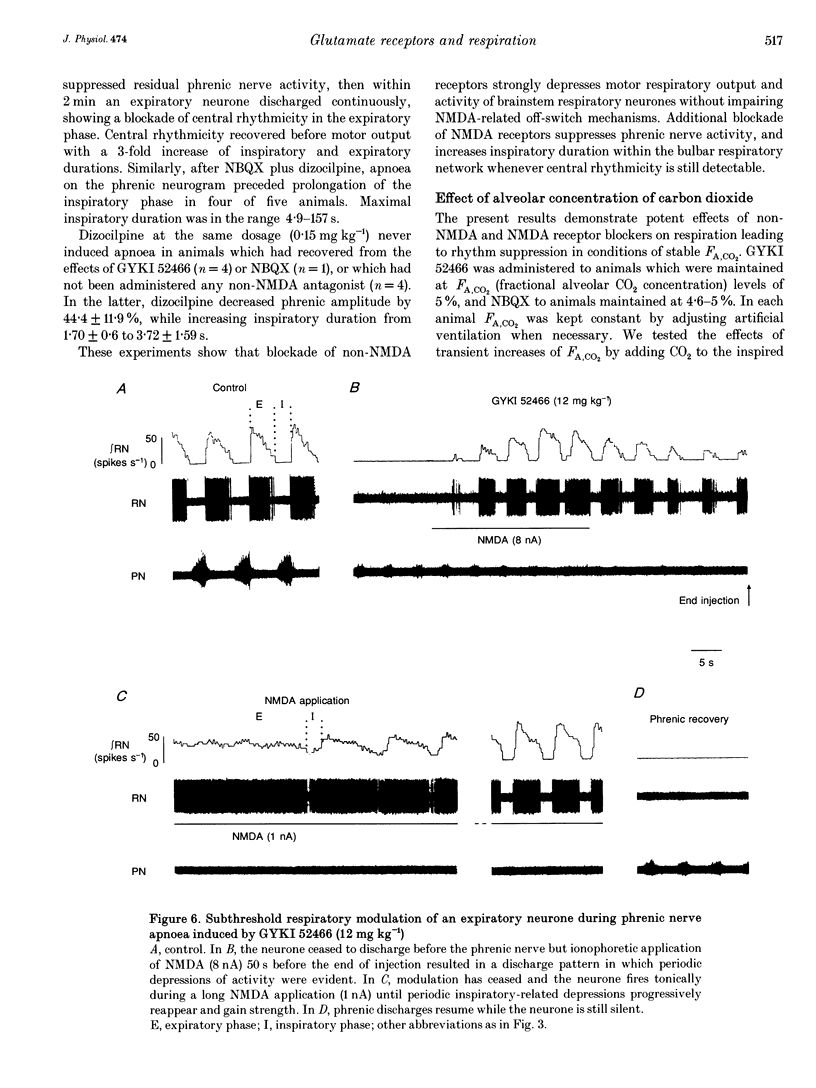
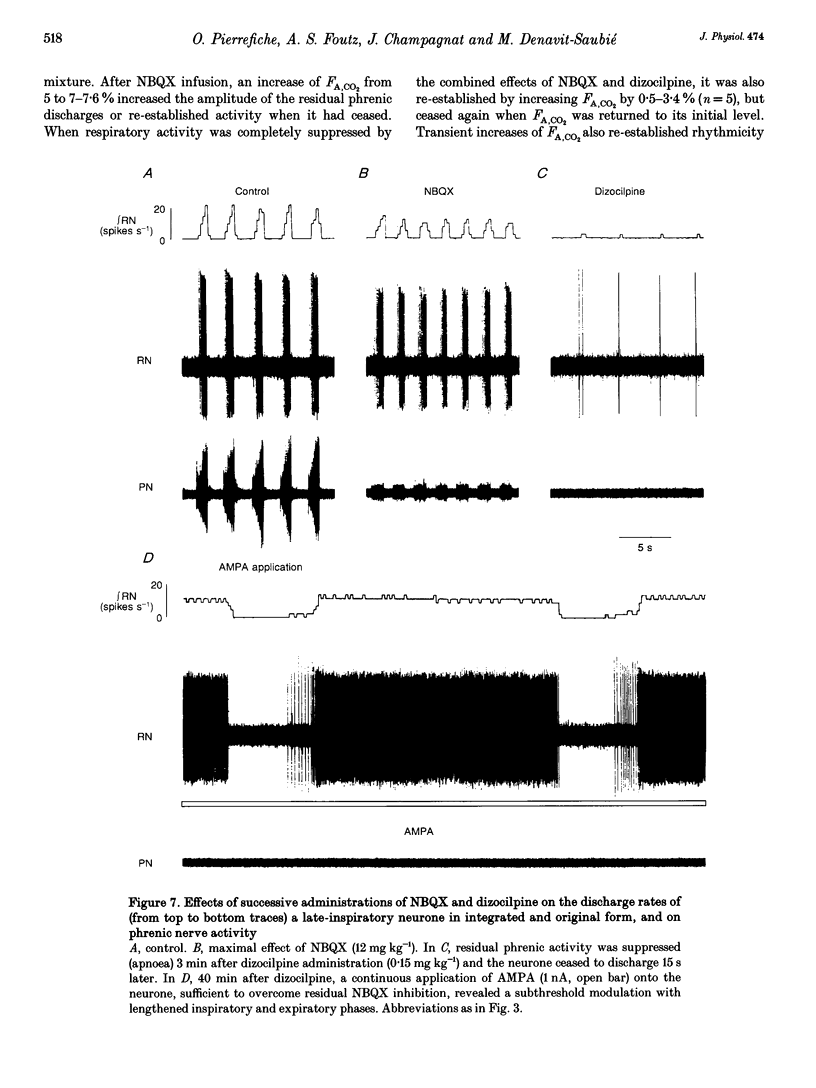
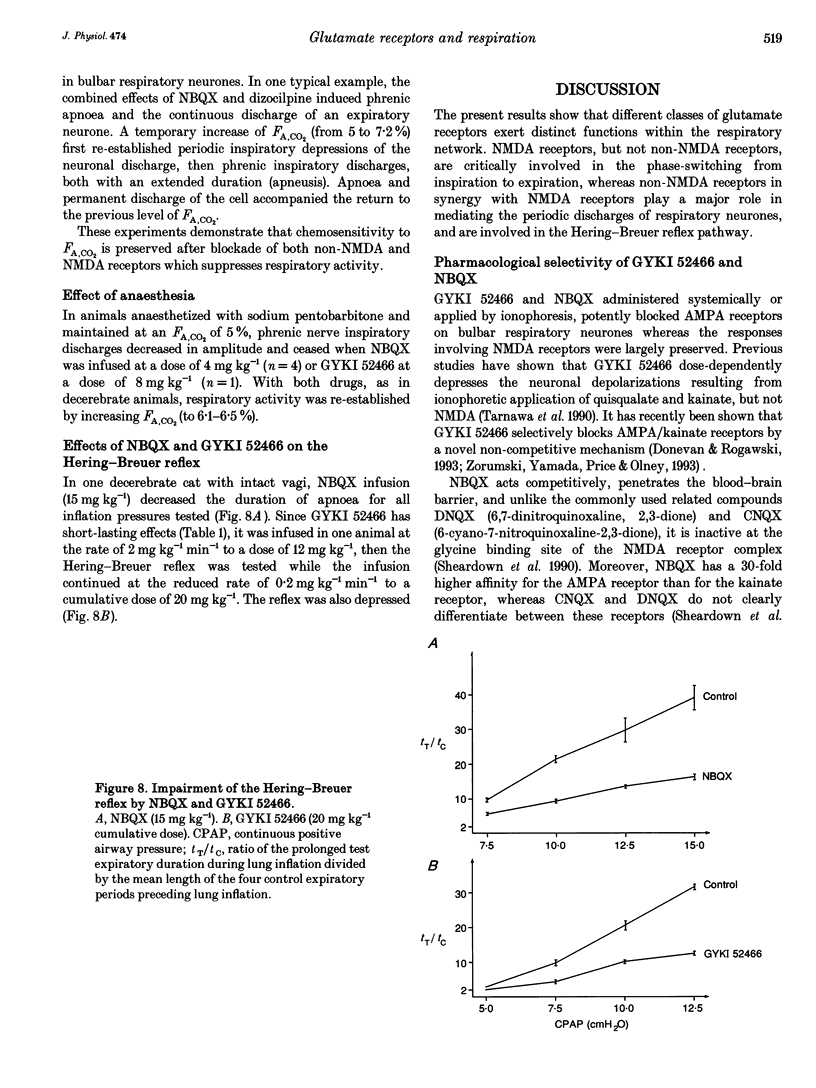
Abstract
1. Activation of N-methyl-D-aspartate (NMDA) glutamate receptors in the brainstem network of respiratory neurones is required to terminate inspiration in the absence of lung afferents, but it is not required in the inspiratory motor act of lung inflation. In the present study we examined the involvement of non-NMDA ionotropic glutamate receptors in these two mechanisms in the adult mammal. 2. Adult cats were either decerebrated or anaesthetized with sodium pentobarbitone, paralysed and ventilated. Inspiratory motor output was recorded from the phrenic nerve and central respiratory activity from neurones in the bulbar ventral respiratory group. 3. In decerebrate vagotomized cats, ionophoretic application of 2,3-dihydroxy-6-nitro-7-sulphamoylbenzo(F)quinoxaline (NBQX) onto single respiratory neurones decreased their spontaneous discharge rate and abolished the excitatory effect of exogenously applied (RS) alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) but not NMDA. 4. In these animals, intravenous infusion (12 mg kg-1) of the non-NMDA receptor blockers GYKI 52466 (1-(4-aminophenyl)-4-methyl-7,8-methylene-dioxy-5-H-2,3-benzodi aze pine) or NBQX: (1) decreased (in 10/15 cats) or abolished (in 5/15 cats) the inspiratory-related discharge