Abstract
Yeast contains at least two complex forms of RNA polymerase II (Pol II), one including the Srbps and a second biochemically distinct form defined by the presence of Paf1p and Cdc73p (X. Shi et al., Mol. Cell. Biol. 17:1160–1169, 1997). In this work we demonstrate that Ccr4p and Hpr1p are components of the Paf1p-Cdc73p-Pol II complex. We have found many synthetic genetic interactions between factors within the Paf1p-Cdc73p complex, including the lethality of paf1Δ ccr4Δ, paf1Δ hpr1Δ, ccr4Δ hpr1Δ, and ccr4Δ gal11Δ double mutants. In addition, paf1Δ and ccr4Δ are lethal in combination with srb5Δ, indicating that the factors within and between the two RNA polymerase II complexes have overlapping essential functions. We have used differential display to identify several genes whose expression is affected by mutations in components of the Paf1p-Cdc73p-Pol II complex. Additionally, as previously observed for hpr1Δ, deleting PAF1 or CDC73 leads to elevated recombination between direct repeats. The paf1Δ and ccr4Δ mutations, as well as gal11Δ, demonstrate sensitivity to cell wall-damaging agents, rescue of the temperature-sensitive phenotype by sorbitol, and reduced expression of genes involved in cell wall biosynthesis. This unusual combination of effects on recombination and cell wall integrity has also been observed for mutations in genes in the Pkc1p-Mpk1p kinase cascade. Consistent with a role for this novel form of RNA polymerase II in the Pkc1p-Mpk1p signaling pathway, we find that paf1Δ mpk1Δ and paf1Δ pkc1Δ double mutants do not demonstrate an enhanced phenotype relative to the single mutants. Our observation that the Mpk1p kinase is fully active in a paf1Δ strain indicates that the Paf1p-Cdc73p complex may function downstream of the Pkc1p-Mpk1p cascade to regulate the expression of a subset of yeast genes.
Initiation of eukaryotic mRNA synthesis in vitro requires RNA polymerase II (Pol II) and the general transcription factors (GTFs), including TBP, TFIIB, TFIIE, TFIIF, and TFIIH (reviewed in references 46 and 49). Regulation of transcription, however, requires additional cofactors to mediate the communication between DNA-binding activators or repressors, and Pol II and the GTFs. These cofactors include the TAFs associated with TBP to form TFIID (reviewed in references 17 and 54) and the mediator proteins, including the Srbps, associated with Pol II to form the “holoenzyme” (reviewed in reference 30). Recent work from several laboratories has shown that multiple forms of these complexes exist in different cell types and under different growth conditions (reviewed in reference 6). The different cofactor complexes therefore contribute to the complex regulatory patterns of eukaryotic genes.
We have reported the isolation and characterization of a novel form of Pol II in yeast distinct from the Srbp containing “holoenzyme” (51, 58). This Pol II complex contains the GTFs TFIIB and TFIIF but lacks TBP and TFIIH (51). The Gal11p-Sin4p-Rgr1p subcomplex is found in both forms of Pol II (35, 51). The products of the CDC73 and PAF1 genes are present in the novel form of Pol II but are not found in the Srbp-containing holoenzyme. Cdc73p and Paf1p are localized in the nucleus, and deletion of either gene causes pleiotropic phenotypes, including temperature sensitivity and slow growth (52). In contrast to the requirement for some of the Srbps for transcription of most yeast genes (55), the expression of only a few genes is affected by mutations in PAF1 and CDC73 (51, 52).
Why does yeast contain at least two complex initiating forms of Pol II? In this work we have begun to answer this question by identifying two additional proteins found uniquely in the Paf1p-Cdc73p complex but not in the Srbp-containing form of Pol II. These proteins, Ccr4p and Hpr1p, have both been implicated in the transcription of subsets of yeast genes (11, 63). Both factors have also been demonstrated to have genetic interactions with components of the transcription machinery (16, 38). By analyzing the phenotypes of single and multiple mutants of these Pol II-associated proteins, we have found that the complex appears to play a role both in recombination and in the expression of genes involved in cell wall biosynthesis. A feature that links these two seemingly disparate properties is a known connection to the protein kinase C–mitogen-activated protein (MAP) kinase signaling pathway (23, 25).
In yeast the protein kinase C homologue encoded by the PKC1 gene has been shown to play an essential role in maintenance of cell integrity. A mutation in PKC1 leads to cell lysis, which can be rescued by the osmotic stabilizer sorbitol (32, 47). Pkc1p is activated in response to alterations in the cell membrane caused by heat shock, hypoosmotic shock, or α-factor treatment (27, 62). Activated Pkc1p initiates the sequential activation of its downstream MAP kinase cascade, including Bck1p, Mkk1p-Mkk2p, and Mpk1p (Slt2p) (reviewed in reference 33). The status of the membrane may be signaled to Pkc1p by the putative transmembrane protein Slg1p (18) through a step requiring Rho1p, a small GTP-binding protein (45). Activation of Pkc1p is critically important for cell cycle progression (34), where it plays a role in bud emergence in response to signals from Cdc28p (18, 43). Pkc1p is also sensitive to the mating pathway of yeast via direct communication with the Ste20p-activated MAP kinase cascade (5). In both bud emergence and the mating pathway, activation of Pkc1p and the MAP kinase cascade controls increased synthesis of gene products required for the newly made cell walls (25).
Pkc1p also plays a less-well-characterized role in recombination in yeast. Huang and Symington (22, 23) found that mutations in PKC1 led to increased rates of mitotic recombination but that, unlike the case for expression of the cell wall biosynthetic genes, a direct connection to the MAP kinase cascade was not evident. Our discovery of a Pol II complex containing factors that affect both the expression of cell wall biosynthetic genes and frequency of recombination therefore may begin to resolve these two very different functions of Pkc1p. Our results are consistent with the possibility that the Pol II complex containing Cdc73p, Paf1p, Ccr4p, and Hpr1p functions, at least in part, to transmit signals from the PKC1 kinase cascade to target genes involved in recombination and cell wall integrity.
MATERIALS AND METHODS
Strains and media.
The Saccharomyces cerevisiae strains used in this study are shown in Table 1. Strains YJJ564, YJJ577, YJJ662, YJ664, YJJ665, YJJ681, YJJ691, YJJ693, YJJ832, YJJ854, YJJ875, YJJ879, YJJ898, YJJ899, YJJ932, YJJ935, YJJ952, YJJ954, YJJ956, and YJJ1027 were all derived from the homozygous diploid YJJ453 and are therefore isogenic. YJJ998 was created by mating YJJ577 with YJJ662 and then disrupting a single copy of the MPK1 gene in the diploid by using the construct described below. YJJ1027 and YJJ756 are isogenic with YJJ755. The HKY strains used for the recombination analyses are isogenic (16). Yeast strains were grown in YPD or synthetic medium prepared according to standard methods (19).
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| YJJ453 | MATa/α leu2Δ1/leu2Δ1 his3Δ200/his3Δ200 ura3-52/ura3-52 | 56 |
| YJJ564 | MATa leu2Δ1 his3Δ200 ura3-52 gal11Δ::LEU2 | 52 |
| YJJ577 | MATα leu2Δ1 his3Δ200 ura3-52 paf1Δ::HIS3 | 52 |
| YJJ662 | MATa leu2Δ1 his3Δ200 ura3-52 | 52 |
| YJJ664 | MATa leu2Δ1 his3Δ200 ura3-52 paf1Δ::HIS3 | 52 |
| YJJ665 | MATa leu2Δ1 his3Δ200 ura3-52 cdc73Δ::HIS3 | 52 |
| YJJ681 | MATα leu2Δ1 his3Δ200 ura3-52 cdc73Δ::HIS3 | X. Shi |
| YJJ691 | MATa leu2Δ1 his3Δ200 ura3-52 cdc73Δ::HIS3 (pEGST-CDC73) | 52 |
| YJJ693 | MATa leu2Δ1 his3Δ200 ura3-52 cdc73Δ::HIS3 (pEGST) | 52 |
| YJJ755 | MATa bar1 his6 his7 leu2 ura3 pep4 prb1 trp1 | R. Sclafani |
| YJJ756 | MATa bar1 his6 his7 leu2 ura3 pep4 prb1 trp1 paf1Δ::TRP1 | This work |
| YJJ832 | MATa leu2Δ1 his3Δ200 ura3-52 sin4::LEU2 | This work |
| YJJ854 | MATa leu2Δ1 his3Δ200 ura3-52 trg2Δ::HIS3 (pEGST-TFG2) | 52 |
| YJJ855 | MATa leu2Δ1 his3Δ200 ura3-52 (pEGST) | 52 |
| YJJ875 | MATa leu2Δ1 his3Δ200 ura3-52 srb5Δ::URA3 | This work |
| YJJ879 | MATa leu2Δ1 his3Δ200 ura3-52 ccr4Δ::URA3 | This work |
| YJJ898 | MATa leu2Δ1 his3Δ200 ura3-52 hpr1Δ::HIS3 | This work |
| YJJ899 | MATα leu2Δ1 his3Δ200 ura3-52 hpr1Δ::HIS3 | This work |
| YJJ932 | MATα leu2Δ1 his3Δ200 ura3-52 ccr4Δ::URA3 | This work |
| YJJ935 | MATα leu2Δ1 his3Δ200 ura3-52 srb5Δ::URA3 | This work |
| YJJ956 | MATa leu2Δ1 his3Δ200 ura3-52 srb5Δ::HIS3 | This work |
| YJJ952 | MATα leu2Δ1 his3Δ200 ura3-52 hpr1Δ::HIS3 (pEGST-HPR1) | This work |
| YJJ954 | MATα leu2Δ1 his3Δ200 ura3-52 hpr1Δ::HIS3 (pEGST) | This work |
| YJJ998 | MATa/MATα leu2Δ1/leu2Δ1 his3Δ200/his3Δ200 ura3-52/ura3-52 PAF1/paf1Δ::HIS3 MPK1/mpk1Δ::URA3 | This work |
| YJJ1027 | MATα his3Δ1 leu2 trp1 ura3 can1 cyh2 gal1 | R. Sclafani |
| YJJ1036 | MATa/MATα bar1 his3Δ1 his6 his7 leu2/leu2 ura3/ura3 pep4 prb1 trp1/trp1 can1 cyh2 gal1 | This work |
| HKY870-12A | MATa leu2-k::ADE2-URA3::leu2-k ura3-1 leu2-3,112 his3-11,15 trp1-1 ade2-1 can1-100 | 15 |
| HFY998-2C | MATa leu2-k::ADE2-URA3::leu2-k ura3-1 leu2-3,112 his3-11,15 trp1-1 ade2-1 can1-100 hpr1::HIS3 | 15 |
| HFY2074 | MATa leu2-k::ADE2-URA3::leu2-k ura3-1 leu2-3,112 his3-11,15 trp1-1 ade2-1 can1-100 paf1::HIS3 | 15 |
| HFY2059-1A | MATa leu2-k::ADE2-URA3::leu2-k ura3-1 leu2-3,112 his3-11,15 trp1-1 ade2-1 can1-100 cdc73::HIS3 | 15 |
| HFY2085 | MATa leu2-k::ADE2-URA3::leu2-k ura3-1 leu2-3,112 his3-11,15 trp1-1 ade2-1 can1-100 ccr4::HIS3 | 15 |
| HFY2162 | MATα leu2-k::ADE2-URA3::leu2-k ura3-1 leu2-3,112 his3-11,15 trp1-1 ade2-1 can1-100 sin4::TRP1 | 15 |
| HFY2069 | MATa leu2-k::ADE2-URA3::leu2-k ura3-1 leu2-3,112 his3-11,15 trp1-1 ade2-1 can1-100 srb5::HIS3 | 15 |
Protein-protein interaction assays.
