelts. Such garments might have looked comparable to those of the Inuit, who sewed fur bearer pelts into the fringes of parkas whose base material was typically comprised of ungulate hide [11] and used them for hats and mittens. The cold conditions of the North American Younger Dryas in northerly latitudes likely inspired a greater reliance on such garments, and the sparse Early Paleoindian archaeological record suggests a relative abundance of bone needles and fur bearers in Younger Dryas-aged sites relative to periods before and after.
Finally, our results have some relevance to the debate surrounding Early Paleoindian subsistence. The presence of fur bearers in Early Paleoindian sites has been argued to support the existence of a ‘broad spectrum’ subsistence base characterized by generalist rather than specialist diets [57, 58]. Our results are a good reminder that foragers use animal products for a wide range of purposes other than subsistence, and that the mere presence of animal bones in an archaeological site need not be indicative of diet.
Supporting information
(PDF)
Acknowledgments
The Strock family has been gracious in allowing investigations of La Prele on their land. We thank the Science Initiative and the Center for Advanced Scientific Instrumentation at the University of Wyoming for use of the Zeiss Xradia Versa 610
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding for this project includes the National Science Foundation (award #1947297), the Wyoming Cultural Trust Fund, the Quest Archaeological Research Program at Southern Methodist University, the National Geographic Society, Ed and Shirley Cheramy, and the George C. Frison Institute for Archaeology and Anthropology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ruxton GD, Wilkinson DM. Thermoregulation and endurance running in extinct hominins: Wheeler’s models revisited. J Hum Evol. 2011;61(2): 169–75. doi: 10.1016/j.jhevol.2011.02.012 [DOI] [PubMed] [Google Scholar]
- 2.Wheeler PE. The thermoregulatory advantages of large body size for hominids foraging in savannah environments. J Hum Evol. 1992;23(4): 351–62. [Google Scholar]
- 3.Wheeler PE. The influence of the loss of functional body hair on the water budgets of early hominids. J Hum Evol. 1992;23(5): 379–88. [Google Scholar]
- 4.Wheeler PE. The thermoregulatory advantages of hominid bipedalism in open equatorial environments: the contribution of increased convective heat loss and cutaneous evaporative cooling. J Hum Evol. 1991;21(2): 107–15. [Google Scholar]
- 5.Aiello LC, Wheeler P. Neanderthal thermoregulation and the glacial climate. In: van Andel TH, Davies W, editors. Neanderthals and Modern Humans in the European Landscape During the Last Glaciation. Cambridge: McDonald Institute for Archaeological Research; 2003. pp. 147–66. [Google Scholar]
- 6.Pelton SR. A Thermoregulatory Framework for Hominin Research: Archaeological and Ethnographic Studies with Implications for Global Human Dispersal. PhD Dissertation, the University of Wyoming. 2018.