of the phrenic nerve; (2) did not prolong the inspiratory phase; (3) reduced or abolished the spontaneous discharge of respiratory neurones; and (4) profoundly decreased the excitatory effects of AMPA but not NMDA ionophoresed onto these neurones. When both the phrenic nerve and the recorded respiratory neurone were silenced, neuronal excitation by ionophoretic application of NMDA first revealed a subthreshold respiratory modulation without lengthening of the inspiratory phase, then respiratory modulation became undetectable. 5. Additional blockade of NMDA receptors by a small dose (0.15 mg kg-1) of dizocilpine (MK-801), abolished the phrenic nerve activity which persisted after NBQX (apnoea), but the discharge or the subthreshold modulation of the bulbar respiratory neurones showed a lengthening of the inspiratory phase (apneusis). 6. Elevation of FA,CO2 increased or re-established phrenic nerve discharges after blockade of non-NMDA receptors or of both NMDA and non-NMDA receptors. 7. Small doses of NBQX or GYKI 52466 induced apnoea in five of five cats anaesthetized with sodium pentobarbitone. 8. In decerebrate animals with intact vagi, GYKI 52466 and NBQX depressed the Hering-Breuer expiratory-lengthening reflex. 9. The results suggest that: (1) there is a specialization of different classes of glutamate receptors participating in timing mechanisms and transmission within the mammalian respiratory network. Neural transmission predominantly involves activation of non-NMDA receptors, acting in synergy with NMDA receptors.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


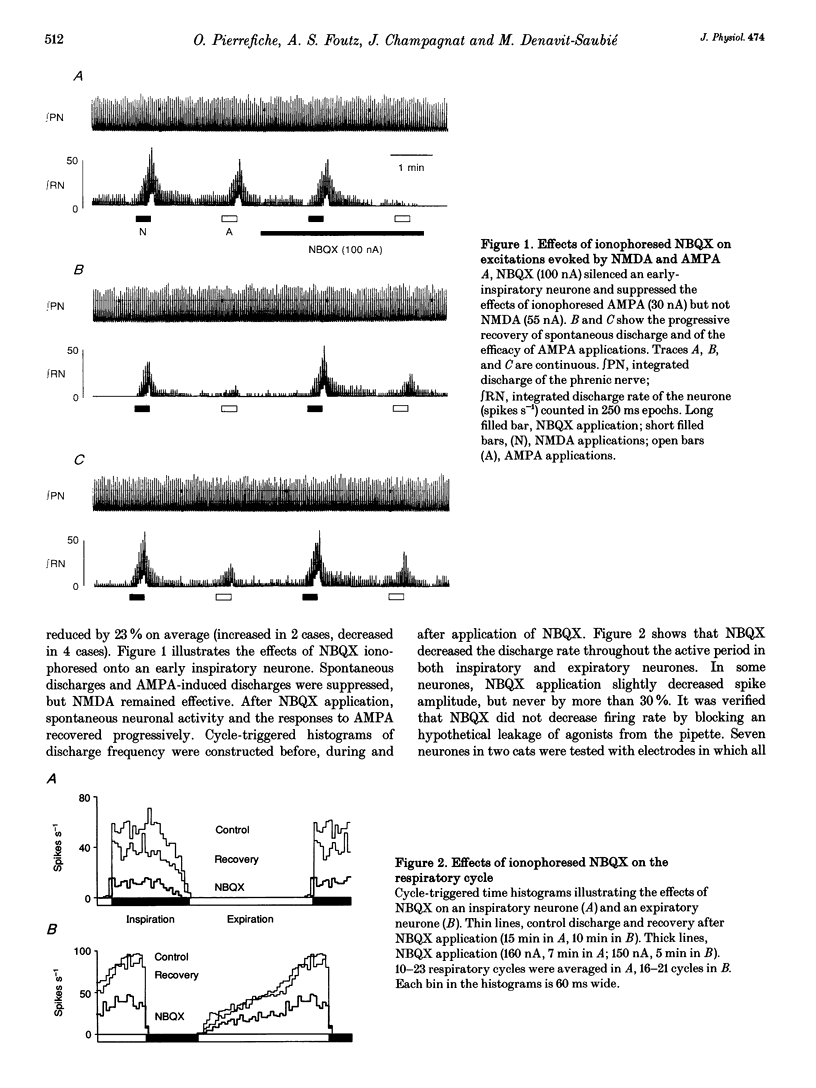
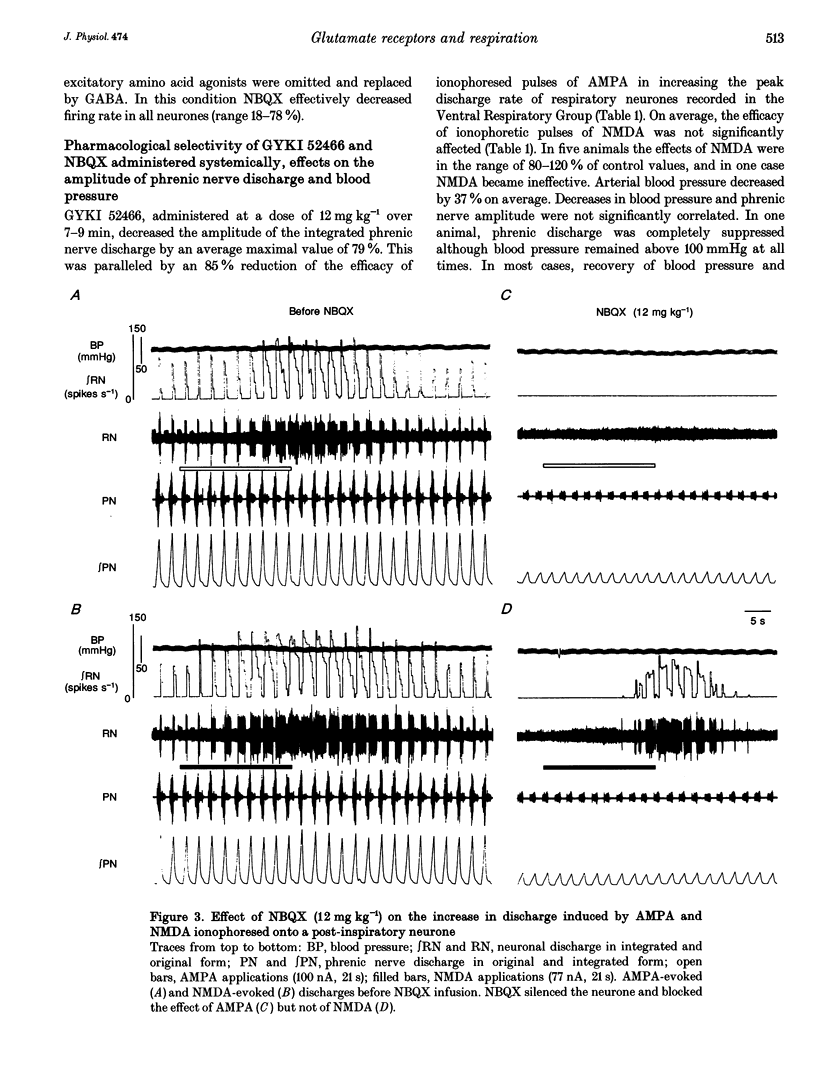
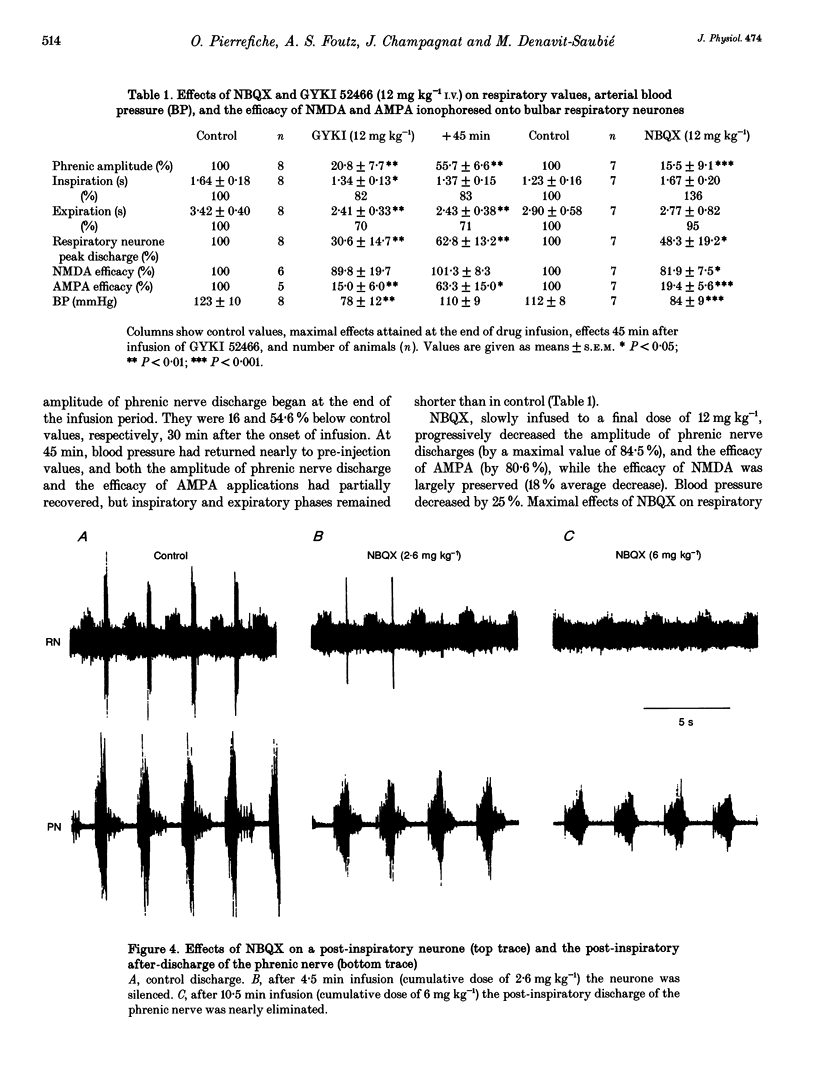
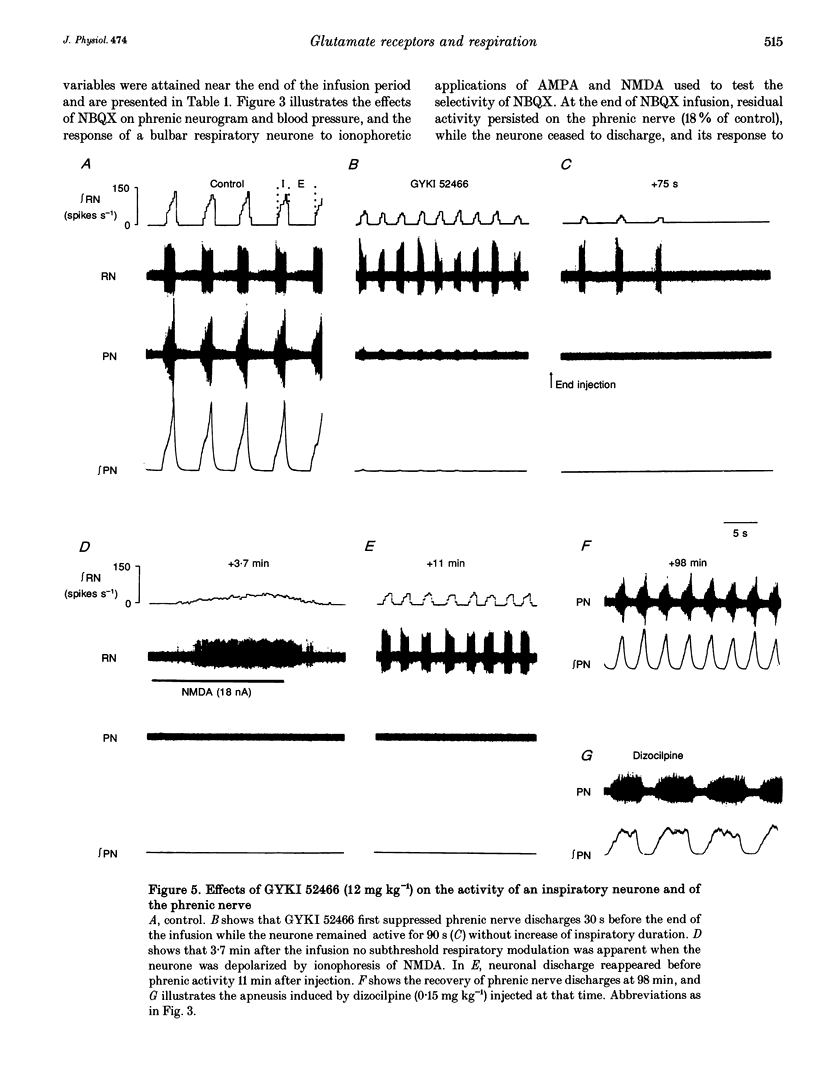





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams T. P., Hornby P. J., Walton D. P., Taveira DaSilva A. M., Gillis R. A. An excitatory amino acid(s) in the ventrolateral medulla is (are) required for breathing to occur in the anesthetized cat. J Pharmacol Exp Ther. 1991 Dec;259(3):1388–1395. [PubMed] [Google Scholar]
- Bonham A. C., Coles S. K., McCrimmon D. R. Pulmonary stretch receptor afferents activate excitatory amino acid receptors in the nucleus tractus solitarii in rats. J Physiol. 1993 May;464:725–745. doi: 10.1113/jphysiol.1993.sp019660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgard E. C., Hablitz J. J. Developmental changes in NMDA and non-NMDA receptor-mediated synaptic potentials in rat neocortex. J Neurophysiol. 1993 Jan;69(1):230–240. doi: 10.1152/jn.1993.69.1.230. [DOI] [PubMed] [Google Scholar]
- Böhmer G., Schmid K., Baumann M. Evidence for a respiration-modulated cholinergic action on the activity of medullary respiration-related neurons in the rabbit. An iontophoretic study. Pflugers Arch. 1989 Oct;415(1):72–80. doi: 10.1007/BF00373143. [DOI] [PubMed] [Google Scholar]
