Abstract
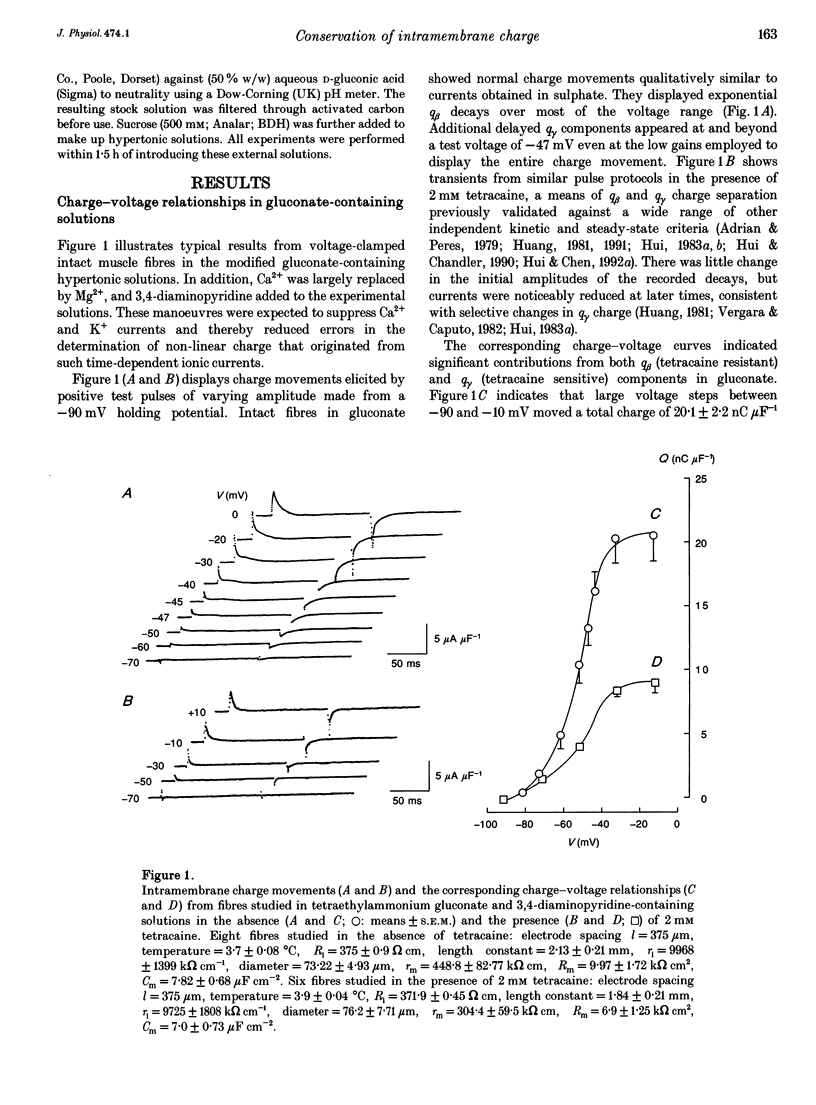
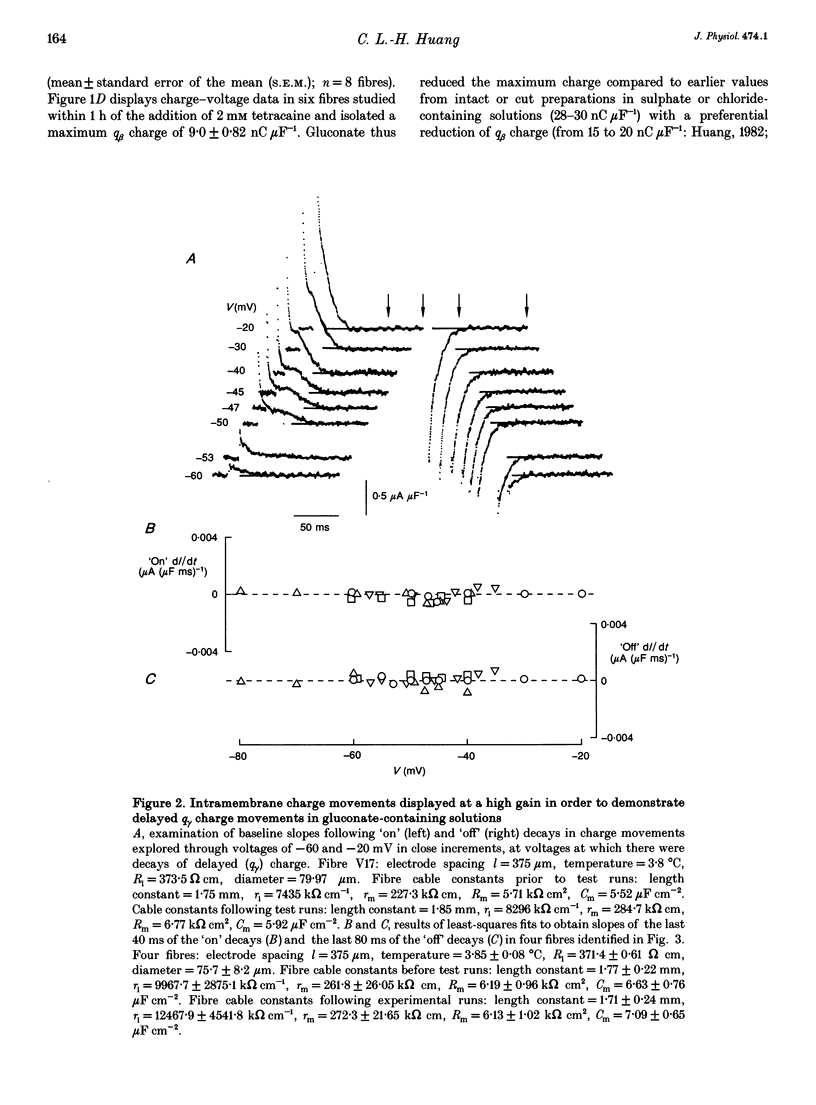
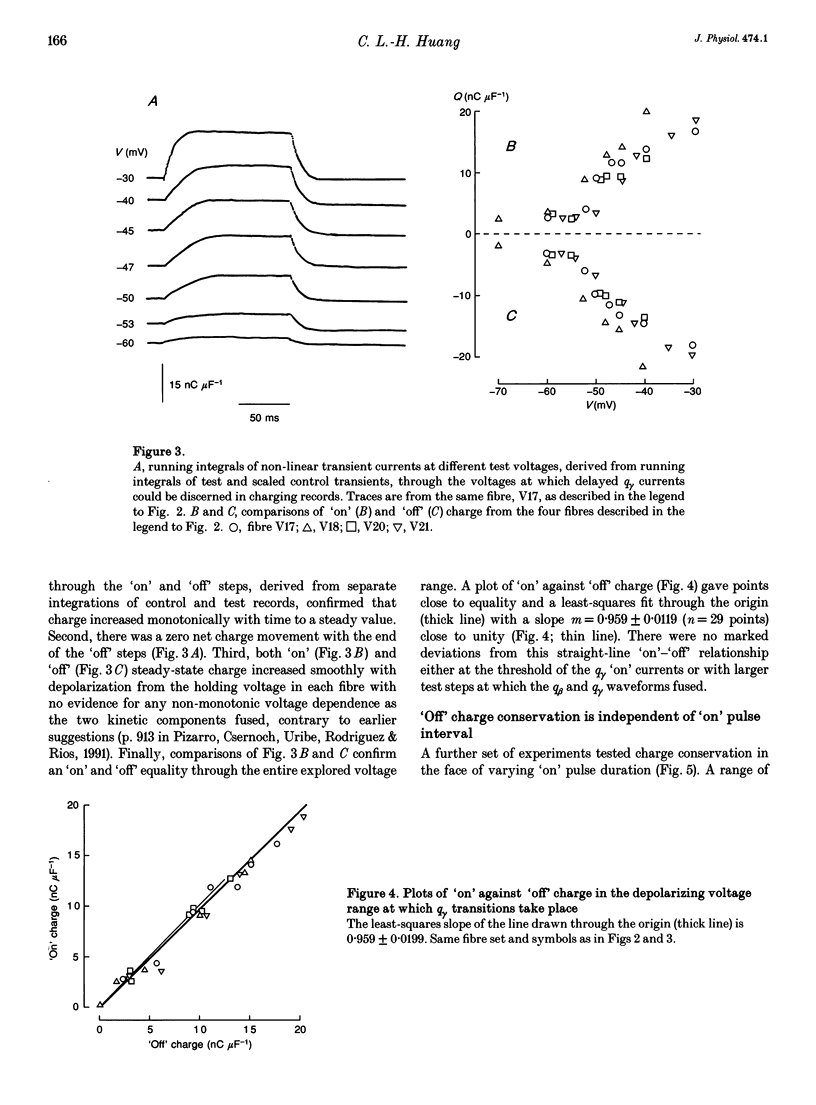
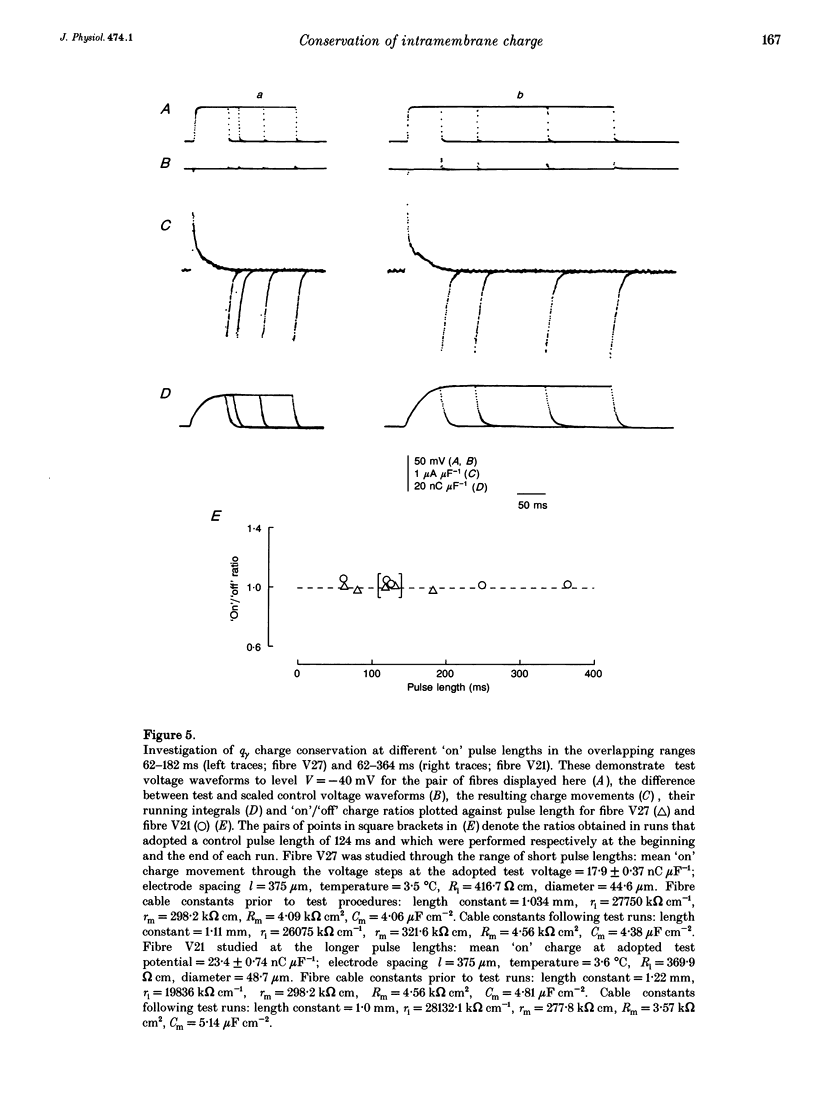
1. The conservation of intramembrane charge was investigated in intact voltage-clamped frog skeletal muscle fibres under conditions that minimized time-dependent ionic currents and so facilitated precise determination of capacitative charge. 2. Prolonged (q gamma) transients were demonstrated in 3,4-diaminopyridine and tetraethyl-ammonium gluconate-containing low [Ca2+] solutions in response to 125 ms pulses that explored the voltage range -90 to -20 mV. The tetracaine-sensitive, q gamma, component then accounted for a significant proportion (over 50%) of available charge. 3. Both delayed 'on' q gamma currents and 'off' current tails decayed to steady direct current (DC) baselines without significant residual ionic current slopes in the chosen extracellular solutions. This suggested that the current transients represented capacitative decays. It was also compatible with the precise determination of effective charge by integration. 4. The advent of 'on' q gamma current was accompanied by increased 'off' charge. Thus, charge was conserved through all 'on' and 'off' steps and through test voltages that extended from the threshold appearance of q gamma as a slow transient to its full merger with the earlier q beta decay at stronger depolarizations. 5. Charge conservation persisted through a wide range of 'on' pulse durations between 60 and 370 ms and was therefore independent of the interval following the q gamma decay. 6. The quantity of q gamma charge remained a monotonic single-valued function of test voltage, whether this potential was reached directly from the -90 mV holding potential or following a prepulse to -10 mV. 7. These findings suggest that the q gamma charge movement represents the electrical signature of an intramembrane entity whose transitions are primarily driven by, and therefore conserved with, the steady-state potential.