Strains containing glutathione S-transferase (GST)-tagged forms of Tfg2p and Cdc73p have been previously described (51). hpr1Δ strains containing the pGEST vector or a GST-Hpr1p construct were created as follows. The HPR1 coding region was amplified by PCR with primer 1 (5′-CGCGGATCCATGTCTAATACCGAGGAATTG-3′) and primer 2 (5′-GCGGGATCCTTATTTCATATCTTGGGTAGATG-3′). Both primers are flanked by BamHI sites. The PCR product was digested with BamHI and ligated into pJJ560 (pGEST vector) to make an in-frame GST-HPR1 fusion under the control of a GAL promoter. The pGEST vector and the pGEST-Hpr1p construct were transformed into strain YJJ899 to create strains YJJ954 and YJJ952, respectively. The presence of the GST-Hpr1p construct, but not the vector alone, corrected the slow growth and temperature-sensitive (ts) defect of the hpr1Δ strain. The expression of GST-HPR1 in the hpr1Δ strain was confirmed by Western blot with antibody directed against Hpr1p. Transcriptionally active whole-cell extracts were prepared from yeast strains YJJ691, YJJ693, YJJ854, YJJ855, YJJ952, and YJJ954 as described previously (61). The protein concentration of the whole-cell extracts were measured by using reagents from Bio-Rad. Equal amounts of the extracts were mixed with glutathione-agarose beads in SK(20) (20 mM HEPES [pH 7.9], 20% glycerol, 10 mM MgSO4, 10 mM EGTA, 5 mM dithiothreitol [DTT], 20 mM potassium acetate, 1 mM phenylmethylsulfonyl fluoride, 0.5 μg of leupeptin per ml, 0.4 μg of bestatin per ml, 0.35 μg of pepstatin A per ml), incubated at 4°C for 2 to 4 h, and washed once with SK(20) and several times with SK(200) (30 mM HEPES [pH 7.9], 10% glycerol, 1 mM EDTA, 1 mM DTT, 200 mM potassium acetate, and protease inhibitors as specified above). The glutathione-agarose beads were spun down briefly and eluted with the sample buffer. The samples were resolved on a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel (20). Western blot analysis was performed as described by using either alkaline phosphatase or enhanced chemiluminescence (ECL) for detection (20). The sources of the antibodies used were as follows: anti-Paf1p (52), anti-Cdc73p (51), anti-Hpr1p- from M. Christman, anti-Ccr4p (42), anti-Gal11p- from T. Fukasawa, anti-Srb5p- from R. Young, and anti-TFIIS and anti-TBP (57).
Differential display and yeast mRNA analysis.
Total yeast RNA was isolated as described previously (13). Differential display analysis has been described (51). RNA samples for Northern blots were quantitated by measuring the optical density at 260 nm, equal amounts of RNA were run on 1% agarose-formaldehyde gels, and RNA blots were prepared according to standard methods (50). [α-32P]dATP-labeled probes were prepared by random priming (50). An 18S rRNA oligonucleotide probe was labeled with T4 kinase and [γ-32P]ATP (50). Northern blots were quantitated by PhosphorImager analysis.
Double deletion and tetrad analysis.
Appropriate deletion strains were crossed to obtain the desired diploid strains, which were sporulated, and at least 30 tetrads for each diploid were dissected (19). The genotypes of the individual spores were determined by analysis of the different markers used to replace the deleted genes. In some cases, PCR was used to confirm the genotype of the spores.
Construction of deletion strains.
SRB5 coding and flanking regions were amplified by using primer 1 (5′-TGCAGCAGCTAAACCTCCAC-3′) and primer 2 (5′-GACGATGACGAAGAGCTAC-3′). The PCR product was ligated into pGEM-T (Promega) vector. Primer 3 (5′-ACGCAAGCTTTTCTTCTTAATATGGAATAC-3′) and primer 4 (5′-GACGAAGCTTATAATCATTGGCACCTGG-3′) were used to amplify the resulting plasmid. The PCR product was then cut with HindIII and ligated with the 1.2-kb HindIII URA3 fragment from YEp24 (4) to give pJJ995. pJJ995 was cut with HindIII, blunt ended with Klenow, and ligated with the BamHI/XhoI-cut and Klenow-treated 1.4-kb HIS3 fragment from YIp1 (53) to give pJJ1072. pJJ995 or pJJ1072 was cut with SpeI/SacII and used to transform YJJ453. The MPK1 deletion construct was made as follows. MPK1 coding and flanking regions were amplified with primer 1 (5′-ATGGCTGATAAGATAGAGAGG-3′) and primer 2 (5′-AGGAATTCAAGAGGCGATAAC-3′). The PCR product was ligated into the PCRII (Invitrogen) vector. The resulting plasmid was partially digested with HindIII, and the 4.2-kb fragment was ligated with the 1.2-kb HindIII URA3 fragment from YEp24 (4) to give pJJ1143. pJJ1143 was cut with EcoRI and used to transform YJJ453. The CCR4 deletion construct was as described earlier (42). The SIN4 deletion construct was derived from M1381 containing sin4Δ::LEU2 (from D. Stillman) which is similar to the sin4Δ::TRP1 disruption described by Jiang and Stillman (26). The HPR1 deletion construct hpr1Δ::HIS3 was as described earlier (2). The deletion constructs described above were transformed into diploid strain YJJ453. The paf1Δ::TRP1 disruptor was made as follows. Primer P1 (5′-CTTAGCACAACTGAATTCGAAAGG-3′) and primer P4 (5′-ATACGAATGATGTTAATGGAGACTCCAGGATTGTCGACT-3′) were used to PCR amplify the paf1Δ::HIS3 construct from genomic DNA (YJJ664). The PCR product was subcloned into the pGEMT vector (Promega) to give pJJ902. pJJ902 was cut with XhoI/BamHI to release the 1.2-kb HIS3 fragment. The vector fragment was gel purified and ligated with the 900-bp SalI/BglII TRP1 fragment, which resulted in pJJ904. pJJ904 was linearized with SpeI/SacII and used to transform yeast strain YJJ755 to obtain paf1Δ strain YJJ756. The pkc1Δ::LEU2 disruptor was made as follows. The PKC1 coding and flanking sequences were amplified from yeast genomic DNA (YJJ755) by PCR with primer 1 (5′-AACTGCAGCATGAGTTTTTCACAATTGTAG-3′) and primer 2 (5′-AACTGCAGTCATGGCATGACCTTTTCTTC-3′).
The PCR product was cut with PstI, gel purified, and ligated into pJJ998 (a modified pGEM-T vector) at the PstI sites. The resulting plasmid pJJ1193 was cut with StuI to release a 1.3-kb internal PKC1 fragment, and the vector fragment was gel purified and ligated to the 2.2-kb HpaI LEU2 fragment from YEp13. The resulting plasmid pJJ1217 was linearized with PstI prior to transforming a diploid yeast strain from a cross between YJJ756 and YJJ1027 to obtain the heterozygous diploid YJJ1036. Southern blotting and PCR analysis were used to confirm the gene replacements. Sporulation and tetrad dissection were used to obtain haploid deletion strains (19).
Enzymatic assays.
CYC1 and FKS1 promoter-lacZ fusion reporter plasmids used in β-galactosidase assays were as previously described (25, 28). Yeast cells transformed with the plasmids (19) were grown in Ura-Casamino Acid media supplemented with 4% glucose. Extract preparation and β-galactosidase assays were performed as described elsewhere (44). Mpk1p kinase assays with hemagglutin (HA)-tagged Mpk1p to phosphorylate MBP were as described by Zarzov et al. (62).
Determination of recombination rates.
Recombination rates were calculated according to the median method of Lea and Coulson (31) as described previously (1).
RESULTS
Ccr4p and Hpr1p are in the Paf1p-Cdc73p-Pol II complex.
Paf1p and Cdc73p were originally identified as proteins associated with a transcriptionally active form of Pol II immobilized by an antibody directed against the nonphosphorylated form of the C-terminal domain (CTD [58]). The proteins bound to Pol II and eluted with moderate salt included TFIIB, the subunits of TFIIF, TFIIS, Paf1p, Cdc73p, Gal11p, and Sin4p, as well as approximately 13 other polypeptides, but did not include TBP, TFIIH, the Srbps or the Swip-Snfp factors (58). To confirm that these Pol II-associated proteins defined a form of Pol II distinct from the Srbp-containing holoenzyme, we created strains bearing GST-tagged forms of Cdc73p and Paf1p functionally replacing the CDC73 and PAF1 genes in yeast cells and analyzed the composition of the complexes isolated by using glutathione-agarose chromatography (51). These experiments confirmed that Cdc73p and Paf1p are found in a distinct complex with Pol II, TFIIB, TFIIF, Gal11p, and Sin4p. Elongation factor TFIIS apparently defines at least one additional form of Pol II, since it was not in any of the GST-tagged complexes nor is it present in the Srbp-containing holoenzyme (51).