- Böhmer G., Schmid K., Schauer W. Evidence for an involvement of NMDA and non-NMDA receptors in synaptic excitation of phrenic motoneurons in the rabbit. Neurosci Lett. 1991 Sep 16;130(2):271–274. doi: 10.1016/0304-3940(91)90413-n. [DOI] [PubMed] [Google Scholar]
- Carlà V., Moroni F. General anaesthetics inhibit the responses induced by glutamate receptor agonists in the mouse cortex. Neurosci Lett. 1992 Oct 26;146(1):21–24. doi: 10.1016/0304-3940(92)90162-z. [DOI] [PubMed] [Google Scholar]
- Champagnat J., Denavit-Saubié M., Moyanova S., Rondouin G. Involvement of amino acids in periodic inhibitions of bulbar respiratory neurones. Brain Res. 1982 Apr 15;237(2):351–365. doi: 10.1016/0006-8993(82)90447-4. [DOI] [PubMed] [Google Scholar]
- Connelly C. A., Dobbins E. G., Feldman J. L. Pre-Bötzinger complex in cats: respiratory neuronal discharge patterns. Brain Res. 1992 Sep 11;590(1-2):337–340. doi: 10.1016/0006-8993(92)91118-x. [DOI] [PubMed] [Google Scholar]
- Dev N. B., Loeschcke H. H. A cholinergic mechanism involved in the respiratory chemosensitivity of the medulla oblongata in the cat. Pflugers Arch. 1979 Feb 14;379(1):29–36. doi: 10.1007/BF00622901. [DOI] [PubMed] [Google Scholar]
