Abstract
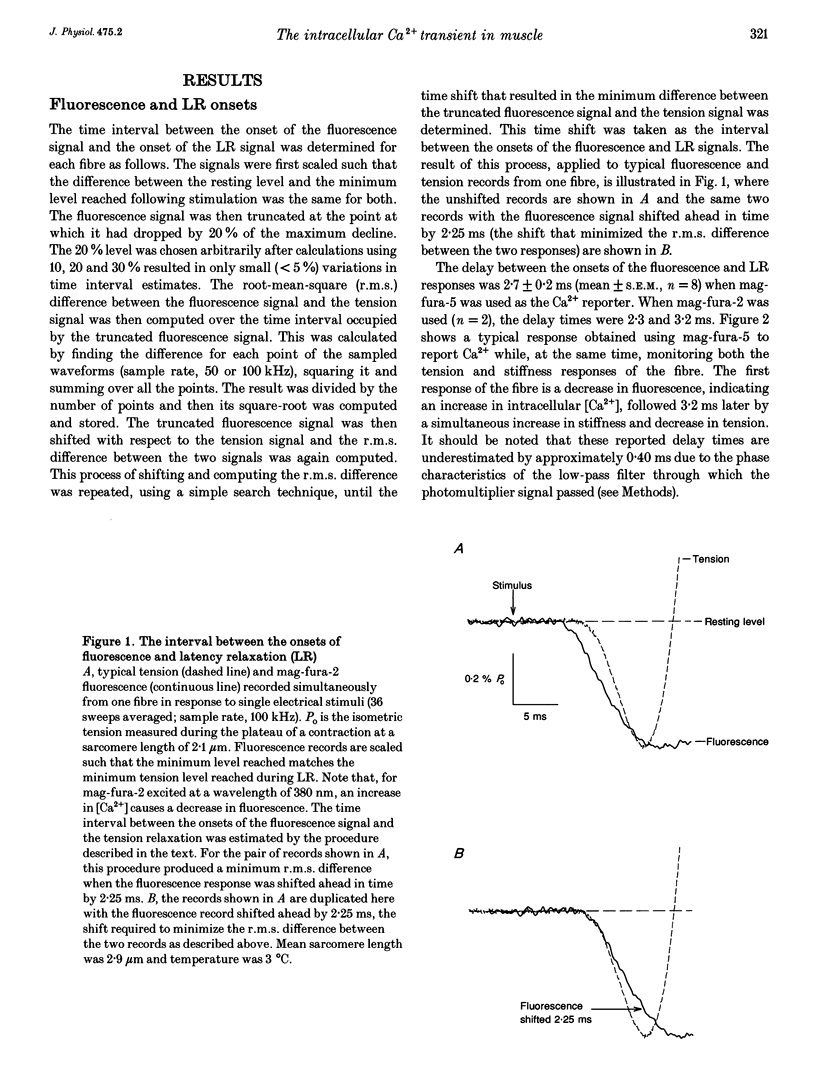
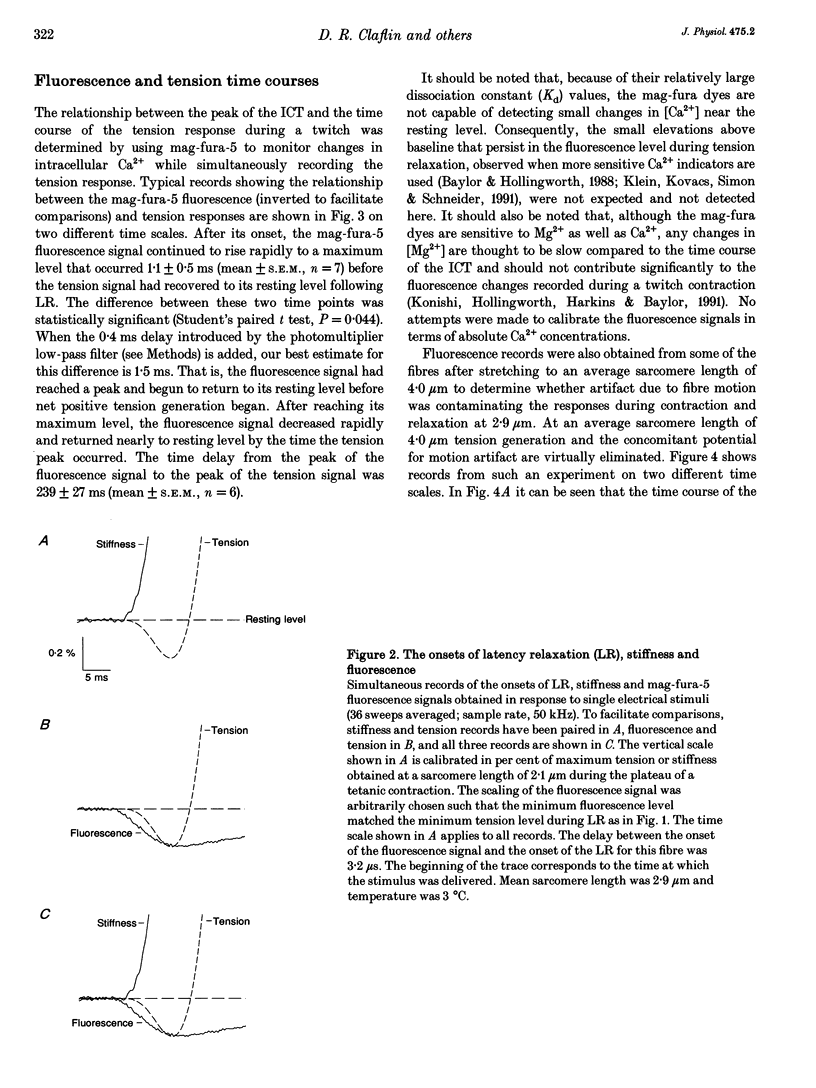
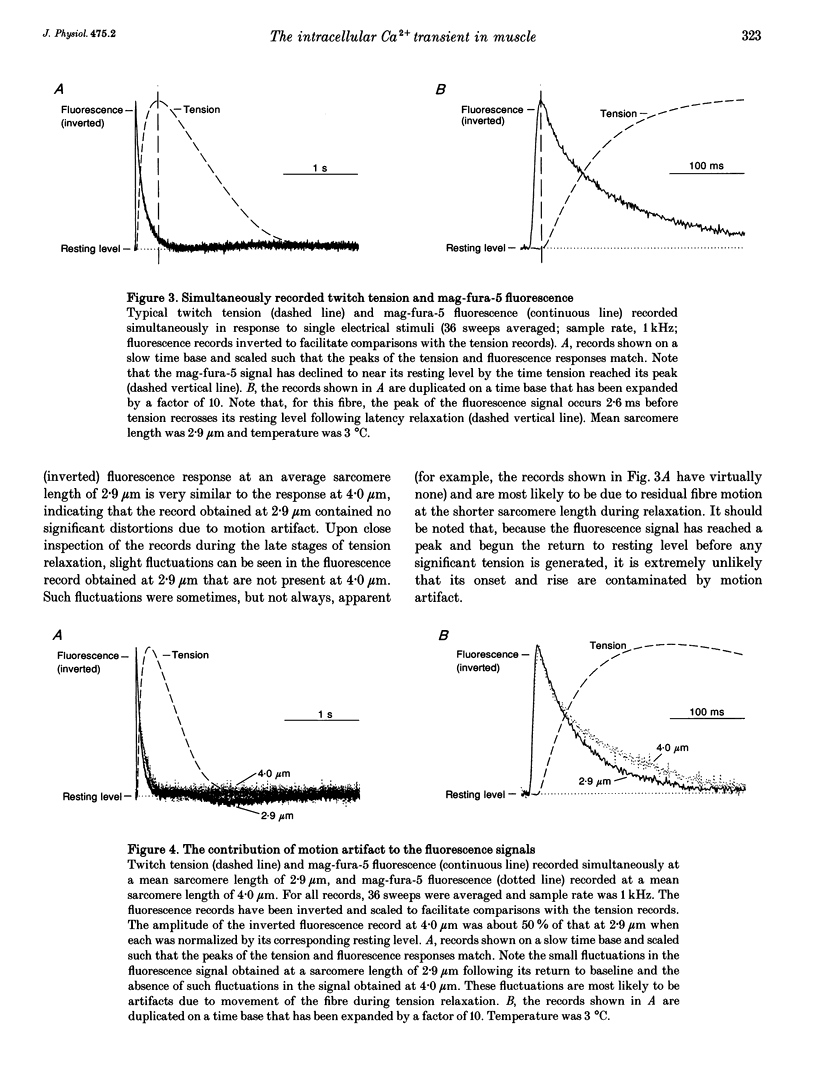
1. The purpose of this study was to determine, with high temporal resolution, the relationship between the intracellular Ca2+ transient (ICT) and the mechanical responses of intact, single skeletal muscle fibres of frogs following stimulation by a single, brief depolarization. 2. The time course of the ICT was monitored using the Ca(2+)-sensitive fluorescent dyes mag-fura-2 (furaptra) and mag-fura-5. The mag-fura dyes have a low affinity for Ca2+ and have been shown to track the ICT with no appreciable kinetic delay. Continuous records of mag-fura fluorescence, tension and stiffness responses were obtained simultaneously at high time resolution at a sarcomere length of 2.9 microns. Experimental temperature was 3 degrees C. 3. When a delay of 0.4 ms due to the low-pass filter associated with the photodetector was included, the onset of the fluorescence response preceded the onset of latency relaxation (the small fall in tension that precedes positive tension generation) by 3.1 +/- 0.2 ms (mean +/- S.E.M., n = 8). After its onset, the mag-fura fluorescence signal continued to change rapidly (indicating increasing intracellular [Ca2+]) to an extreme level that occurred 1.5 +/- 0.5 ms (mean +/- S.E.M., n = 7) before tension had recovered to its resting level following latency relaxation. The time delay from the extreme of the fluorescence signal to the peak of the tension signal was 239 +/- 27 ms (mean +/- S.E.M., n = 6).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Mulligan I. P., Lea T. J. Ca2+ and activation mechanisms in skeletal muscle. Q Rev Biophys. 1991 Feb;24(1):1–73. doi: 10.1017/s0033583500003267. [DOI] [PubMed] [Google Scholar]