- 7.Gilligan I. The prehistoric development of clothing: Archaeological implications of a thermal model. J Archaeol Method Theory. 2010;17: 15–80. [Google Scholar]
- 8.Hoffecker JF. A prehistory of the north: human settlement of the higher latitudes. Rutgers University Press; 2005. [Google Scholar]
- 9.Rodriguez J, Willmes C, Mateos A. Shivering in the Pleistocene. Human adaptations to cold exposure in Western Europe from MIS 14 to MIS 11. J Hum Evol. 2021;153: 102966. doi: 10.1016/j.jhevol.2021.102966 [DOI] [PubMed] [Google Scholar]
- 10.Gagge AP, Burton AC, Bazett HC. A practical system of units for the description of the heat exchange of man with his environment. Science. 1941;94(2445): 428–30. doi: 10.1126/science.94.2445.428 [DOI] [PubMed] [Google Scholar]
- 11.Issenman B. Sinews of survival: The living legacy of Inuit clothing. UBC Press; 1997. [Google Scholar]
- 12.Formicola V, Buzhilova AP. Double child burial from Sunghir (Russia): Pathology and inferences for Upper Paleolithic funerary practices. Am J Phys Anth. 2004;124(3): 189–98. doi: 10.1002/ajpa.10273 [DOI] [PubMed] [Google Scholar]
- 13.d’Errico F, Borgia V, Ronchitelli A. Uluzzian bone technology and its implications for the origin of behavioural modernity. Quat Int. 2012;259: 59–71. [Google Scholar]
- 14.Gilligan I. Neanderthal extinction and modern human behaviour: the role of climate change and clothing. World Archaeol. 2007;39(4): 499–514. [Google Scholar]
- 15.Klein RG. The human career: Human biological and cultural origins. University of Chicago Press; 2009. [Google Scholar]
- 16.Wang W, Bae C, Xu X. Chinese prehistoric eyed bone needles: a review and assessment. J World Prehist. 2020;33: 385–423. [Google Scholar]
- 17.Gilligan I, d’Errico F, Doyon L, Wang W, Kuzmin YV. Paleolithic eyed needles and the evolution of dress. Sci Adv. 2024;10(26): eadp2887. doi: 10.1126/sciadv.adp2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyman RL. North American Paleoindian eyed bone needles: Morphometrics, sewing, and site structure. Am Antiq. 2015;80(1): 146–60. [Google Scholar]
- 19.Osborn AJ. Eye of the needle: cold stress, clothing, and sewing technology during the Younger Dryas cold event in North America. Am Antiq. 2014;79(1): 45–68. [Google Scholar]
- 20.Mota-Rojas D, Titto CG, de Mira Geraldo A, Martínez-Burnes J, Gómez J, Hernández-Ávalos I, et al. Efficacy and function of feathers, hair, and glabrous skin in the thermoregulation strategies of domestic animals. Animals. 2021;11(12): 3472. doi: 10.3390/ani11123472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly RL. The lifeways of hunter-gatherers: the foraging spectrum. Cambridge University Press; 2013. [Google Scholar]
- 22.Bettinger RL. Hunter-gatherer foraging: five simple models. Eliot Werner Publications; 2009. [Google Scholar]
- 23.Winterhalder BP. Canadian fur bearer cycles and Cree-Ojibwa hunting and trapping practices. Am Nat. 1980;115(6): 870–879. [Google Scholar]
- 24.Morin E, Bird D, Winterhalder B, Bliege Bird R. Deconstructing hunting returns: Can we reconstruct and predict payoffs from pursuing prey? J Archaeol MethodTheory. 2022;29(2): 561–623. [Google Scholar]
- 25.Collard M, Tarle L, Sandgathe D, Allan A. Faunal evidence for a difference in clothing use between Neanderthals and early modern humans in Europe. J Anthropol Archaeol. 2016;44: 235–46. [Google Scholar]
- 26.Cueto M, Camaros E, Castanos P, Ontanon R, Arias P. Under the skin of a lion: unique evidence of Upper Paleolithic exploitation and use of cave lion (Panthera spelaea) from the Lower Gallery of La Garma (Spain). PLoS One. 2016;11(10): e0163591. doi: 10.1371/journal.pone.0163591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soffer O. The Upper Paleolithic of the central Russian plain. Elsevier; 2013. [Google Scholar]
- 28.Surovell TA, Pelton SR, Mackie ME, Mahan CM, O’Brien MJ, Kelly RL, et al. The La Prele Mammoth Site, Converse County, Wyoming, USA. In: Konidaris GE, Barkai R, Tourloukis V, Havarti K, editors. Human-elephant interactions: From past to present. Tubingen University Press, 2021. pp. 303–320. [Google Scholar]