Full text
PDF


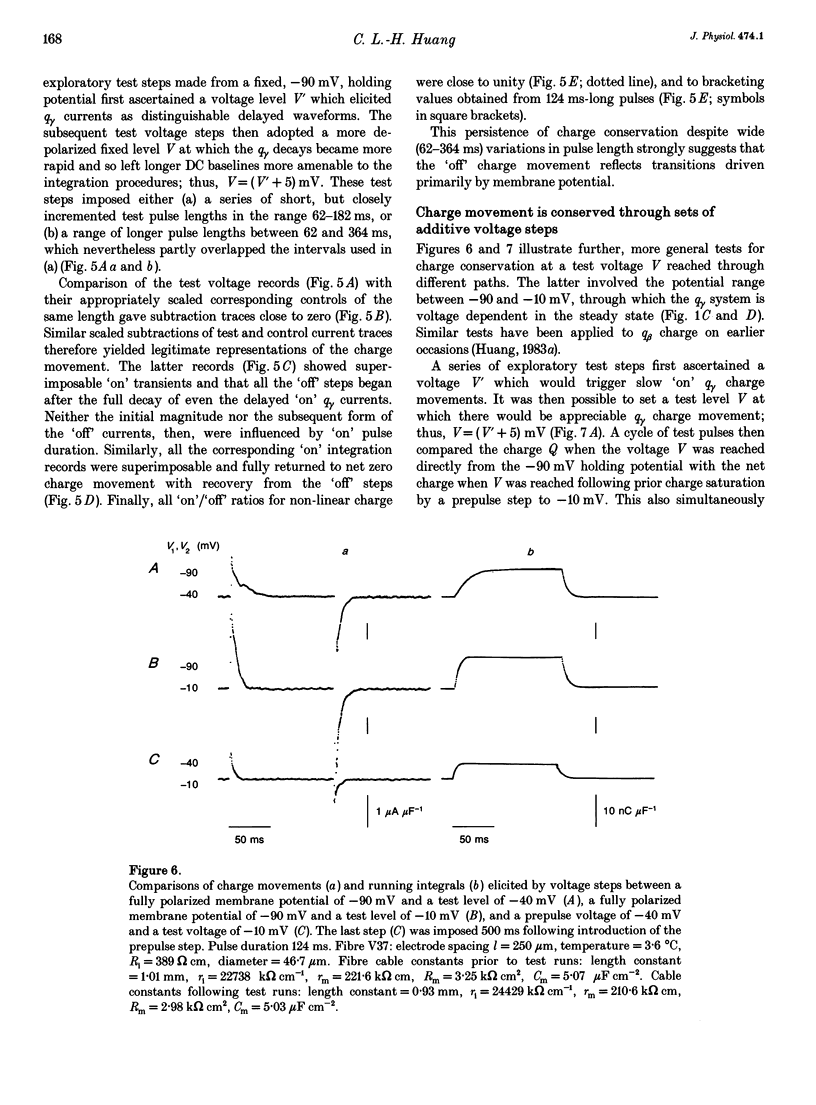
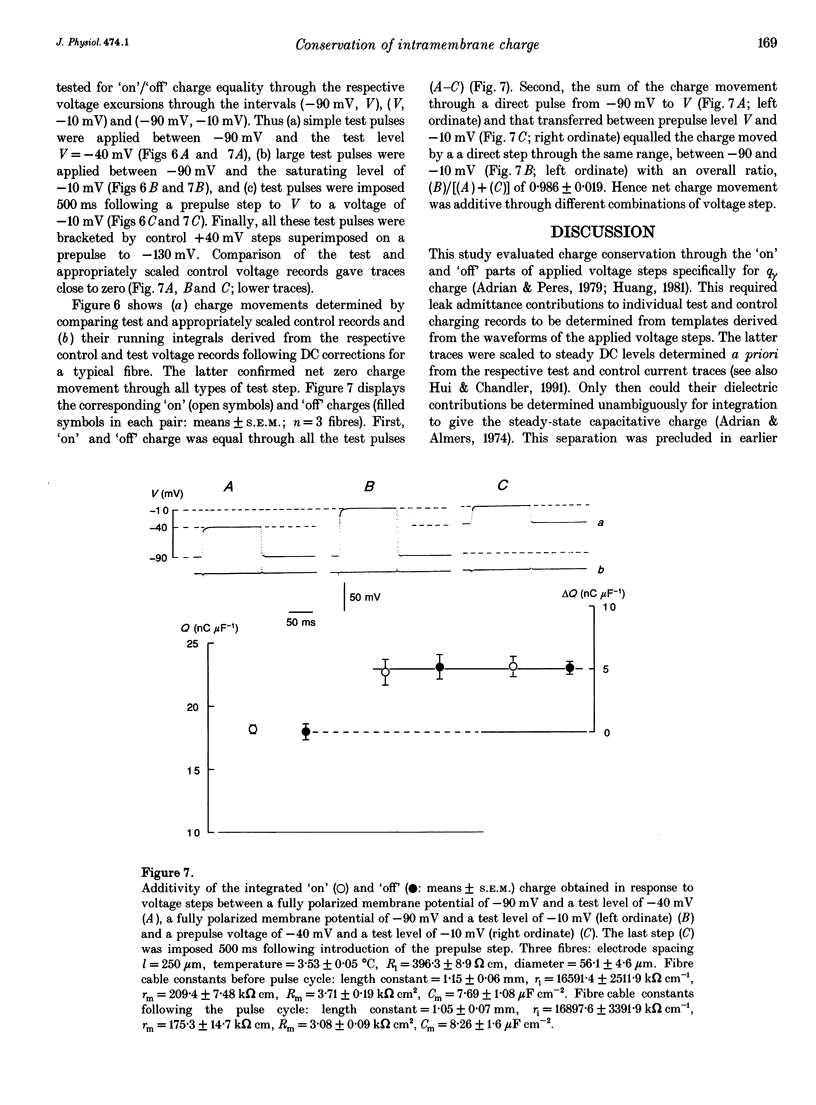


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Almers W. Charge movement in the membrane of striated muscle. J Physiol. 1976 Jan;254(2):339–360. doi: 10.1113/jphysiol.1976.sp011235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Almers W. Membrane capacity measurements on frog skeletal muscle in media of low ion content. J Physiol. 1974 Mar;237(3):573–605. doi: 10.1113/jphysiol.1974.sp010499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H. Charge movement in the membrane of striated muscle. Annu Rev Biophys Bioeng. 1978;7:85–112. doi: 10.1146/annurev.bb.07.060178.000505. [DOI] [PubMed] [Google Scholar]
- Adrian R. H., Peres A. Charge movement and membrane capacity in frog muscle. J Physiol. 1979 Apr;289:83–97. doi: 10.1113/jphysiol.1979.sp012726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Hui C. S. Membrane capacitance in frog cut twitch fibers mounted in a double vaseline-gap chamber. J Gen Physiol. 1990 Aug;96(2):225–256. doi: 10.1085/jgp.96.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. A non-linear voltage dependent charge movement in frog skeletal muscle. J Physiol. 1976 Jan;254(2):245–283. doi: 10.1113/jphysiol.1976.sp011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernoch L., Pizarro G., Uribe I., Rodríguez M., Ríos E. Interfering with calcium release suppresses I gamma, the "hump" component of intramembranous charge movement in skeletal muscle. J Gen Physiol. 1991 May;97(5):845–884. doi: 10.1085/jgp.97.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García J., Pizarro G., Ríos E., Stefani E. Effect of the calcium buffer EGTA on the "hump" component of charge movement in skeletal muscle. J Gen Physiol. 1991 May;97(5):885–896. doi: 10.1085/jgp.97.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nakajima S. The effect of diameter on the electrical constants of frog skeletal muscle fibres. J Physiol. 1972 Feb;221(1):105–120. doi: 10.1113/jphysiol.1972.sp009742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. 'Off' tails of intramembrane charge movements in frog skeletal muscle in perchlorate-containing solutions. J Physiol. 1987 Mar;384:491–509. doi: 10.1113/jphysiol.1987.sp016466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Dielectric components of charge movements in skeletal muscle. J Physiol. 