- Ashley C. C., Ridgway E. B. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1970 Jul;209(1):105–130. doi: 10.1113/jphysiol.1970.sp009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Use of metallochromic dyes to measure changes in myoplasmic calcium during activity in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:139–177. doi: 10.1113/jphysiol.1982.sp014368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Quinta-Ferreira M. E., Hui C. S. Comparison of isotropic calcium signals from intact frog muscle fibers injected with Arsenazo III or Antipyrylazo III. Biophys J. 1983 Oct;44(1):107–112. doi: 10.1016/S0006-3495(83)84282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin D. R., Morgan D. L., Julian F. J. Earliest mechanical evidence of cross-bridge activity after stimulation of single skeletal muscle fibers. Biophys J. 1990 Mar;57(3):425–432. doi: 10.1016/S0006-3495(90)82559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I., Lännergren J. I. Arsenazo III calcium transients and latency relaxation in frog skeletal muscle fibres at different sarcomere lengths. J Physiol. 1984 Oct;355:323–344. doi: 10.1113/jphysiol.1984.sp015422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension transients during the rise of tetanic tension in frog muscle fibres. J Physiol. 1986 Mar;372:595–609. doi: 10.1113/jphysiol.1986.sp016027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. The relation between stiffness and filament overlap in stimulated frog muscle fibres. J Physiol. 