Abstract
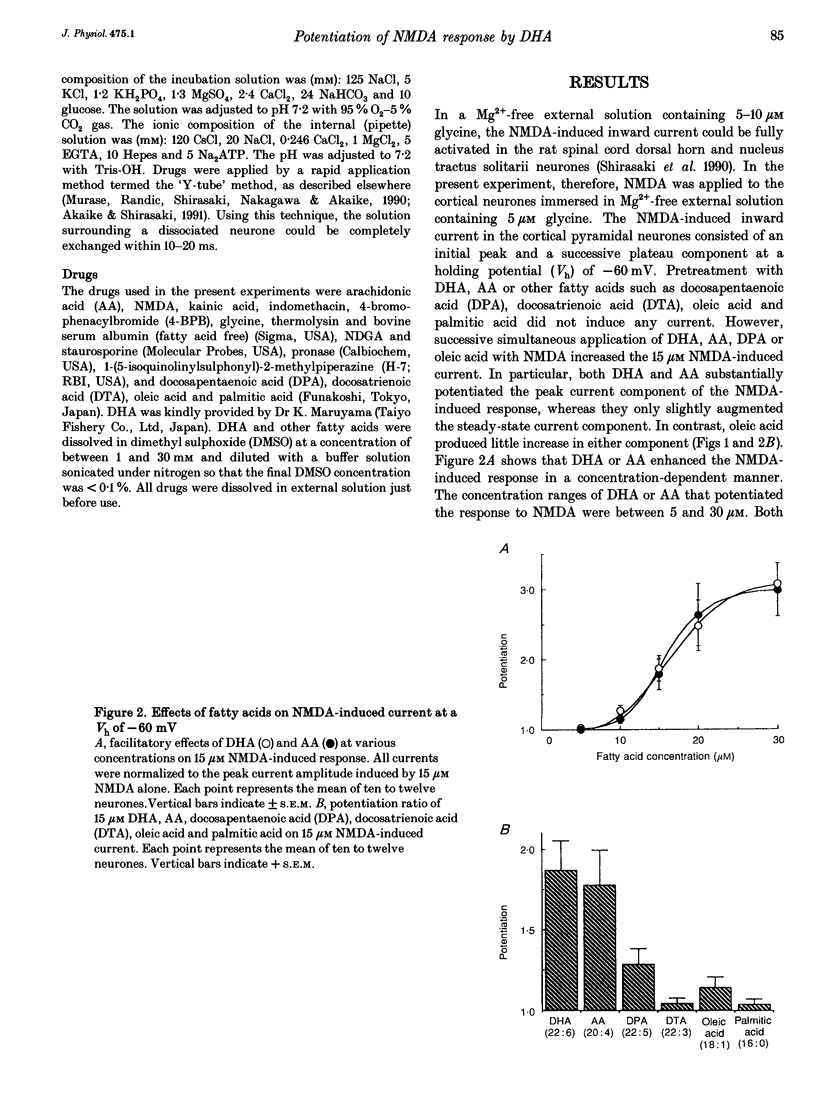
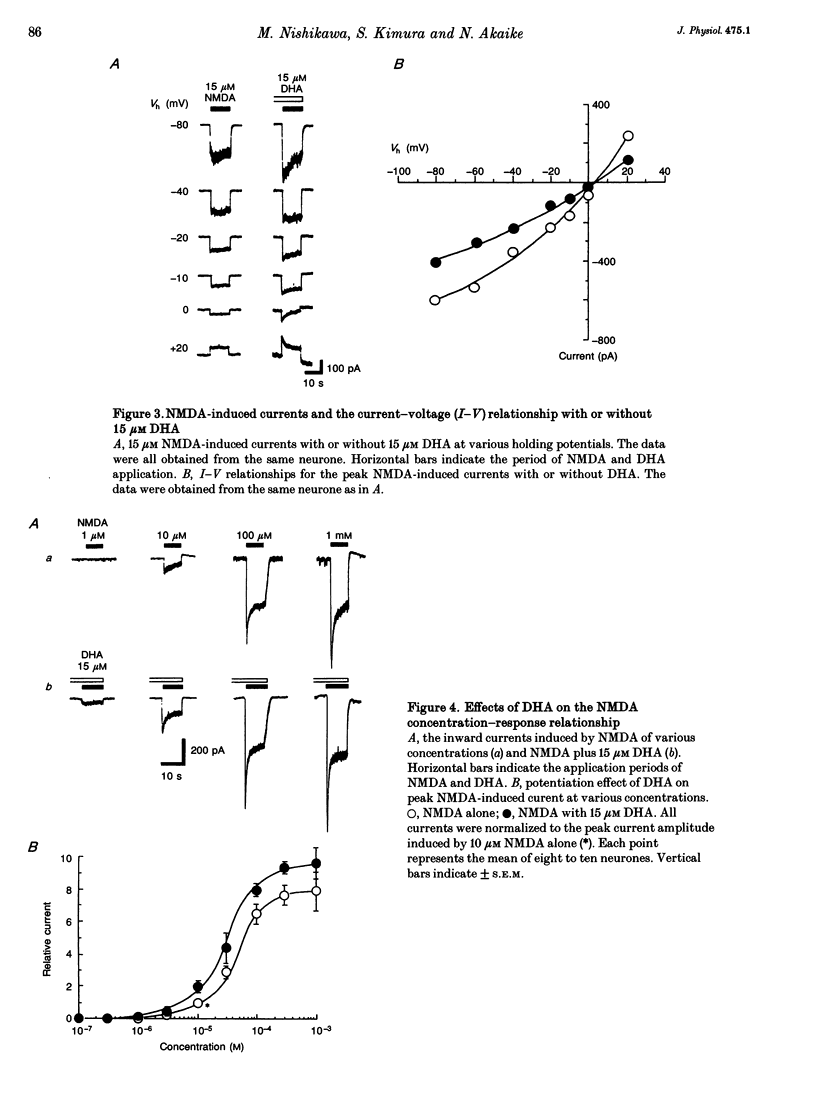
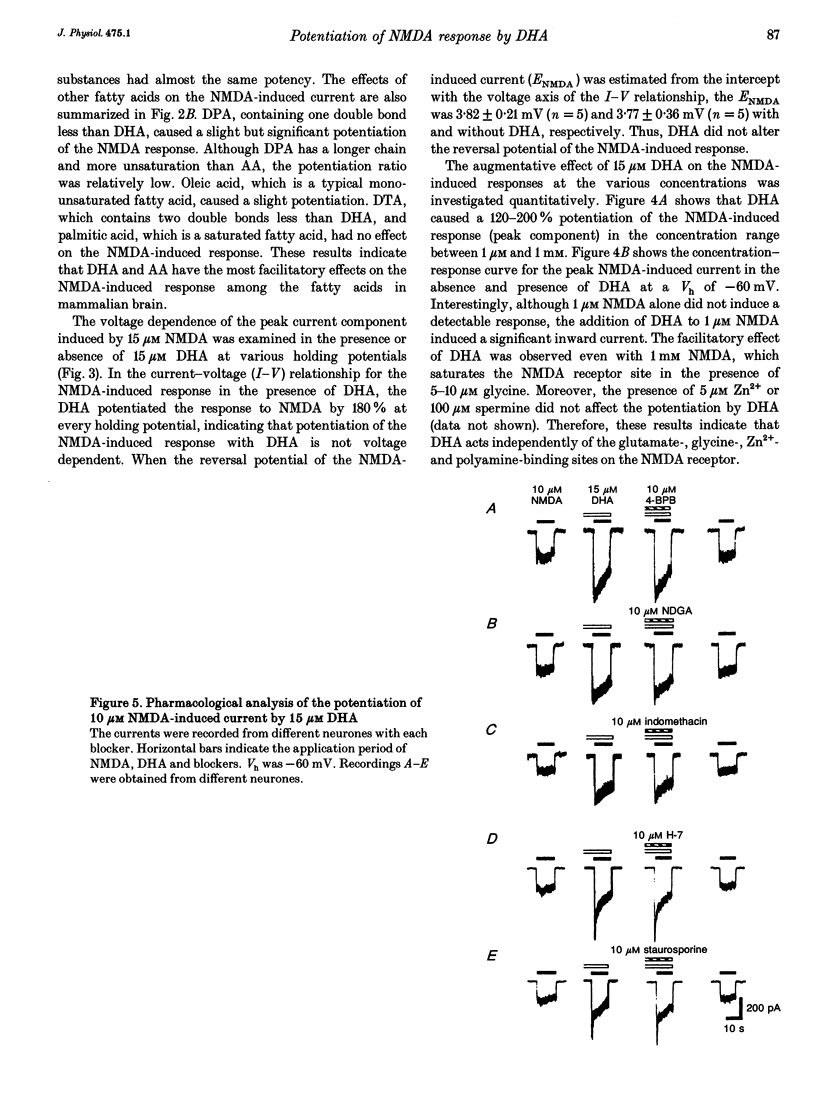
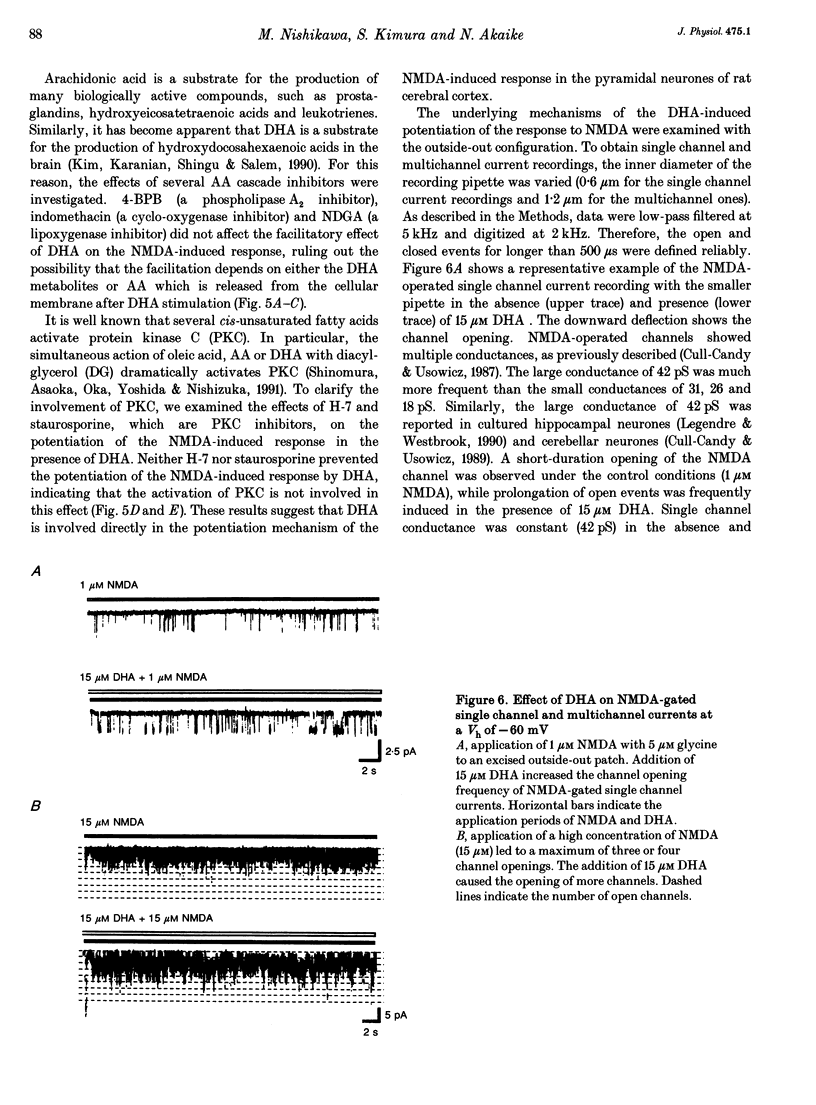
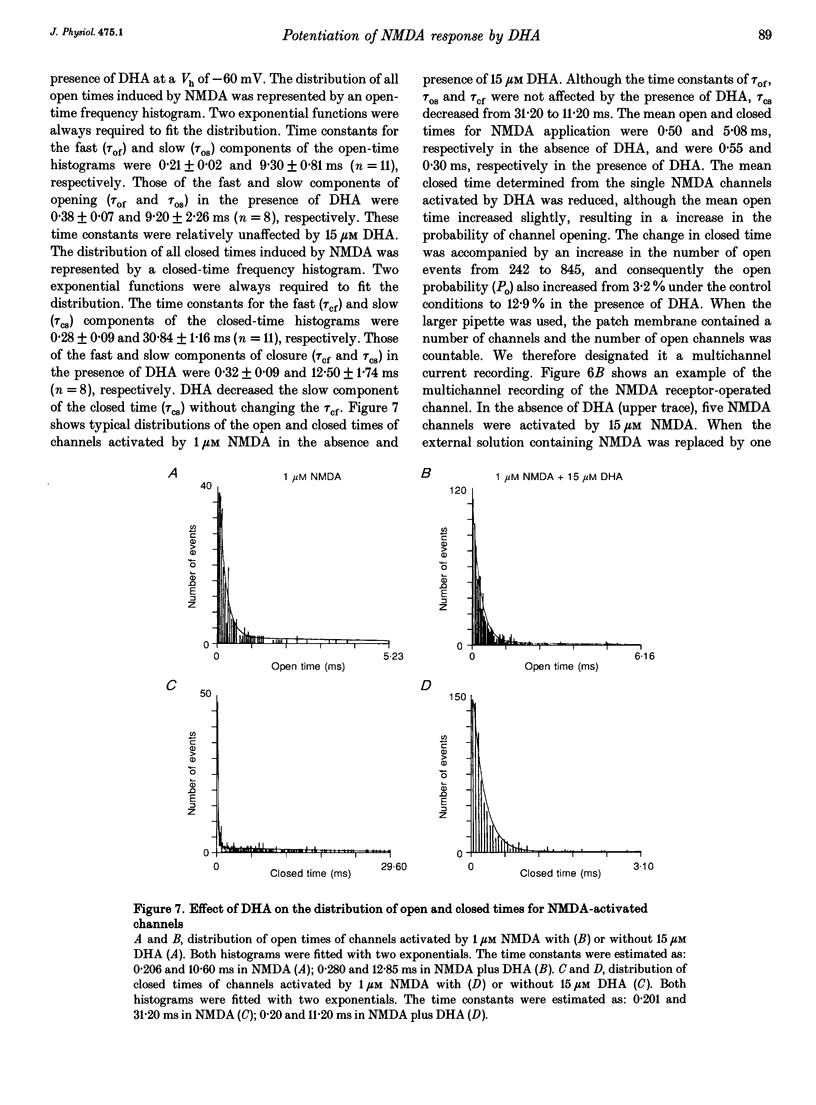
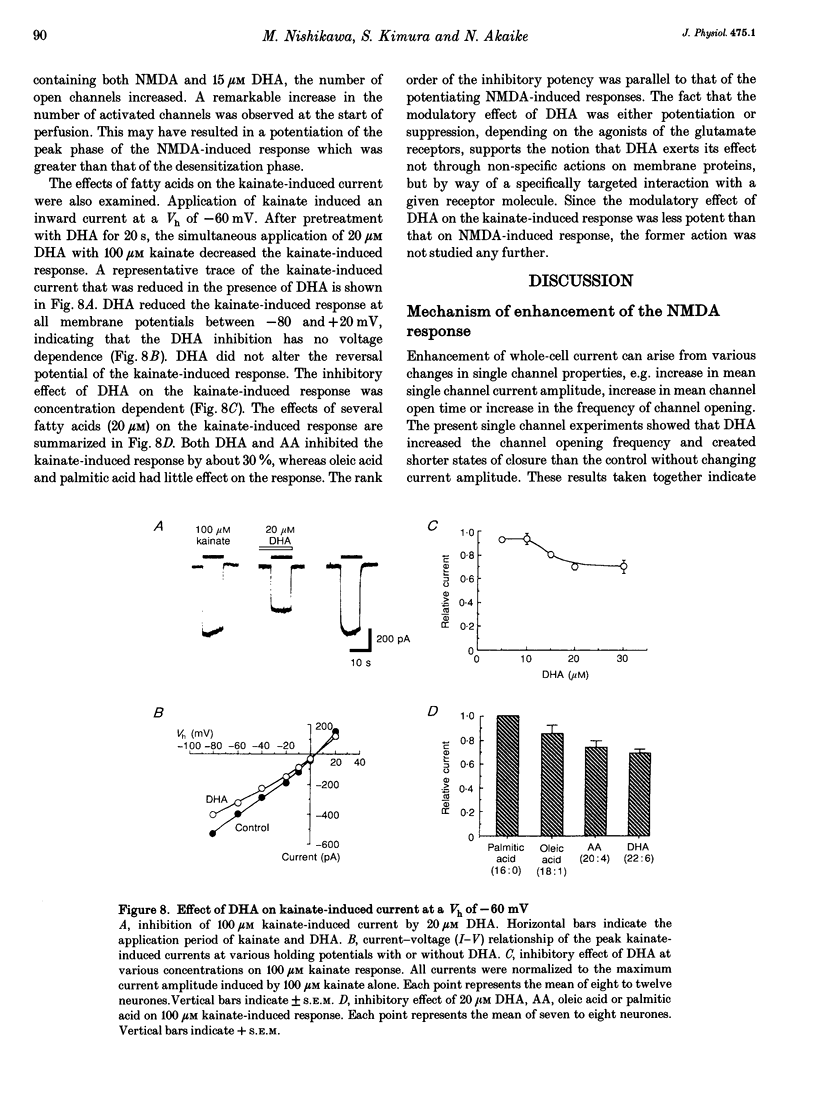
1. The effect of docosahexaenoic acid (DHA) on N-methyl-D-aspartic acid (NMDA) responses in the presence of glycine was investigated in pyramidal neurons acutely dissociated from rat cerebral cortex in whole-cell and single channel configurations. 2. DHA potentiated the NMDA-induced response but reduced the non-NMDA (kainate-induced) response in a concentration-dependent manner at a holding potential of -60 mV under voltage-clamp conditions. 3. Arachidonic acid (AA) also potentiated the NMDA-induced response in a manner similar to DHA. Oleic acid caused a slight potentiation. However, other polyunsaturated and saturated fatty acids had no such effects. 4. The facilitatory action of DHA on the NMDA-induced response was not affected by adding inhibitors of cyclo-oxygenase, lipoxygenase or phospholipase A2, suggesting that DHA may exert its facilitatory effect directly on the NMDA receptor. 5. The facilitatory action of DHA was observed in the presence of a saturating dose of NMDA. Moreover, a detailed analysis of the NMDA receptor-operated single channel currents revealed that, in the presence of DHA, the open probability of the channel