- Dillon G. H., Welsh D. E., Waldrop T. G. Modulation of respiratory reflexes by an excitatory amino acid mechanism in the ventrolateral medulla. Respir Physiol. 1991 Jul;85(1):55–72. doi: 10.1016/0034-5687(91)90006-5. [DOI] [PubMed] [Google Scholar]
- Donevan S. D., Rogawski M. A. GYKI 52466, a 2,3-benzodiazepine, is a highly selective, noncompetitive antagonist of AMPA/kainate receptor responses. Neuron. 1993 Jan;10(1):51–59. doi: 10.1016/0896-6273(93)90241-i. [DOI] [PubMed] [Google Scholar]
- Eaton S. A., Salt T. E. Thalamic NMDA receptors and nociceptive sensory synaptic transmission. Neurosci Lett. 1990 Mar 14;110(3):297–302. doi: 10.1016/0304-3940(90)90863-5. [DOI] [PubMed] [Google Scholar]
- Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog Neurobiol. 1990;35(6):429–450. doi: 10.1016/0301-0082(90)90030-k. [DOI] [PubMed] [Google Scholar]
- Foutz A. S., Champagnat J., Denavit-Saubié M. Involvement of N-methyl-D-aspartate (NMDA) receptors in respiratory rhythmogenesis. Brain Res. 1989 Oct 23;500(1-2):199–208. doi: 10.1016/0006-8993(89)90314-4. [DOI] [PubMed] [Google Scholar]
- Foutz A. S., Champagnat J., Denavit-Saubié M. N-methyl-D-aspartate (NMDA) receptors control respiratory off-switch in cat. Neurosci Lett. 1988 May 3;87(3):221–226. doi: 10.1016/0304-3940(88)90452-1. [DOI] [PubMed] [Google Scholar]