- 29.Allaun SA, Surovell TA, Vance Haynes C, Pelton SR, Mackie ME, Kelly RL, et al. The geochronological and geoarchaeological context of the Clovis-age La Prele Mammoth Site (48CO1401), Converse County, Wyoming. PaleoAmerica. 2023;9(3): 174–193. [Google Scholar]
- 30.Buckley M, Gu M, Shameer S, Patel S, Chamberlain AT. High‐throughput collagen fingerprinting of intact microfaunal remains; a low‐cost method for distinguishing between murine rodent bones. Rapid Commun Mass Spectrom. 2016;30(7): 805–812. doi: 10.1002/rcm.7483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Sluis LG, Hollund HI, Buckley M, De Louw PGB, Rijsdijk KF, Kars H. Combining histology, stable isotope analysis and ZooMS collagen fingerprinting to investigate the taphonomic history and dietary behaviour of extinct giant tortoises from the Mare aux Songes deposit on Mauritius. Palaeogeogr, Palaeoclimatol, Palaeoecol. 2014;416: 80–91. [Google Scholar]
- 32.Buckley M, Collins M, Thomas-Oates J, Wilson JC. Species identification by analysis of bone collagen using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Rapid Comm Mass Spectrom. 2009;23(23): 3843–3854. doi: 10.1002/rcm.4316 [DOI] [PubMed] [Google Scholar]
- 33.Welker F, Soressi M, Rendu W, Hublin JJ, Collins M. Using ZooMS to identify fragmentary bone from the late Middle/Early Upper Palaeolithic sequence of Les Cottés, France. J Archaeol Sci. 2015;54: 279–286. [Google Scholar]
- 34.Brown S, Hebestreit S, Wang N, Boivin N, Douka K, Richter Korzow K. Zooarchaeology by Mass Spectrometry (ZooMS)-Pretreatment protocols for bone material. Protocols io [Online] [Internet]. 2020. [cited 2024 Mar 17]; Available from: https://scholar.archive.org/work/4q3ase4txvhvndwhxbsrfejleu/access/wayback/https://www.protocols.io/view/zooarchaeology-by-mass-spectrometry-zooms-pretreat-bf5djq26.pdf [Google Scholar]
- 35.Welker F, Hajdinjak M, Talamo S, Jaouen K, Dannemann M, David F, et al. Palaeoproteomic evidence identifies archaic hominins associated with the Châtelperronian at the Grotte du Renne. Proc Natl Acad Sci USA. 2016;113(40): 11162–11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckley M, Harvey VL, Chamberlain AT. Species identification and decay assessment of Late Pleistocene fragmentary vertebrate remains from Pin Hole Cave (Creswell Crags, UK) using collagen fingerprinting. Boreas. 2017;46(3): 402–411. [Google Scholar]
- 37.Brown S, Douka K, Collins MJ, Richter KK. On the standardization of ZooMS nomenclature. J Proteomics. 2021;235: 104041. doi: 10.1016/j.jprot.2020.104041 [DOI] [PubMed] [Google Scholar]
- 38.Mackie ME, Surovell TA, Pelton SR, O’Brien M, Kelly RL, Frison GC, et al. Spatial Analysis of a Clovis Hearth Centered Activity Area at the La Prele Mammoth Site, Converse County, Wyoming. In: Carlson KA, Bement LC, editors. Diversity in Open Air Site Structure Across the Pleistocene/Holocene Boundary. Boulder: University Press of Colorado; 2022. pp. 103–121. [Google Scholar]
- 39.Perri AR, Feuerborn TR, Frantz LA, Larson G, Malhi RS, Meltzer DJ, et al. Dog domestication and the dual dispersal of people and dogs into the Americas. Proc Natl Acad Sci USA. 2021;118(6): e2010083118. doi: 10.1073/pnas.2010083118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frison GC, Stanford, Dennis J. The Agate Basin Site: A Record of the Paleoindian Occupation of the Northwestern High Plains. New York: Academic Press; 1982. [Google Scholar]
- 41.Wilmsen EN, Robert FH Jr. Lindenmeier, 1934–1974: Concluding report on investigations. Smithsonian Contributions to Anthropology 24; 1978. [Google Scholar]
- 42.Buchanan B, David Kilby J, Hamilton MJ, LaBelle JM, Meyer KA, Holland-Lulewicz J, et al. Bayesian revision of the Folsom age range using IntCal20. PaleoAmerica. 2021;7(2): 133–44. [Google Scholar]
- 43.Surovell TA, Waguespack NM. Human prey choice in the late Pleistocene and its relation to megafaunal extinctions. In: Haynes G, editor. American megafaunal extinctions at the end of the Pleistocene. Springer; 2009. pp. 77–105. [Google Scholar]
- 44.Amick DS. Folsom diet breadth and land use in the American Southwest. PhD Dissertation; The University of New Mexico; 1994.