Like Cdc73p and Paf1p, Ccr4p and Hpr1p have both been reported to affect the expression of subsets of yeast genes (11, 63, 37, 38), to be required for growth at high temperature (42, 14), and to be associated with large complexes of proteins distinct from the Srbp-containing holoenzyme (63, 38). We therefore tested the possibility that Ccr4p and Hpr1p were also present in the GST-tagged Cdc73p-Pol II complex. As shown in Fig. 1A, both Ccr4p and Hpr1p are found in the GST-Cdc73p complex (Fig. 1A, lane 8) and in a complex isolated via a GST tag on the Tfg2p subunit of TFIIF (Fig. 1A, lane 4); neither protein is found in the GST alone control lanes (Fig. 1A, lanes 2 and 6). In addition, we have found that a GST-tagged form of Paf1p colocalizes with Hpr1p and Ccr4p (data not shown) and that a GST-tagged Hpr1p construct copurifies with Pol II, Cdc73p, Paf1p, and Ccr4p (Fig. 1B, lane 4, and data not shown).
FIG. 1.
Hpr1p and Ccr4p are present in the Paf1p-Cdc73p-Pol II complex. Proteins separated on SDS polyacrylamide gels were transferred and probed with antibodies directed against Hpr1p and Ccr4p as indicated. ECL was used for antibody detection. (A) Transcription-competent whole-cell extracts (WCE) were isolated and used as a source to purify GST-tagged Tfg2p, Cdc73p, and associated proteins by glutathione agarose chromatography as described in Materials and Methods. Lanes 1, 3, 5, and 7 (labeled IP) contain the input WCE from the indicated strains; lanes 2, 4, 6, and 8 (labeled B) contain the proteins bound to the glutathione agarose beads. Lanes 1 and 2 (labeled WT-GST) are from wild-type (YJJ662) cells transformed with the GST vector alone; lanes 3 and 4 (labeled GST-TFG2) are from the tfg2Δ mutant complemented with GST-Tfg2p (YJJ854); lanes 5 and 6 (labeled cdc73Δ-GST) are from cdc73Δ (YJJ665) mutant cells transformed with the GST vector; and lanes 7 and 8 (labeled GST-CDC73) are from the cdc73Δ mutant complemented by GST-Cdc73p (YJJ691). (B) Transcription-competent WCE were isolated and used as a source to purify GST-tagged Hpr1p and associated proteins by glutathione agarose chromatography as described in Materials and Methods. Lanes labeled IP and B are as described in panel A. Lanes 1 and 2 (labeled hpr1Δ-GST) are from an hpr1Δ strain transformed with the GST vector alone (YJJ954); lanes 3 and 4 (labeled GST-HPR1) are from an hpr1Δ strain transformed with GST-Hpr1p (YJJ952). (C) Fractions from antibody affinity chromatography performed as described in Wade et al. (58). Lanes: WCE, 40 μg of protein from a transcription competent WCE; α-ς70, 10 μl of the salt-eluted fraction from a control column containing antibody directed against the ς70 subunit of E. coli RNA polymerase; α-CTD, 10 μl of the salt-eluted fraction from a column containing antibody directed against the C-terminal domain of the largest subunit of RNA Pol II.
We also looked for Hpr1p and Ccr4p in the original collection of affinity-isolated proteins associated with Pol II used to identify Paf1p and Cdc73p (58). As shown in Fig. 1C, both proteins are present in the fraction affinity purified with the anti-CTD antibody (lane 3) but not in a control fraction (lane 2). The association of Ccr4p and Hpr1p with the Paf1p-Cdc73p-Pol II complex is quite stable, since it is resistant to washing with 0.5 M potassium acetate and 0.5 M ammonium sulfate (data not shown). Therefore, analyzing complexes isolated by two very different purification strategies—GST-tagged complexes and an antibody affinity isolated Pol II complex—we find that Paf1p, Cdc73p, Hpr1p, and Ccr4p are found together in a stable complex with Pol II, TFIIB, TFIIF, and the Gal11p and Sin4p subcomplex. Although we cannot entirely rule out that we are looking at multiple complexes by these techniques, the fact that Paf1p, Cdc73p, and Hpr1p each colocalizes with each other and with Ccr4p is consistent with these factors being present in one Pol II-associated complex.
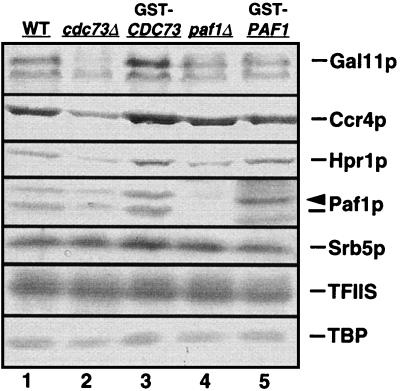
We have shown that Cdc73p can associate directly with purified RNA Pol II (51), but we do not have any additional information about the assembly of this complex of proteins. We therefore analyzed the effect of mutating the CDC73 and PAF1 genes on the abundance of the other factors in the complex. In particular, we were interested in the possibility that the elimination of these proteins might destabilize other complex components. We found that the cdc73Δ and paf1Δ mutations did not reduce the abundance of transcripts from the GAL11, CCR4, HPR1, or SRB5 genes. Mutations in PAF1 and CDC73 also had little or no effect on each other’s transcript abundance (data not shown). However, as shown in Fig. 2, the cdc73Δ mutation but not the paf1Δ mutation appears to significantly reduce the abundance of Gal11p and Ccr4p and, to a lesser extent, Paf1p and Hpr1p (Fig. 2, lane 2) when compared to either the isogenic wild-type strain (Fig. 2, lane 1), to the deletion strain complemented by the GST-tagged form of Cdc73p (Fig. 2, lane 3), or to either of the paf1Δ strains (Fig. 2, lanes 4 and 5).
FIG. 2.
A mutation in the CDC73 gene affects the abundance of Ccr4p, Hpr1p, Gal11p, and Paf1p. The abundance of the indicated proteins was analyzed as described in Materials and Methods in different transcription-competent WCEs. Antibodies were detected with alkaline phosphatase. Lanes 1, 2, and 4 show WCEs from wild-type (WT; YJJ662), cdc73Δ (YJJ665), and paf1Δ (YJJ664) cells, respectively, transformed with the GST vector alone. Lanes 3 and 5 show cdc73Δ and paf1Δ mutant strains complemented by GST-Cdc73p (YJJ691) and GST-Paf1p (YJJ676), respectively. The arrowhead above Paf1p points to the position of the GST-Paf1p protein seen in lane 5. Proteins absent from the Paf1p-Cdc73p-Pol II complex, including Srb5p, TFIIS, and TBP, are used as loading controls.
Protein levels of factors not found in the Paf1p-Cdc73p-Pol II complex, including TBP, TFIIS, and Srb5p, remain unchanged in the cdc73Δ strain (Fig. 2, compare lane 2 with lanes 1, 3, 4, and 5). Since the cdc73Δ mutation did not reduce mRNA abundance for any of the genes analyzed, these results suggest that Cdc73p may play a role in the assembly or stabilization of the Pol II complex. The reduced abundance of Gal11p, Ccr4p, Hpr1p, and Paf1p in the cdc73Δ strain further supports the idea that these proteins are present in the same complex.
Identification of transcripts affected by mutations in Paf1p-Cdc73p-Pol II complex components.
Mutations in PAF1 and CDC73 affect the transcript abundance of only a very few yeast genes (51, 52). In general, mutation of PAF1 affects the expression of more transcripts than mutation of CDC73, a finding consistent with its more severe phenotype. For example, we have previously shown that inducible GAL gene expression measured in the chromosomal GAL7+10 genes and in a GAL promoter-reporter construct is reduced five- to eightfold in paf1Δ but is relatively unaffected by cdc73Δ (52). We have also analyzed the inducible expression of the GAL1,7+10 genes in isogenic ccr4Δ and hpr1Δ strains. We found that GAL gene induction in a ccr4Δ strain was actually enhanced in both rate and extent, while there was little or no effect on GAL gene expression in an hpr1Δ strain (data not shown). Therefore, although these proteins are clearly found together in a complex, their effects on gene expression are not equivalent.
We have previously reported the use of differential display (36) to identify genes whose expression is differentially affected by mutations in PAF1, CDC73, and GAL11 (51). We have extended this analysis to the identification of genes differentially expressed in ccr4Δ, hpr1Δ, and srb5Δ strains. A partial collection of the genes identified is shown in Fig. 3. Although some of the effects shown in Fig. 3 appear to be subtle (less than twofold), note that the data represent the averages of six to nine independent repetitions of the RNA analyses. Therefore, as indicated by the use of error bars, the differences shown are in many cases significant relative to the levels in the wild-type strain. These studies have identified some known genes, including CYC1, RSP7B, PRS1, and PFK26, and some open reading frames of unknown function. As described above for the inducible GAL1,7+10 genes, the effects on transcript abundance are not the same for mutations in different genes of the complex. The most common pattern of expression observed is that transcripts are reduced in abundance two- to fourfold in paf1Δ (note CYC1, RSP7B, PRS1, YBR265, and YNR067c), and there are several examples where transcript abundance is significantly increased in hpr1Δ (note PRS1, PFK26, and YLR346c). However, as previously reported, in some cases transcript abundance increases in paf1Δ (note the fourfold increase of YLR346c). In most of the examples shown the cdc73Δ and srb5Δ mutations have little effect on transcript abundance, although expression of YNR067c is reduced in these backgrounds. As previously observed by Liu et al. (38), ccr4Δ leads to both positive (YLR346c) and negative (CYC1) effects on transcription.
FIG. 3.
Transcripts identified by differential display are differentially expressed in isogenic paf1Δ, cdc73Δ, gal11Δ, srb5Δ, ccr4Δ, and hpr1Δ mutant strains. Differential display was performed as described in Materials and Methods. DNA encoding the differentially expressed transcripts was cloned and sequenced to identify the yeast gene. RNA was isolated from the indicated isogenic strains and probed for transcripts from each gene as described in Materials and Methods. Abundance was determined with a PhosphorImager and was normalized to the signal for 18S rRNA. The data is presented relative to a transcript abundance in wild type set as 1, which is shown as a dashed line in each panel. The results shown represent the averages and standard deviations from six to nine separate RNA isolations. The yeast strains used for RNA isolation were paf1Δ-YJJ664, cdc73Δ-YJJ665, gal11Δ-YJJ564, srb5Δ-YJJ875, ccr4Δ-YJJ879, and hpr1Δ-YJJ898.