1981 Feb;311:219–249. doi: 10.1113/jphysiol.1981.sp013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL A. V. The development of the active state of muscle during the latent period. Proc R Soc Lond B Biol Sci. 1950 Oct 13;137(888):320–329. doi: 10.1098/rspb.1950.0043. [DOI] [PubMed] [Google Scholar]
- Klein M. G., Kovacs L., Simon B. J., Schneider M. F. Decline of myoplasmic Ca2+, recovery of calcium release and sarcoplasmic Ca2+ pump properties in frog skeletal muscle. J Physiol. 1991 Sep;441:639–671. doi: 10.1113/jphysiol.1991.sp018771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Hollingworth S., Harkins A. B., Baylor S. M. Myoplasmic calcium transients in intact frog skeletal muscle fibers monitored with the fluorescent indicator furaptra. J Gen Physiol. 1991 Feb;97(2):271–301. doi: 10.1085/jgp.97.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. L., Claflin D. R., Julian F. J. Tension in frog single muscle fibers while shortening actively and passively at velocities near Vu. Biophys J. 1990 May;57(5):1001–1007. doi: 10.1016/S0006-3495(90)82619-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade P., Vergara J. Arsenazo III and antipyrylazo III calcium transients in single skeletal muscle fibers. J Gen Physiol. 1982 Apr;79(4):679–707. doi: 10.1085/jgp.79.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju B., Murphy E., Levy L. A., Hall R. D., London R. E. A fluorescent indicator for measuring cytosolic free magnesium. Am J Physiol. 1989 Mar;256(3 Pt 1):C540–C548. doi: 10.1152/ajpcell.1989.256.3.C540. [DOI] [PubMed] [Google Scholar]