increased without changing the conductance, indicating that DHA may act by binding directly to a novel site on the NMDA receptor or by altering the lipid environment of the NMDA receptor and thereby potentiating the response to NMDA. 6. The results are discussed in terms of the possibility that DHA may play an important role in the genesis of long-term potentiation, at least that involving the activation of NMDA receptors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artola A., Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987 Dec 17;330(6149):649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Douglas R. M., Errington M. L., Lynch M. A. Correlation between long-term potentiation and release of endogenous amino acids from dentate gyrus of anaesthetized rats. J Physiol. 1986 Aug;377:391–408. doi: 10.1113/jphysiol.1986.sp016193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford M. A., Casperd N. M., Sinclair A. J. The long chain metabolites of linoleic avid linolenic acids in liver and brain in herbivores and carnivores. Comp Biochem Physiol B. 1976;54(3):395–401. doi: 10.1016/0305-0491(76)90264-9. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. On the multiple-conductance single channels activated by excitatory amino acids in large cerebellar neurones of the rat. J Physiol. 1989 Aug;415:555–582. doi: 10.1113/jphysiol.1989.sp017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumuis A., Pin J. P., Oomagari K., Sebben M., Bockaert J. Arachidonic acid released from striatal neurons by joint stimulation of ionotropic and metabotropic quisqualate receptors. Nature. 1990 Sep 13;347(6289):182–184. doi: 10.1038/347182a0. [DOI] [PubMed] [Google Scholar]
- Dumuis A., Sebben M., Haynes L., Pin J. P., Bockaert J. NMDA receptors activate the arachidonic acid cascade system in striatal neurons. Nature. 1988 Nov 3;336(6194):68–70. doi: 10.1038/336068a0. [DOI] [PubMed] [Google Scholar]
- Hadjiagapiou C., Spector A. A. Docosahexaenoic acid metabolism and effect on prostacyclin production in endothelial cells. Arch Biochem Biophys. 1987 Feb 15;253(1):1–12. doi: 10.1016/0003-9861(87)90631-x. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harris E. W., Ganong A. H., Cotman C. W. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984 Dec 3;323(1):132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Ito C., Wakamori M., Akaike N. Dual effect of glycine on isolated rat suprachiasmatic neurons. Am J Physiol. 1991 Feb;260(2 Pt 1):C213–C218. doi: 10.1152/ajpcell.1991.260.2.C213. [DOI] [PubMed] [Google Scholar]
- Kauer J. A., Malenka R. C., Nicoll R. A. NMDA application potentiates synaptic transmission in the hippocampus. Nature. 1988 Jul 21;334(6179):250–252. doi: 10.1038/334250a0. [DOI] [PubMed] [Google Scholar]