Although we cannot rule out that some of the changes in transcript abundance may be due to secondary effects, we can confirm, as previously demonstrated for the paf1Δ reduction of GAL7+10 transcription (52), that the effects are at the level of initiation of transcription by using a promoter fusion-reporter construct as shown in Table 2. Using the CYC1 promoter-regulatory region fused to β-galactosidase, we have confirmed the reduction in expression seen by the RNA analysis shown in Fig. 3. Liu et al. (38) have reported that ccr4Δ reduces expression of a CYC1-lacZ reporter construct about eightfold, which is somewhat greater than the fivefold reduction in CYC1 mRNA levels seen in Fig. 3. The effect of paf1Δ is also more dramatic with the reporter construct, with expression reduced over 12-fold compared to the 4-fold reduction seen in the RNA analysis. In the srb5Δ strain we saw no differences in CYC1-lacZ expression relative to the wild-type strain (data not shown), a result consistent with the results presented in Fig. 3. It is therefore clear that the reduced abundance of CYC1 mRNA seen in the ccr4Δ and paf1Δ strains in Fig. 3 is due to effects on the promoter-driven initiation of transcription.
TABLE 2.
Expression of promoter-reporter constructs is reduced in the paf1Δ straina
| Strain | β-Galactosidase activity (U/mg) with promoter:
|
|
|---|---|---|
| CYC1 | FKS1 | |
| Wild type | 24,000 ± 1,500 | 20,000 ± 3,200 |
| paf1Δ | 1,900 ± 370 | 2,600 ± 150 |
Extracts from wild-type (YJJ662) and paf1Δ mutant (YJJ664) strains bearing the pF712-380 pFKS1-lacZ reporter plasmid (25) or the AJ-1 CYC1-lacZ reporter plasmid (28) were prepared and assayed as described in Materials and Methods. β-Galactosidase activity (± the standard error of the mean) is expressed in units per milligram of protein, where 1 U is defined as the A420 × 103 per minute. Values are derived from replicate assays of at least three different transformants.
Genetic interactions between PAF1, CDC73, CCR4, and HPR1.
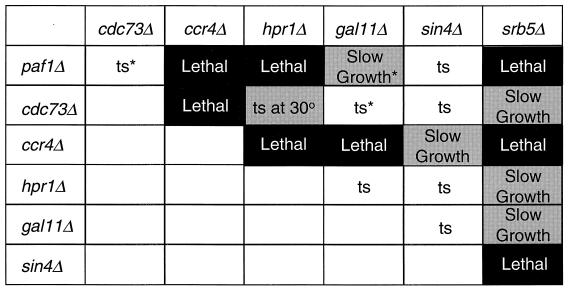
We have previously reported that a paf1Δ cdc73Δ double mutant is viable and does not show an enhanced phenotype (51), while a paf1Δ gal11Δ double mutant demonstrates a dramatically enhanced slow-growth phenotype (52). These data are consistent with the idea that Paf1p and Gal11p have at least partially overlapping essential functions in the yeast cell, with Paf1p and Cdc73p participating in the same pathway such that a double mutant is no more deleterious than either single mutation. To determine the genetic interactions between PAF1, CDC73, and the newly identified components of the Pol II complex, CCR4 and HPR1, we created double mutants in an isogenic background as described in Materials and Methods. Many of the double-mutant analyses were repeated in other genetic backgrounds with identical results. We also evaluated the effects of combining mutations in these genes with mutations in other holoenzyme components, including gal11Δ, sin4Δ, and srb5Δ. Based on the results of 30 or more tetrads for each doubly mutant diploid, we found extensive evidence for genetic interactions, as summarized in Fig. 4. We found that combining a paf1Δ mutation with either ccr4Δ or hpr1Δ results in lethality. Deletion of CCR4 is also lethal in combination with cdc73Δ, gal11Δ, or hpr1Δ and has an enhanced phenotype with sin4Δ. In each case the nonviable spores did germinate and formed microcolonies. The combination of cdc73Δ and hpr1Δ results in an enhanced ts phenotype in which the permissive temperature is reduced from 30 to 22°C. These results, like our earlier analysis of the paf1Δgal11Δ double mutant (51), support the idea that the components of this novel Pol II complex have redundant essential functions for the yeast cell.
FIG. 4.
Synthetic genetic interactions between pairwise combinations of factors in RNA Pol II complexes. Tetrad analysis of heterozygous diploids obtained by sporulating crosses between isogenic single deletion mutants and tetrad dissection. At least 30 tetrads were dissected for each diploid analyzed. All of the single deletion mutants are ts at 38°C. The growth phenotypes of the double-deletion mutants are shown in the figure, with shaded areas highlighting significant synthetic interactions (solid box, synthetic lethality; gray box, synthetic enhancement). The genotypes of inviable spores were deduced by the markers used to delete the genes. “Slow growth” means that the double mutants grew significantly more slowly than either of the parents. The “ts at 30°” means that the permissive temperature for the double mutant is reduced to 22°C. The “ts” in an unshaded box indicates that the phenotype of the double mutant was not significantly worse than either of the parent strains. The asterisks refer to previously published results (51, 52) included for completeness. The strains used in the crosses were paf1Δ-YJJ664 or -YJJ577; cdc73Δ-YJJ665 or YJJ681; gal11Δ-YJJ564; sin4Δ-YJJ832 srb5Δ-YJJ956, -YJJ935, or -YJJ875; ccr4Δ-YJJ932 or -YJJ879 and hpr1Δ-YJJ898 or -YJJ899.
In addition to the genetic interactions between factors within the Paf1p-Cdc73p-Pol II complex, we also found dramatic genetic interactions between mutations in SRB5 and mutations in PAF1, CDC73, CCR4, HPR1, GAL11, and SIN4. As described above, most of the double mutants were also created and analyzed in other genetic backgrounds with identical results. The srb5Δ mutation is lethal in combination with paf1Δ, ccr4Δ, or sin4Δ and has an enhanced slow-growth phenotype in combination with cdc73Δ, hpr1Δ, or gal11Δ. This suggests that there are redundant essential functions both within and between the two Pol II complexes.
The Paf1p-Cdc73p-Pol II complex is involved in recombination.
The differential display and genetic analyses described above suggested that factors within the Paf1p-Cdc73p-Pol II complex have some similar functions. Aguilera and Klein (1) reported that the hpr1 mutation leads to increased levels of recombination between direct repeats. We therefore asked if mutations in the other factors in the Paf1p-Cdc73p-Pol II complex demonstrated a similar phenotype. As shown in Table 3, the recombination rates in the paf1Δ and cdc73Δ strains increased 82- and 45-fold, respectively, relative to the wild type, while a 700-fold increase was observed in the hpr1Δ strain. In contrast, recombination rates were essentially unchanged relative to wild type in ccr4Δ, sin4Δ, and srb5Δ strains. This result strongly supports the idea that defects in some components of a Pol II complex, specifically the Paf1p-Cdc73p-Pol II complex, lead to elevated levels of recombination. Whether the complex is directly involved in recombination or the formation of a recombinogenic substrate, or indirectly affecting the expression of genes required for this process is discussed further below.
TABLE 3.
Mutations in HPR1, PAF1, and CDC73 lead to elevated rates of recombination between direct repeats
| Genotypea | Recombination rate (10−6)b | Fold increase |
|---|---|---|
| Wild type | 3.8 ± 1.8 | 1 |
| hpr1Δ | 2,700 ± 1,400 | 700 |
| paf1Δ | 320 ± 50 | 82 |
| cdc73Δ | 170 ± 40 | 45 |
| ccr4Δ | 4.1 ± 1.9 | 1 |
| sin4Δ | 3.0 ± 1.7 | 1 |
| srb5Δ | 9.2 ± 4.8 | 2 |
Strains used in this table are wild type (HKY870-12A), hpr1Δ (HFY988-2C), paf1Δ (HFY2074), cdc73Δ (HFY2059-1A), ccr4Δ (HFY2085), sin4Δ (HFY2162), and srb5Δ (HFY2069).
Rates were calculated as described in Materials and Methods from 5-FOA resistance frequencies of strains carrying the duplication leu2-k::ADE2-URA3::leu2-k. Each rate was calculated from three independent fluctuation tests on three strains of the same genotype and is expressed as the mean rate ± the standard deviation of the three determinations.
Cdc73p, Paf1p, and Ccr4p are required for the integrity of the cell wall.
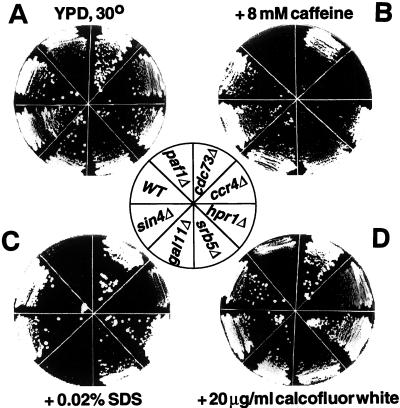
Both paf1Δ and ccr4Δ show enlarged cell morphology and use glycerol as a carbon source very poorly (52, 10). In addition, ccr4Δ is hypersensitive to staurosporine and to 8 mM caffeine and 0.04% SDS (21, 37, 38), an indication of weakened cell walls (8, 39). We therefore tested the possibility that other components of the Paf1p-Cdc73p-Pol II complex might also have cell wall defects. As shown in Fig. 5, both paf1Δ and ccr4Δ are hypersensitive to growth on 8 mM caffeine (Fig. 5B) and somewhat sensitive to growth on 20 μg of calcofluor per ml (Fig. 5D), another assay for cell wall integrity (39, 48). These mutant strains and the gal11Δ strain are inhibited by growth on 0.02% SDS (Fig. 5C). A plasmid expressing PAF1 can correct the caffeine and calcofluor sensitivity phenotypes of paf1Δ, confirming that the phenotypes are caused by deletion of the PAF1 gene (data not shown). In contrast, isogenic cdc73Δ, srb5Δ, hpr1Δ, and sin4Δ strains are no more sensitive to these cell wall-damaging agents than is the wild-type strain (Fig. 5).
FIG. 5.