- 45.Litynski ML. Microfauna Analysis at the La Prele Mammoth Site (48CO1401): Implications for Clovis Diets and Paleoenvironments. MA Thesis, The University of Wyoming. 2023.
- 46.Smith EA. Inujjuamiunt foraging strategies: Evolutionary ecology of an Arctic hunting economy. Transaction Publishers; 1991. [Google Scholar]
- 47.Simms SR. Behavioral ecology and hunter-gatherer foraging: An example from the Great Basin. British Archaeological Reports International Series 381. 1987. [Google Scholar]
- 48.Rood RJ. Archaic communal jackrabbit hunting in central Wyoming: Faunal remains from the Dick Myal Housepit site, 48FR6256. Plains Anthropol. 2018;63(247): 260–278. [Google Scholar]
- 49.Schmitt DN, Madsen DB, Lupo KD. The worst of times, the best of times: Jackrabbit hunting by Middle Holocene human foragers in the Bonneville Basin of western North America. In: Mondini M, Munoz S, Wickler S, editors. Colonization, Migration and Marginal Areas: A Zooarchaeological Approach. Oxford: Oxbow Books; 2004. pp. 86–95. [Google Scholar]
- 50.Stiner MC, Munro ND, Surovell TA. The tortoise and the hare: small-game use, the broad-spectrum revolution, and Paleolithic demography. Curr anthropol. 2000;41(1): 39–79. [PubMed] [Google Scholar]
- 51.Lee Lyman R. Prehistoric Mink (Mustela vison) Trapping on the Northwest Coast. J Field Archaeol. 2007;32(1): 91–95. [Google Scholar]
- 52.Pelton SR, Boyd JR, Rockwell H, Newton C. Younger Dryas-Aged Fluting, Cold, and Time Budgeting in the Great Plains and Rocky Mountains. PaleoAmerica. 2016;2(2): 169–178. [Google Scholar]
- 53.Kornfeld M, Larson ML. Reinvestigation in Context: Paleoindian Prehistory at the Edge of the Rockies. In: Larson ML, Kornfeld M, Frison GC, editors. Hell Gap: A Stratified Paleoindian Campsite at the Edge of the Rockies. Salt Lake City: University of Utah Press; 2009. pp. 3–13. [Google Scholar]
- 54.Flenniken JJ. The experimental replication of Paleo-Indian eyed needles from Washington. Northwest Anthropological Research Notes. 1978;12(1): 61–71. [Google Scholar]
- 55.Hoffman BW. Broken eyes and simple grooves: Understanding Eastern Aleut needle technology through experimental manufacture and use of bone needles. In: Frink L, Shepard RS, Reinhardt GA, editors. Many Faces of Gender: Roles and Relationships Through Time in Indigenous Communities. Boulder: University Press of Colorado; 2002. pp. 151–164. [Google Scholar]
- 56.Surovell TA, Litynski ML, Allaun SA, Buckley M, Schoborg TA, Govaerts JA, et al. Use of hare bone for the manufacture of a Clovis bead. Sci Rep. 2024;14(1): 2937. doi: 10.1038/s41598-024-53390-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cannon MD, Meltzer DJ. Early Paleoindian foraging: examining the faunal evidence for large mammal specialization and regional variability in prey choice. Quat Sci Rev. 2004;23(18–19): 1955–1987. [Google Scholar]
- 58.Byers DA, Ugan A. Should we expect large game specialization in the late Pleistocene? An optimal foraging perspective on early Paleoindian prey choice. J Archaeol Sci. 2005;32(11): 1624–40. [Google Scholar]