- Kim H. Y., Karanian J. W., Shingu T., Salem N., Jr Stereochemical analysis of hydroxylated docosahexaenoates produced by human platelets and rat brain homogenate. Prostaglandins. 1990 Nov;40(5):473–490. doi: 10.1016/0090-6980(90)90110-h. [DOI] [PubMed] [Google Scholar]
- Legendre P., Westbrook G. L. The inhibition of single N-methyl-D-aspartate-activated channels by zinc ions on cultured rat neurones. J Physiol. 1990 Oct;429:429–449. doi: 10.1113/jphysiol.1990.sp018266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Perkel D. J., Mauk M. D., Kelly P. T., Nicoll R. A., Waxham M. N. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature. 1989 Aug 17;340(6234):554–557. doi: 10.1038/340554a0. [DOI] [PubMed] [Google Scholar]
- Martínez M. Severe deficiency of docosahexaenoic acid in peroxisomal disorders: a defect of delta 4 desaturation? Neurology. 1990 Aug;40(8):1292–1298. doi: 10.1212/wnl.40.8.1292. [DOI] [PubMed] [Google Scholar]
- Miller B., Sarantis M., Traynelis S. F., Attwell D. Potentiation of NMDA receptor currents by arachidonic acid. Nature. 1992 Feb 20;355(6362):722–725. doi: 10.1038/355722a0. [DOI] [PubMed] [Google Scholar]
- Moore S. A., Yoder E., Murphy S., Dutton G. R., Spector A. A. Astrocytes, not neurons, produce docosahexaenoic acid (22:6 omega-3) and arachidonic acid (20:4 omega-6). J Neurochem. 1991 Feb;56(2):518–524. doi: 10.1111/j.1471-4159.1991.tb08180.x. [DOI] [PubMed] [Google Scholar]