Mutations in PAF1, CCR4, and GAL11 lead to increased sensitivity to cell wall-damaging agents. Isogenic wild type (WT; YJJ662) and paf1Δ (YJJ664), cdc73Δ (YJJ665), gal11Δ (YJJ564), srb5Δ (YJJ875), ccr4Δ (YJJ879), sin4Δ (YJJ832), and hpr1Δ (YJJ898) were grown on YPD or YPD plus the indicated additions. The cells were allowed to grow at 30°C for 3 to 4 days.
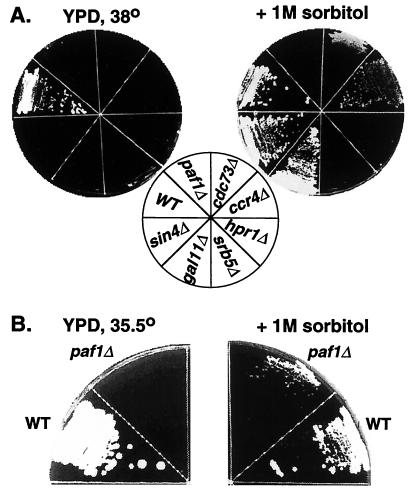
Defects in the yeast cell wall can be compensated for by osmotic stabilizers such as sorbitol. It has previously been shown that the caffeine and temperature sensitivity of ccr4Δ can be corrected by the addition of 1 M sorbitol to the medium (37, 38). We confirmed this observation in the genetic background used in this work and found that the ts phenotype of gal11Δ can also be corrected by sorbitol (Fig. 6A). Although cdc73Δ and sin4Δ are not obviously sensitive to the cell wall-damaging agents shown in Fig. 5, they are at least partially rescued at high temperature by sorbitol (Fig. 6A), indicating that these mutations do cause some cell wall defects. Although paf1Δ cannot be rescued by sorbitol at 38°C (Fig. 6A), it does show partial rescue at 35.5°C (Fig. 6B). We have also found that inclusion of sorbitol in liquid culture medium significantly reduces the doubling time of paf1Δ strains at 30°C (data not shown). Sorbitol is not sufficient to rescue srb5Δ and hpr1Δ, a finding consistent with the fact that they are not sensitive to cell wall-damaging agents. These results demonstrate that at least part of the growth defect in the paf1Δ, ccr4Δ, and possibly the cdc73Δ strains is due to defective cell wall formation.
FIG. 6.
The ts phenotype of paf1Δ, cdc73Δ, ccr4Δ, and gal11Δ can be corrected by the cell wall-stabilizing agent sorbitol. (A) Isogenic wild type (WT; YJJ662), paf1Δ (YJJ664), cdc73Δ (YJJ665), gal11Δ (YJJ564), srb5Δ (YJJ875), ccr4Δ (YJJ879), sin4Δ (YJJ832), and hpr1Δ (YJJ898) strains were grown on YPD or YPD plus 1 M sorbitol at 38°C for 4 days. (B) Isogenic wild-type (WT; YJJ662) and paf1Δ (YJJ664) strains were grown on YPD or YPD plus 1 M sorbitol at 35.5°C for 2.5 days.
Expression of cell wall biosynthetic genes is affected by paf1Δ, cdc73Δ, and ccr4Δ.
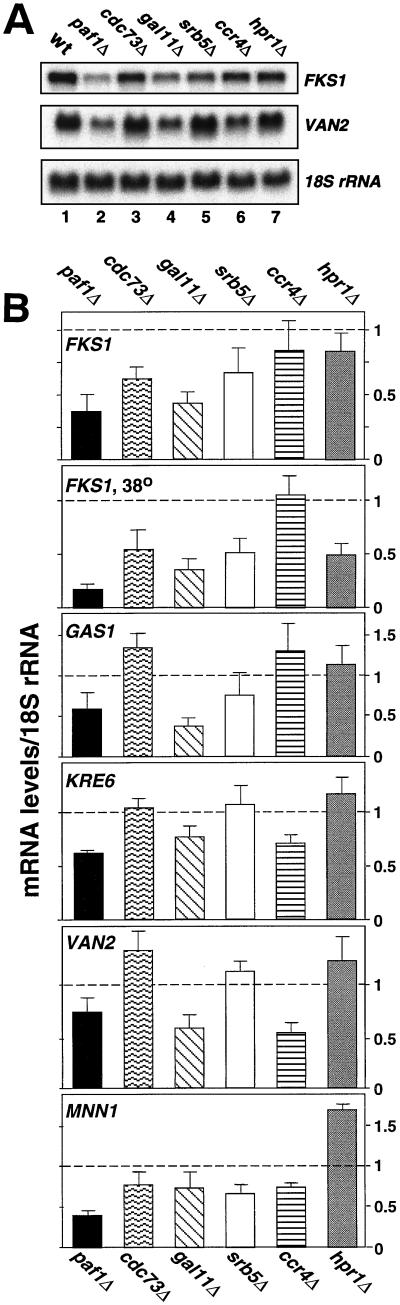
The two phenotypes described above, i.e., increases in recombination between direct repeats and defects in cell wall integrity, have both been observed for mutations in yeast protein kinase C (PKC1) (23, 47). Although the direct connection between recombination and PKC1 is not yet clear, Igual et al. (25) have provided a partial explanation for the cell wall defects by measuring a reduction in expression of several key genes in cell wall biosynthesis (FKS1, MNN1, GAS1, KRE6, and VAN2) in pkc1 and mpk1 mutant strains. We therefore analyzed the effects of mutations in the Paf1p-Cdc73p-Pol II complex on the expression of the FKS1, MNN1, GAS1, KRE6, and VAN2 genes as shown in Fig. 7. A representative example of some of the RNA analyses are shown in Fig. 7A, and a quantitation of three independent sets of samples are shown in Fig. 7B. We found that the paf1Δ mutation causes a two- to threefold decrease in the expression of several of these genes at the permissive temperature, and a four- to fivefold reduction in FKS1 expression at 38°C. For comparison, Igual et al. (25) reported that mutations in PKC1 and in MPK1 (SLT2), the terminal MAP kinase in the PKC1 signaling cascade, reduced FKS1 mRNA abundance from 1.5- to 2-fold at 30°C and from 3- to 4-fold at 38°C. In addition, they found that MNN1 and GAS1 expression was reduced two- to threefold, while there was little affect on the abundance of the KRE6 and VAN2 mRNAs (25).
FIG. 7.
Expression of genes involved in cell wall biosynthesis is reduced in paf1Δ ccr4Δ and gal11Δ mutants. (A) Total yeast RNA was prepared from isogenic mutant strains and probed for the indicated genes as described in Materials and Methods. (B) The results shown are based on three or more independently isolated sets of RNA. The RNA abundance was normalized to 18S rRNA, and the wild-type value is set as 1, which is shown as a dashed line in each panel. The RNA in the panel labeled FKS1 at 38°C was isolated from cells shifted from 30 to 38°C and incubated for 5 h. The yeast strains used for RNA isolation were wild type-YJJ662, paf1Δ-YJJ664, cdc73Δ-YJJ665, gal11Δ-YJJ564, srb5Δ-YJJ875, ccr4Δ-YJJ879, and hpr1Δ-YJJ898.
FKS1 expression is not significantly reduced in the ccr4Δ strain, a finding consistent with the observation of Liu et al. (38) that expression from an FKS1 promoter-reporter construct is relatively unaffected by a mutation in CCR4. However, expression of VAN2 and KRE6 is reduced in the ccr4Δ strain and expression of FKS1, GAS1, and VAN2 is reduced in the gal11Δ strain. Although the cdc73Δ mutation has little effect on most of the cell wall genes assayed, it does cause an almost twofold reduction in FKS1 expression, which may help to explain the rescue by sorbitol described above. The hpr1Δ and srb5Δ strains show little effect on expression of any of these genes at 30°C, a finding that is consistent with their resistance to cell wall-damaging agents. We also confirmed that the effect of the paf1Δ mutation was at the level of initiation of transcription using an FKS1-promoter-lacZ reporter construct and measuring β-galactosidase activity in the mutant strain. As shown in Table 2, we observed an almost eightfold reduction in activity from the reporter construct in the paf1Δ strain relative to the wild type, which is very similar to the effect observed for a mutation in PKC1 (25). These results are consistent with the theory that the Paf1p-Cdc73p-Pol II complex is involved in the Pkc1p-dependent pathway affecting cell wall biosynthesis.
The Paf1p-Cdc73p-Pol II complex functions in the Pkc1p-Mpk1p MAP kinase cascade.
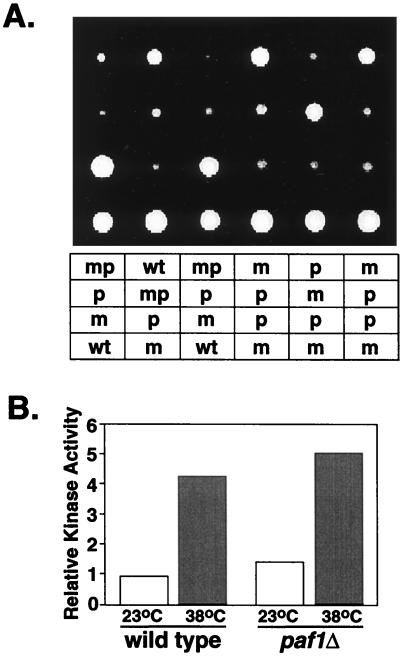
If the Paf1p-Cdc73p-Pol II complex functions in the same pathway as the Pkc1p-MAP kinase cascade, then combining a mutation in PKC1 or MPK1 with a PAF1 mutation should not have a more deleterious effect on cell wall integrity than either single mutation. The phenotype of mutations in MPK1 are less severe and more variable in different strains than is the phenotype of a mutation in PKC1 (43). We therefore created paf1Δ mpk1Δ double mutants in two different genetic backgrounds which varied in the extent of the effect of the mpk1Δ mutation. Spores dissected from both mpk1Δ-MPK1 paf1Δ-PAF1 heterozygous diploids were all viable (Fig. 8A). The double-mutant spores were slow growing, ts and caffeine sensitive, and indistinguishable from the paf1Δ parent. We also created paf1Δ pkc1Δ double mutants and again found that all of the doubly mutant spores were viable and dependent on sorbitol like the pkc1Δ parent (data not shown). The paf1Δ pkc1Δ double mutant did grow somewhat more slowly than either of the single mutations. However, when we analyzed the abundance of FKS1 mRNA in the paf1Δ mpk1Δ and paf1Δ pkc1Δ double mutants we found no additional reduction in expression relative to the single mutants (data not shown). We conclude from these results that the similar effects of the PAF1 gene and the PKC1 and MPK1 genes on cell wall biosynthesis are due to their participation in the same regulatory pathway.