1981;313:187–205. doi: 10.1113/jphysiol.1981.sp013658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Experimental analysis of alternative models of charge movement in frog skeletal muscle. J Physiol. 1983 Mar;336:527–543. doi: 10.1113/jphysiol.1983.sp014596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L., Peachey L. D. A reconstruction of charge movement during the action potential in frog skeletal muscle. Biophys J. 1992 May;61(5):1133–1146. doi: 10.1016/S0006-3495(92)81923-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L., Peachey L. D. Anatomical distribution of voltage-dependent membrane capacitance in frog skeletal muscle fibers. J Gen Physiol. 1989 Mar;93(3):565–584. doi: 10.1085/jgp.93.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Pharmacological separation of charge movement components in frog skeletal muscle. J Physiol. 1982 Mar;324:375–387. doi: 10.1113/jphysiol.1982.sp014118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Separation of intramembrane charging components in low-calcium solutions in frog skeletal muscle. J Gen Physiol. 1991 Aug;98(2):249–263. doi: 10.1085/jgp.98.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. The differential effects of twitch potentiators on charge movements in frog skeletal muscle. J Physiol. 1986 Nov;380:17–33. doi: 10.1113/jphysiol.1986.sp016269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Time domain spectroscopy of the membrane capacitance in frog skeletal muscle. J Physiol. 1983 Aug;341:1–24. doi: 10.1113/jphysiol.1983.sp014789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Voltage-dependent block of charge movement components by nifedipine in frog skeletal muscle. J Gen Physiol. 1990 Sep;96(3):535–557. doi: 10.1085/jgp.96.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S., Chandler W. K. Intramembranous charge movement in frog cut twitch fibers mounted in a double vaseline-gap chamber. J Gen Physiol. 1990 Aug;96(2):257–297. doi: 10.1085/jgp.96.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S., Chandler W. K. Q beta and Q gamma components of intramembranous charge movement in frog cut twitch fibers. J Gen Physiol. 1991 Sep;98(3):429–464. doi: 10.1085/jgp.98.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S., Chen W. Effects of conditioning depolarization and repetitive stimulation on Q beta and Q gamma charge components in frog cut twitch fibers. J Gen Physiol. 1992 Jun;99(6):1017–1043. doi: 10.1085/jgp.99.6.