- Murase K., Randic M., Shirasaki T., Nakagawa T., Akaike N. Serotonin suppresses N-methyl-D-aspartate responses in acutely isolated spinal dorsal horn neurons of the rat. Brain Res. 1990 Aug 13;525(1):84–91. doi: 10.1016/0006-8993(90)91323-9. [DOI] [PubMed] [Google Scholar]
- Neuringer M., Connor W. E., Lin D. S., Barstad L., Luck S. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4021–4025. doi: 10.1073/pnas.83.11.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada D., Yamagishi S., Sugiyama H. Differential effects of phospholipase inhibitors in long-term potentiation in the rat hippocampal mossy fiber synapses and Schaffer/commissural synapses. Neurosci Lett. 1989 May 22;100(1-3):141–146. doi: 10.1016/0304-3940(89)90674-5. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Greengard P. Lipoxygenase metabolites of arachidonic acid in neuronal transmembrane signalling. Trends Pharmacol Sci. 1990 Sep;11(9):367–373. doi: 10.1016/0165-6147(90)90182-8. [DOI] [PubMed] [Google Scholar]
- Shinomura T., Asaoka Y., Oka M., Yoshida K., Nishizuka Y. Synergistic action of diacylglycerol and unsaturated fatty acid for protein kinase C activation: its possible implications. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5149–5153. doi: 10.1073/pnas.88.12.