FIG. 8.
Interactions between PAF1 and MPK1. (A) A strain heterozygous for paf1Δ and mpk1Δ (YJJ998) was sporulated, and tetrads were dissected. The figure shows 6 of 30 tetrads, all of which showed similar results. The genotype of the spores was determined from the markers associated with the deletions. wt, Wild type; m, mpk1Δ::URA3; p, paf1Δ::HIS3; mp, mpk1Δpaf1Δ. (B) Cell extracts were prepared from wild type (WT; YJJ755) and paf1Δ (YJJ756) strains containing a 3HA-tagged form of Mpk1p and grown at the indicated temperatures. The tagged Mpk1p was isolated and used to phosphorylate the MAP kinase substrate MBP as described by Zarzov et al. (62). The data represent the phosphorylation of MBP normalized to the amount of HA-tagged Mpk1p in each extract.
Does the Paf1p-Cdc73p-Pol II complex function up- or downstream of the MAP kinase cascade? To position the Pol II complex in this pathway, we asked if the paf1Δ phenotype could be corrected by overexpression of Pkc1p or Mpk1p. We found that the addition of neither single- nor high-copy plasmid forms of PKC1 nor high-copy forms of MPK1 would correct the phenotype of a mutation in PAF1 (data not shown), which is consistent with the possibility that the Paf1p-Cdc73p-Pol II complex functions downstream of the PKC1-MPK1 cascade. If the Paf1p-Cdc73p-Pol II complex does function downstream of the kinase cascade, then the terminal kinase, Mpk1p, should be fully active in paf1Δ and cdc73Δ strains under conditions which activate Pkc1p (heat shock). We confirmed this supposition by measuring the activity of a tagged form of Mpk1p isolated from paf1Δ and cdc73Δ strains. In both cases, Mpk1p was as active in the mutant strains as in the wild-type strain (Fig. 8B and data not shown). Paf1p and Cdc73p therefore appear to function downstream of the activation of the kinase cascade, presumably at the level of transcription of the genes affected by Pkc1p and Mpk1p.
DISCUSSION
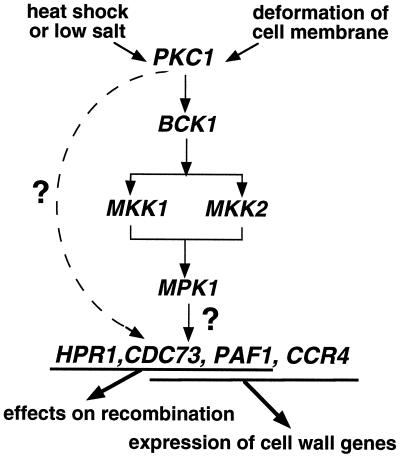
A large and growing collection of proteins has been identified as mediators or coactivators critical for communicating signals from transcriptional activators and repressors to RNA Pol II (reviewed in reference 6). In this work we have extended the description of a unique collection of proteins associated with Pol II, each of which appears to function as a transcriptional mediator. The first components of this complex to be identified, Paf1p and Cdc73p, are not encoded by essential genes but are required for the normal expression of a subset of yeast genes (51, 52). In this work we have shown that Ccr4p and Hpr1p, also encoded by nonessential genes, are also components of this Pol II complex, where they play a role in the expression of subsets of yeast genes. Although mutations in PAF1, CDC73, HPR1, and CCR4 lead to complex changes in expression patterns and, in some cases, to very different phenotypes, we have established that many of the functions of the Pol II complex are consistent with a specific role in the protein kinase C signaling cascade. In particular we have found that Paf1p, Ccr4p, and possibly Cdc73p play a role in the expression of genes required for cell wall biosynthesis and that Paf1p, Cdc73p, and Hpr1p are required for normal levels of recombination between direct repeats. As outlined in the introduction, similar effects on both the expression of cell wall biosynthetic genes and on recombination have been associated with defects in PKC1 (see Fig. 9).
FIG. 9.
A model for the interactions between the Paf1p-Cdc73p-Pol II transcription complex and the Pkc1p-Mpk1p protein kinase cascade. An explanation of the model is provided in the text.
Paf1p, Cdc73p, Hpr1p, and Ccr4p are found together in a complex with Pol II.
We have shown that Hpr1p and Ccr4p are present in the previously described Paf1p-Cdc73p-Pol II complex. Two very different isolation procedures, affinity isolation of a transcriptionally active form of RNA Pol II and isolation of GST-tagged forms of Cdc73p, Paf1p, Hpr1p, and Tfg2p have established that these four proteins exist in a complex with RNA Pol II, TFIIB, TFIIF, and the Gal11p-Rgr1p-Sin4p subcomplex. None of these proteins is present in the Srbp-containing form of Pol II (38, 51, 63), which further supports our earlier evidence that the complex is biochemically distinct from the mediator-containing “holoenzyme.” We cannot currently rule out the possibility that more than one complex containing subsets of these proteins exists in yeast cells, but the fact that Hpr1p and Ccr4p colocalize with tagged forms of both Cdc73p and Paf1p, and the fact that Ccr4p, Paf1p, and Cdc73p are all found in a tagged Hpr1p complex is consistent with this being a single complex. Does this complex, like the Srbp-containing form of Pol II (29), have homologues in other eukaryotes? Currently, there are no examples of homologues of Paf1p, Cdc73p, or Hpr1p, although homologues of Ccr4p can be found in the database. Since the Paf1p-Cdc73p form of Pol II is present in low amounts in yeast cells (51), the failure to have observed homologues in other systems may be due to low abundance of the gene products or a restricted distribution of the factors.
Although a major fraction of Paf1p and Cdc73p appears to be in the Pol II-associated complex (51), a smaller fraction of Hpr1p colocalizes with Paf1p and Cdc73p (Fig. 1), and Ccr4p is clearly not exclusively in this Pol II complex. There is evidence that Ccr4p is present in at least one other distinct complex in the yeast cell associated with Caf1p, Not1p, Not2p, and Not3p (21, 38). We have found little or no Caf1p, Not1p, Not2p, or Not3p in the Paf1p-Cdc73p-Pol II complex or in the original collection of affinity-isolated proteins associated with Pol II (data not shown), so the Pol II complex described in this work is clearly distinct from the Not complex. Consistent with the evidence that Ccr4p is present in more than one complex, it also appears to play multiple roles in the expression of genes, based on the fact that many of its properties are quite different than those of Paf1p. For example, despite some overlap, the spectrum of transcripts affected by the two genes is quite different (Fig. 3 and 7 and data not shown). In addition, a mutation in CCR4 causes sensitivity to staurosporine (21) and to 6-azauracil (data not shown); neither of these phenotypes is observed in paf1 or cdc73 mutant strains; hpr1 mutants are slightly sensitive to 6-azauracil but not to staurosporine (7; data not shown). Finally, a mutation in CCR4 leads to subtle defects in the cell cycle (37), while we have not observed any cell cycle effects of mutations in PAF1 or CDC73.
Phenotypes of paf1, cdc73, hpr1, and ccr4 mutations are consistent with a role in Pkc1p signaling.
Although the phenotypes of mutations in the different Pol II-associated factors are not identical, there are some shared patterns, including the facts that mutations in PAF1 and CCR4 cause sensitivity to caffeine, calcofluor, and SDS (Fig. 5); the ts phenotype of mutations in CDC73 and CCR4, and to a lesser extent, PAF1 can be rescued by sorbitol (Fig. 6); and mutations in HPR1, PAF1, and CDC73 all result in elevated rates of recombination (Table 3).
The mechanism for the sensitivity to the cell wall-damaging agents can be directly explained, at least in part, by the effects of the mutations on the expression of certain genes involved in cell wall biosynthesis. For example, mutations in FKS1 and KRE6 are hypersensitive to calcofluor (8, 39); a PAF1 mutation reduces the abundance of both mRNAs, and a CCR4 mutation reduces the abundance of KRE6 mRNA (Fig. 7). In addition, a mutation in GAS1, whose mRNA abundance is reduced by paf1, causes sensitivity to SDS (39). The complete explanation of the altered patterns of expression leading to the sensitivity to cell wall-damaging agents is, however, far more complex than that shown in Fig. 7. Lussier et al. (39) recently reported the identification of 82 yeast genes whose products are important for cell wall integrity. It is interesting that the genetic screen used in this analysis did not identify PAF1 or CCR4, confirming the authors’ conclusion that there are many additional genes yet to be identified. Knowledge of the expression patterns of many or all of these genes will be required to understand the defects in the various pathways. It is, however, useful to note that the patterns that we observe for the cell wall biosynthetic genes shown in Fig. 7 are very similar to the alterations in mRNA abundance observed by Igual et al. (25) for mutations in PCK1 and MPK1, indicating that much of the effect of the mutations in the kinase-encoding genes can be explained by the properties of the Pol II-associated factors.
Connections between transcription and recombination.
To establish that the effects of the mutations in the Pol II-associated factors were at the level of transcriptional initiation, we used promoter-reporter constructs to confirm the results of the RNA analysis described above. Although the results of the β-galactosidase reporter assays agreed with the mRNA analyses, the effects were more dramatic in the reporter assays. For example a PAF1 mutation reduces the level of FKS1 mRNA 2.5-fold but reduces the level of expression from the FKS1 promoter construct nearly 8-fold (Fig. 7 and Table 2). We saw similar effects with CYC1 mRNA and a CYC1 reporter construct with 3- to 4-fold and 12-fold reductions, respectively, in a paf1 background (Fig. 3 and Table 2). This same phenomenon has been observed by Fan and Klein (14) and Chavez and Aguilera (7) for expression of GAL10 and GAL1 mRNAs versus expression from promoter-fusion constructs in an HPR1 mutant background. Chavez and Aguilera (7) have shown that the discrepancy is due to an elongation pause site in the lacZ coding region causing reduced abundance of full-length RNA in hpr1 cells. Does the fact that we see a similar discrepancy for paf1 indicate that these genes play a direct role in transcriptional elongation? This seems unlikely since we have shown that unlike GAL1 expression in hpr1, the level of GAL1,7+10, CYC1, and FKS1 mRNAs (plus many other mRNAs shown in Fig. 3 and 7) are significantly reduced in paf1 and, in some cases, the ccr4 and cdc73 backgrounds. It is probable that the additional effects that we see with the reporter constructs are due to secondary effects on elongation through the lacZ gene.