- Foutz A. S., Champagnat J., Denavit-Saubié M. Respiratory effects of the N-methyl-D-aspartate (NMDA) antagonist, MK-801, in intact and vagotomized chronic cats. Eur J Pharmacol. 1988 Sep 13;154(2):179–184. doi: 10.1016/0014-2999(88)90095-7. [DOI] [PubMed] [Google Scholar]
- Greer J. J., Smith J. C., Feldman J. L. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiol. 1991 Jun;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz A., Zieglgänsberger W., Färber G. Microelectrophoretic studies concerning the spread of glutamic acid and GABA in brain tissue. Exp Brain Res. 1969;9(3):221–235. doi: 10.1007/BF00234456. [DOI] [PubMed] [Google Scholar]
- Jung R., Bruce E. N., Katona P. G. Cardiorespiratory responses to glutamatergic antagonists in the caudal ventrolateral medulla of rats. Brain Res. 1991 Nov 15;564(2):286–295. doi: 10.1016/0006-8993(91)91465-d. [DOI] [PubMed] [Google Scholar]
- Kawasaki M., Heiligenberg W. Different classes of glutamate receptors and GABA mediate distinct modulations of a neuronal oscillator, the medullary pacemaker of a gymnotiform electric fish. J Neurosci. 1990 Dec;10(12):3896–3904. doi: 10.1523/JNEUROSCI.10-12-03896.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Feldman J. L., Smith J. C. Excitatory amino acid-mediated transmission of inspiratory drive to phrenic motoneurons. J Neurophysiol. 1990 Aug;64(2):423–436. doi: 10.1152/jn.1990.64.2.423. [DOI] [PubMed] [Google Scholar]