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S., Chen W. Separation of Q beta and Q gamma charge components in frog cut twitch fibers with tetracaine. Critical comparison with other methods. J Gen Physiol. 1992 Jun;99(6):985–1016. doi: 10.1085/jgp.99.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S. Differential properties of two charge components in frog skeletal muscle. J Physiol. 1983 Apr;337:531–552. doi: 10.1113/jphysiol.1983.sp014640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S. Factors affecting the appearance of the hump charge movement component in frog cut twitch fibers. J Gen Physiol. 1991 Aug;98(2):315–347. doi: 10.1085/jgp.98.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S. Pharmacological studies of charge movement in frog skeletal muscle. J Physiol. 1983 Apr;337:509–529. doi: 10.1113/jphysiol.1983.sp014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving M., Maylie J., Sizto N. L., Chandler W. K. Intrinsic optical and passive electrical properties of cut frog twitch fibers. J Gen Physiol. 1987 Jan;89(1):1–40. doi: 10.1085/jgp.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving M., Maylie J., Sizto N. L., Chandler W. K. Simultaneous monitoring of changes in magnesium and calcium concentrations in frog cut twitch fibers containing antipyrylazo III. J Gen Physiol. 1989 Apr;93(4):585–608. doi: 10.1085/jgp.93.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Schneider M. F., Simon B. J., Szucs G. Intramembrane charge movement and calcium release in frog skeletal muscle. J Physiol. 1986 Apr;373:481–511. doi: 10.1113/jphysiol.1986.sp016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro G., Csernoch L., Uribe I., Rodríguez M., Ríos E. The relationship between Q gamma and Ca release from the sarcoplasmic reticulum in skeletal muscle. J Gen Physiol. 1991 May;97(5):913–947. doi: 10.1085/jgp.97.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Szücs G., Csernoch L., Magyar J., Kovács L. Contraction threshold and the "hump" component of charge movement in frog skeletal muscle. J Gen Physiol. 1991 May;97(5):897–911. doi: 10.1085/jgp.97.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara J., Caputo C. Effects of tetracaine on charge movements and calcium signals in frog skeletal muscle fibers. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1477–1481. doi: 10.1073/pnas.80.5.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]