Chavez and Aguilera (7) have also speculated that the elongation pause site in lacZ is the cause of the increased levels of recombination seen in the constructs used for analyzing changes in resolution of direct repeats. Mutations in PAF1, like mutations in HPR1, increase recombination and show enhanced effects with lacZ reporter constructs relative to results with direct RNA analysis. Therefore, our results are consistent with the idea that transcriptional pauses in the lacZ gene correlate with increased recombination. We cannot rule out the possibility that mutated forms of the Paf1p-Cdc73p-Pol II complex are directly involved in a transcriptional pause leading to increased levels of recombination. However, this complex also contains initiation factors TFIIB and TFIIF and was originally identified in association with the nonphosphorylated, initiating form of RNA Pol II (51, 58). In addition, mutations in PAF1 and CDC73 do not result in sensitivity to 6-azauracil (data not shown), a phenotype often indicative of defects in transcriptional elongation (3, 7). It is therefore equally likely at this point that the effects of Hpr1p, Paf1p, and Cdc73p on recombination may be indirect through changes in expression of factors required for transcriptional elongation or recombination. Since a mutation in HPR1 often leads to increased mRNA abundance (see Fig. 3 and 7), perhaps increased expression of a factor responsible for formation of a recombinogenic substrate leads to the dramatic increases in recombination.
The Paf1p-Cdc73p-Pol II complex is downstream of the Pkc1p-MAP kinase cascade.
As described above, many of the phenotypes of mutations in the PKC1-MPK1 pathway are mimicked by mutations in PAF1, CDC73, CCR4, and HPR1. The observation that paf1 mpk1 and paf1 pkc1 double mutants do not demonstrate an enhanced phenotype (Fig. 8 and data not shown), strongly supports the theory that all of these factors function in the same pathway. Our results do not rule out the possibility that the Pol II complex is integrating inputs from more than one signaling pathway. The fact that many double mutants of factors within the complex (paf1 ccr4, paf1 hpr1, cdc73 ccr4, and ccr4 hpr1) are synthetically lethal and cannot be rescued by sorbitol is consistent with this view. Our results are therefore in agreement with the conclusions of Hata et al. (21), who determined that Ccr4p might be functioning in both the Pkc1p and an independent Caf1p(Pop2p) pathway. The existence of distinct complexes and pathways is also consistent with the fact that Caf1p is not present in the Paf1p-Cdc73p-Pol II complex.
Where is the Paf1p-Cdc73p-Pol II complex in the signaling pathway? The following points support the model shown in Fig. 9, placing the Pol II complex downstream of the kinase cascade in a position to directly affect the transcription of target genes. First, we have found that overexpression of Pkc1p or Mpk1p does not suppress a paf1 defect (data not shown). Second, although the abundance of PKC1 mRNA is reduced about twofold in paf1 cells, we have found little or no effect of mutations in the other polymerase-associated factors on the expression of PKC1 (data not shown), thus indicating that the defect is not due to critically reduced levels of Pkc1p. Third, as shown in Fig. 8B, the terminal MAP kinase, Mpk1p, is fully activated by heat stress in a paf1 strain, indicating that the paf1 defect is not due to failure of the kinase cascade to be activated. Finally, although the phenotypes of paf1 encompass many of the defects seen in a pkc1 mutant strain, mutations in HPR1 and CCR4 each only encompass a subset of the phenotypes, indicating that these factors are more likely to be downstream of Pkc1p.
One difficulty in trying to determine the order of the factors in this pathway is the variable phenotype of mutations in MPK1. As demonstrated by Madhani et al. (41), complete deletion of a MAP kinase as done in this study and in many other analyses of the PKC1-MPK1 pathway can result in inaccurate conclusions about the necessity for the kinase. It is possible that when the kinase protein is absent other related gene products can compensate for the deficiency, whereas when a defective form of the kinase protein is present a negative phenotype might be observed. Since another potential MAP kinase encoded by the YKL161C gene (24) has also been shown to play a role in cell wall integrity (60), it may be that the role of both kinases will have to be evaluated to arrive at a clear picture of the biochemical signaling pathway. These same issues may help to explain why Huang and Symington (22, 23) found that mutations in PKC1, but not in other downstream components of the pathway, lead to increases in recombination. Redundant factors in the cascade may mask the effects on recombination in the deletion strains used for these analyses. Without further clarification of this issue, we have used a separate dashed line in Fig. 9 to indicate the uncertainty in the path of the signal from Pkc1p to the Pol II complex for this activity.
There are two possible models for how the Pol II-associated factors respond to the signals from the kinase cascade. First, Mpk1p may directly phosphorylate one or more of the Pol II-associated factors and alter their activity or ability to assemble into the complex. We have, in fact, observed that Cdc73p is a substrate for Mpk1p in vitro (data not shown). However, we have not detected any direct association of Mpk1p with the polymerase-associated factors. A second possibility, in which the Paf1p-Cdc73p-Pol II complex responds to changes in the phosphorylation state of known targets of Mpk1p, currently seems more likely. Mpk1 is known to associate with and/or phosphorylate several transcriptional regulatory factors, including the SBF complex composed of Swi4p and Swi6p (40), Rlm1p (12, 59, 60), and the HMG-1-like proteins Nhp6Ap and Nhp6Bp (9). There is evidence that each of these factors plays a role in the Pkc1p-Mpk1p pathway leading to maintenance of cell wall integrity. Ultimately, it will be interesting to study the interactions between the Paf1p-Cdc73p-Pol II complex and these regulatory factors to determine the role of the polymerase-associated factors in the expression of Pkc1p-responsive genes.
ACKNOWLEDGMENTS
We thank A. Johnson, L. Johnston, D. Stillman, and M. Snyder for disruption and expression constructs; R. Sclafani for helpful advice and yeast strains; M. Christman for the anti-Hpr1p antibody; R. Young for the anti-Srb5p antibody; T. Fukasawa for the anti-Gal11p antibody; and L. Johnston for the RNA probes.
These studies were supported by NIH grants GM 38101 (J.A.J.), GM 30439 (H.K.), and GM 41215 (C.L.D.).
REFERENCES
- 1.Aguilera A, Klein H L. Genetic control of intrachromosomal recombination in Saccharomyces cerevisiae. I. Isolation and genetic characterization of hyper-recombination mutations. Genetics. 1988;119:779–790. doi: 10.1093/genetics/119.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilera A, Klein H L. HPR1, a novel yeast gene that prevents intrachromosomal excision recombination shows carboxy-terminal homology to the Saccharomyces cerevisiae TOP1 gene. Mol Cell Biol. 1990;10:1439–1451. doi: 10.1128/mcb.10.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archambault J, Lacroute F, Ruet A, Friesen J D. Genetic interaction between transcription elongation factor TFIIS and RNA polymerase II. Mol Cell Biol. 1992;12:4142–4152. doi: 10.1128/mcb.12.9.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botstein D, Falco S C, Stewart S E, Brennan M, Scherer S, Stinchcomb D T, Struhl K, Davis R W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979;8:17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- 5.Buehrer B M, Errede B. Coordination of the mating and cell integrity mitogen-activated protein kinase pathways in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:6517–6525. doi: 10.1128/mcb.17.11.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang M, Jaehning J A. A multiplicity of mediators: alternative forms of transcription complexes communicated with transcriptional regulators. Nucleic Acids Res. 1997;25:4861–4865. doi: 10.1093/nar/25.24.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavez S, Aguilera A. The yeast HPR1 gene has a functional role in transcriptional elongation that uncovers a novel source of genome stability. Genes Dev. 1997;11:3459–3470. doi: 10.1101/gad.11.24.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cid V J, Duran A, Del Rey F, Snyder M P, Nombela C, Sanchez M. Molecular basis of cell integrity and morphogenesis in Saccharomyces cerevisiae. Microbiol Rev. 1995;59:345–386. doi: 10.1128/mr.59.3.345-386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costigan C, Kolodrubetz D, Snyder M. NPH6A and NHP6B, which encode HMG1-like proteins, are candidates for downstream components of the yeast SLT2 mitogen-activated protein kinase pathway. Mol Cell Biol. 1994;14:2391–2403. doi: 10.1128/mcb.14.4.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denis C L. Identification of new genes involved in the regulation of yeast alcohol dehydrogenase II. Genetics. 1984;108:833–844. doi: 10.1093/genetics/108.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denis C L, Malvar T. The CCR4 gene from Saccharomyces cerevisiae is required for both nonfermentative and spt-mediated gene expression. Genetics. 1990;124:283–291. doi: 10.1093/genetics/124.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodou E, Treisman R. The Saccharomyces cerevisiae MADS-box transcription factor Rlm1 is a target for the Mpk1 mitogen-activated protein kinase pathway. Mol Cell Biol. 1997;17:1848–1859. doi: 10.1128/mcb.17.4.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elder R T, Loh E Y, Davis R W. RNA from the yeast transposable element Ty1 has both ends in the direct repeats, a structure similar to retrovirus RNA. Proc Natl Acad Sci USA. 1983;80:2432–2436. doi: 10.1073/pnas.80.9.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan H-Y, Klein H L. Characterization of mutations that suppress the temperature-sensitive growth of the hpr1Δ mutant of Saccharomyces cerevisiae. Genetics. 1994;137:945–956. doi: 10.1093/genetics/137.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan, H.-Y., and H. L. Klein. Submitted for publication.