- Mooney R., Konishi M. Two distinct inputs to an avian song nucleus activate different glutamate receptor subtypes on individual neurons. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4075–4079. doi: 10.1073/pnas.88.10.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Pierrefiche O., Foutz A. S., Denavit-Saubié M. Pneumotaxic mechanisms in the non-human primate: effect of the N-methyl-D-aspartate (NMDA) antagonist ketamine. Neurosci Lett. 1990 Oct 30;119(1):90–93. doi: 10.1016/0304-3940(90)90763-y. [DOI] [PubMed] [Google Scholar]
- Pierrefiche O., Schmid K., Foutz A. S., Denavit-Saubie M. Endogenous activation of NMDA and non-NMDA glutamate receptors on respiratory neurones in cat medulla. Neuropharmacology. 1991 May;30(5):429–440. doi: 10.1016/0028-3908(91)90003-t. [DOI] [PubMed] [Google Scholar]
- Richter D. W. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982 Oct;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Salt T. E., Eaton S. A. Sensory Excitatory Postsynaptic Potentials Mediated by NMDA and non-NMDA Receptors in the Thalamus in vivo. Eur J Neurosci. 1991;3(3):296–300. doi: 10.1111/j.1460-9568.1991.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Schweitzer P., Pierrefiche O., Foutz A. S., Denavit-Saubié M. Effects of N-methyl-D-aspartate (NMDA) receptor blockade on breathing pattern in newborn cat. Brain Res Dev Brain Res. 1990 Nov 1;56(2):290–293. doi: 10.1016/0165-3806(90)90095-g. [DOI] [PubMed] [Google Scholar]