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki T., Nakagawa T., Wakamori M., Tateishi N., Fukuda A., Murase K., Akaike N. Glycine-insensitive desensitization of N-methyl-D-aspartate receptors in acutely isolated mammalian central neurons. Neurosci Lett. 1990 Jan 1;108(1-2):93–98. doi: 10.1016/0304-3940(90)90712-i. [DOI] [PubMed] [Google Scholar]
- Stubbs C. D., Smith A. D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984 Jan 27;779(1):89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Söderberg M., Edlund C., Kristensson K., Dallner G. Fatty acid composition of brain phospholipids in aging and in Alzheimer's disease. Lipids. 1991 Jun;26(6):421–425. doi: 10.1007/BF02536067. [DOI] [PubMed] [Google Scholar]
- Tinoco J., Babcock R., Hincenbergs I., Medwadowski B., Miljanich P. Linolenic acid deficiency: changes in fatty acid patterns in female and male rats raised on a linolenic acid-deficient diet for two generations. Lipids. 1978 Jan;13(1):6–17. doi: 10.1007/BF02533360. [DOI] [PubMed] [Google Scholar]
- Williams J. H., Bliss T. V. Induction but not maintenance of calcium-induced long-term potentiation in dentate gyrus and area CA1 of the hippocampal slice is blocked by nordihydroguaiaretic acid. Neurosci Lett. 1988 May 16;88(1):81–85. doi: 10.1016/0304-3940(88)90319-9. [DOI] [PubMed] [Google Scholar]
- Williams J. H., Errington M. L., Lynch M. A., Bliss T. V. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature. 1989 Oct 26;341(6244):739–742. doi: 10.1038/341739a0. [DOI] [PubMed] [Google Scholar]