- 16.Fan H-Y, Chang K K, Klein H L. Mutations in the RNA polymerase II transcription machinery suppress the hyperrecombination mutant hpr1D of Saccharomyces cerevisiae. Genetics. 1996;142:749–759. doi: 10.1093/genetics/142.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodrich J A, Tjian R. TBP-TAF complexes: selectivity factors for eukaryotic transcription. Curr Biol. 1994;6:403–409. doi: 10.1016/0955-0674(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 18.Gray J V, Ogas J P, Kamada Y, Stone M, Levin D E, Herskowitz I. A role for the Pkc1 MAP kinase pathway of Saccharomyces cerevisiae in bud emergence and identification of a putative upstream regulator. EMBO J. 1997;16:4924–4937. doi: 10.1093/emboj/16.16.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guthrie C, Fink G R. Guide to yeast genetics and molecular biology. Methods Enzymol. 1994;194:3–37. [PubMed] [Google Scholar]
- 20.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 21.Hata H, Mitsui H, Liu H, Bai Y, Denis C L, Shimazu Y, Sakai A. Dhh1p, a putative RNA helicase, associates with the general transcription factors Pop2p and Ccr4p from Saccharomyces cerevisiae. Genetics. 1998;148:571–579. doi: 10.1093/genetics/148.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang K N, Symington L S. Suppressors of a Saccharomyces cerevisiae pkc1 mutation identify alleles of the phosphatase PTC1 and of a novel gene encoding a putative basic leucine zipper protein. Genetics. 1995;141:1275–1285. doi: 10.1093/genetics/141.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang K N, Symington L S. Mutation of the gene encoding protein kinase C1 stimulates mitotic recombination in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:6039–6045. doi: 10.1128/mcb.14.9.6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter T, Plowman G D. The protein kinases of budding yeast: six score and more. Trends Biochem Sci. 1997;22:18–22. doi: 10.1016/s0968-0004(96)10068-2. [DOI] [PubMed] [Google Scholar]
- 25.Igual J C, Johnson A L, Johnston L H. Coordinated regulation of gene expression by the cell cycle transcription factor SWI4 and the protein kinase C MAP kinase pathway for yeast cell integrity. EMBO J. 1996;15:5001–5013. [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang Y W, Stillman D J. Involvement of SIN4 global transcriptional regulator in the chromatin structure of Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:4503–4514. doi: 10.1128/mcb.12.10.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamada Y, Jung U S, Piotrowski J, Levin D E. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995;9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- 28.Keleher C A, Redd M J, Schultz J, Carlson M, Johnson A D. Ssn6-Tup1 is a general repressor of transcription in yeast. Cell. 1992;68:709–719. doi: 10.1016/0092-8674(92)90146-4. [DOI] [PubMed] [Google Scholar]
- 29.Koleske A J, Young R A. An RNA polymerase II holoenzyme responsive to activators. Nature. 1994;368:466–469. doi: 10.1038/368466a0. [DOI] [PubMed] [Google Scholar]
- 30.Koleske A J, Young R A. The RNA polymerase II holoenzyme and its implications for gene regulation. Trends Biochem Sci. 1995;3:113–116. doi: 10.1016/s0968-0004(00)88977-x. [DOI] [PubMed] [Google Scholar]
- 31.Lea D E, Coulson C A. The distribution of the numbers of mutants in bacterial populations. J Genet. 1948;49:264–284. doi: 10.1007/BF02986080. [DOI] [PubMed] [Google Scholar]
- 32.Levin D E, Bartlett-Heubusch E. Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J Cell Biol. 1992;116:1221–1229. doi: 10.1083/jcb.116.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin D E, Errede B. The proliferation of MAP kinase signaling pathways in yeast. Curr Biol. 1995;7:197–202. doi: 10.1016/0955-0674(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 34.Levin D E, Fields F O, Kunisawa R, Bishop J M, Thorner J. A candidate protein kinase C gene, PKC1, is required for the S. cerevisiae cell cycle. Cell. 1990;62:213–224. doi: 10.1016/0092-8674(90)90360-q. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Bjorkland S, Jiang Y W, Kim Y-K, Lane W S, Stillman D J, Kornberg R D. Yeast global transcriptional regulators Sin4 and Rgr1 are components of mediator complex/RNA polymerase II holoenzyme. Proc Natl Acad Sci USA. 1995;92:10864–10868. doi: 10.1073/pnas.92.24.10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang P, Pardee A B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- 37.Liu H-Y, Toyn J H, Chiang Y-C, Draper M P, Johnston L H, Denis C L. DBF2, a cell cycle-regulated protein kinase, is physically and functionally associated with the CCR4 transcriptional regulatory complex. EMBO J. 1997;16:5289–5298. doi: 10.1093/emboj/16.17.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu H Y, Badarinarayana V, Audino D C, Rappsilber J, Mann M, Denis C L. The NOT proteins are part of the CCR4 transcriptional complex and affect gene expression both positively and negatively. EMBO J. 1998;17:1096–1106. doi: 10.1093/emboj/17.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lussier M, White A, Sheratin J, di Paolo T, Treadwell J, Southard S, Horenstein C, Shen-Weiner J, Ram A, Kapteyn J, Roemer T, Vo D, Bondoc D, Hall J, Zhong W, Sdicu A, Davies J, Klis F, Robbins P, Bussey H. Large-scale identification of genes involved in cell surface biosynthesis and architecture in Saccharomyces cerevisiae. Genetics. 1997;147:435–450. doi: 10.1093/genetics/147.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madden K, Sheu Y-J, Baetz K, Andrews B, Snyder M. SBF cell cycle regulator as a target of the yeast PKC-MAP kinase pathway. Science. 1997;275:1781–1784. doi: 10.1126/science.275.5307.1781. [DOI] [PubMed] [Google Scholar]
- 41.Madhani H D, Styles C A, Fink G R. MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell. 1997;91:673–684. doi: 10.1016/s0092-8674(00)80454-7. [DOI] [PubMed] [Google Scholar]
- 42.Malvar T, Biron R W, Kaback D B, Denis C L. The Ccr4 protein from Saccharomyces cerevisiae contains a leucine-rich repeat region which is required for its control of ADH2 expression. Genetics. 1992;132:951–962. doi: 10.1093/genetics/132.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin H, Castellanos M C, Cenamor R, Sanchez M, Molina M, Nombela C. Molecular and functional characterization of a mutant allele of the mitogen-activated protein kinase gene SLT2 (MPK1) Curr Genet. 1996;29:516–522. doi: 10.1007/BF02426955. [DOI] [PubMed] [Google Scholar]
- 44.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1972. [Google Scholar]
- 45.Nonaka H, Tanaka K, Hirano H, Fujiwara T, Kohno H, Umikawa M, Mino A, Takai Y. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 1995;14:5931–5938. doi: 10.1002/j.1460-2075.1995.tb00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orphanides G, Lagrange T, Reinberg D. The general transcription factors of RNA polymerase II. Genes Dev. 1996;10:2657–2683. doi: 10.1101/gad.10.21.2657. [DOI] [PubMed] [Google Scholar]
- 47.Paravicini G, Cooper M, Freidli L, Smith D J, Carpentier J-L, Klig L S, Payton M A. The osmotic integrity of the yeast cell requires a functional PKC1 gene product. Mol Cell Biol. 1992;12:4896–4905. doi: 10.1128/mcb.12.11.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ram A F J, Wolters A, denHoopen R, Klis F M. A new approach for isolating cell wall mutants of Saccharomyces cerevisiae by screening for hypersensitivity to calcofluor white. Yeast. 1994;10:1019–1030. doi: 10.1002/yea.320100804. [DOI] [PubMed] [Google Scholar]
- 49.Roeder R G. The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem Sci. 1996;21:327–334. [PubMed] [Google Scholar]
- 50.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 51.Shi X, Chang M, Wolf A J, Chang C-H, Frazer-Abel A A, Wade P A, Burton Z F, Jaehning J A. Cdc73p and Paf1p are found in a novel RNA polymerase II-containing complex distinct from the Srbp-containing holoenzyme. Mol Cell Biol. 1997;17:1160–1169. doi: 10.1128/mcb.17.3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi X, Finkelstein A, Wolf A J, Wade P A, Burton Z F, Jaehning J A. Paf1p, an RNA polymerase II-associated factor in Saccharomyces cerevisiae, may have both positive and negative roles in transcription. Mol Cell Biol. 1996;16:669–676. doi: 10.1128/mcb.16.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Struhl K, Stinchcomb D T, Scherer S, Davis R W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci USA. 1979;76:1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tansey W P, Herr W. TAFs: guilt by association? Cell. 1997;88:729–732. doi: 10.1016/s0092-8674(00)81916-9. [DOI] [PubMed] [Google Scholar]
- 55.Thompson C M, Young R A. General requirement for RNA polymerase II holoenzymes in vivo. Proc Natl Acad Sci USA. 1995;92:4587–4590. doi: 10.1073/pnas.92.10.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ulery T L, Jaehning J A. MTF1, encoding the yeast mitochondrial RNA polymerase specificity factor, is located on chromosome XIII. Yeast. 1994;10:839–841. doi: 10.1002/yea.320100614. [DOI] [PubMed] [Google Scholar]
- 57.Wade P A, Shaffer S D, Jaehning J A. Resolution of transcription factors from a transcriptionally active whole-cell extract from yeast: purification of TFIIB, TBP and RNA polymerase IIa. Pro Exp Pur. 1993;4:290–297. doi: 10.1006/prep.1993.1037. [DOI] [PubMed] [Google Scholar]
- 58.Wade P A, Werel W, Fentzke R C, Thompson N E, Leykam J F, Burgess R R, Jaehning J A, Burton Z F. A Novel Collection of Accessory Factors Associated with Yeast RNA Polymerase II. Protein Expr Purif. 1996;8:85–90. doi: 10.1006/prep.1996.0077. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe Y, Irie K, Matsumoto K. Yeast RLM1 encodes a serum response-like protein that may function downstream of the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol Cell Biol. 1995;15:5740–5749. doi: 10.1128/mcb.15.10.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watanabe Y, Takaesu G, Hafiwara M, Irie K, Matsumoto K. Characterization of a serum response factor-like protein in Saccharomyces cerevisiae, Rlm1, which has transcriptional activity regulated by the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol Cell Biol. 1997;17:2615–2623. doi: 10.1128/mcb.17.5.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woontner M, Wade P A, Bonner J, Jaehning J A. Transcriptional activation in an improved whole-cell extract from Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:4555–4560. doi: 10.1128/mcb.11.9.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zarzov P, Mazzoni C, Mann C. The SLT2 (MPK1) MAP kinase is activated during periods of polarized cell growth. EMBO J. 1996;15:83–91. [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu Y, Peterson C L, Christman M F. HRP1 encodes a global positive regulator of transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:1698–1708. doi: 10.1128/mcb.15.3.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]