- Sheardown M. J., Nielsen E. O., Hansen A. J., Jacobsen P., Honoré T. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science. 1990 Feb 2;247(4942):571–574. doi: 10.1126/science.2154034. [DOI] [PubMed] [Google Scholar]
- Takeda R., Haji A. Synaptic response of bulbar respiratory neurons to hypercapnic stimulation in peripherally chemodenervated cats. Brain Res. 1991 Oct 11;561(2):307–317. doi: 10.1016/0006-8993(91)91609-5. [DOI] [PubMed] [Google Scholar]
- Tell Fabien, Jean André. Activation of N-methyl-d-aspartate Receptors Induces Endogenous Rhythmic Bursting Activities in Nucleus Tractus Solitarii Neurons: An Intracellular Study on Adult Rat Brainstem Slices. Eur J Neurosci. 1991;3(12):1353–1365. doi: 10.1111/j.1460-9568.1991.tb00068.x. [DOI] [PubMed] [Google Scholar]
- WIDDICOMBE J. G. Respiratory reflexes in man and other mammalian species. Clin Sci. 1961 Oct;21:163–170. [PubMed] [Google Scholar]
- Zorumski C. F., Thio L. L. Properties of vertebrate glutamate receptors: calcium mobilization and desensitization. Prog Neurobiol. 1992 Sep;39(3):295–336. doi: 10.1016/0301-0082(92)90020-f. [DOI] [PubMed] [Google Scholar]
- Zorumski C. F., Yamada K. A., Price M. T., Olney J. W. A benzodiazepine recognition site associated with the non-NMDA glutamate receptor. Neuron. 1993 Jan;10(1):61–67. doi: 10.1016/0896-6273(93)90242-j. [DOI] [PubMed] [Google Scholar]


