Abstract
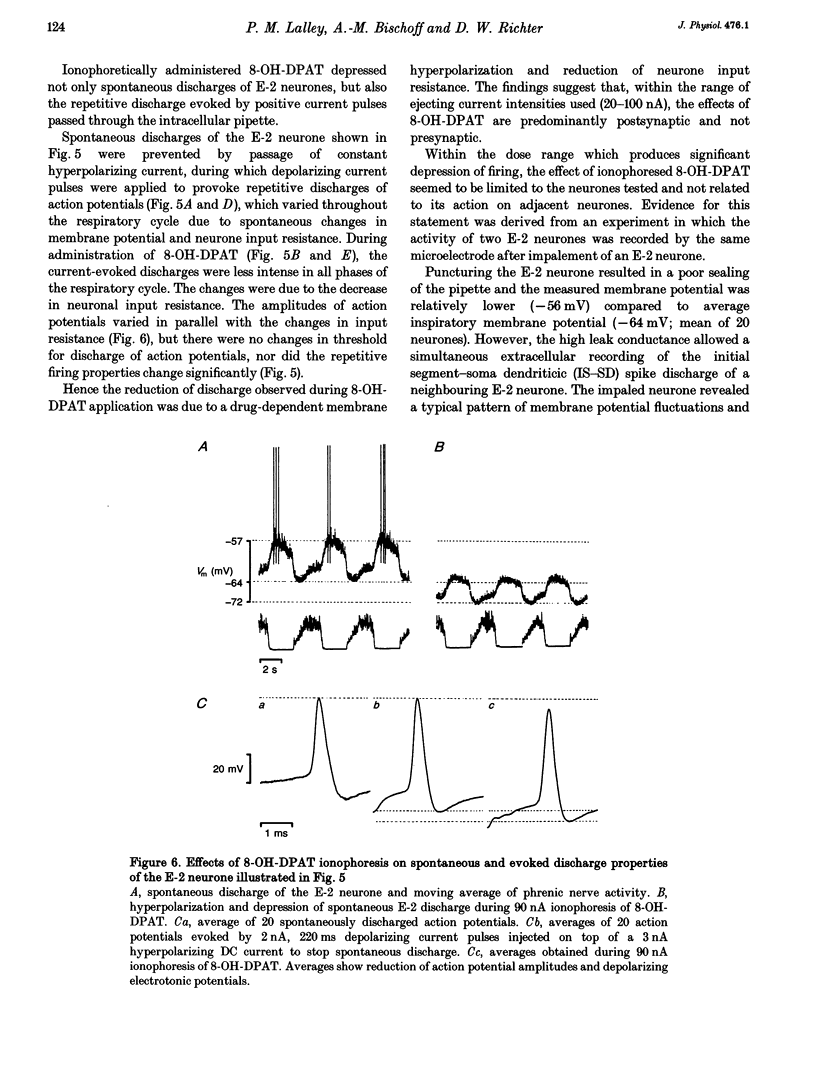
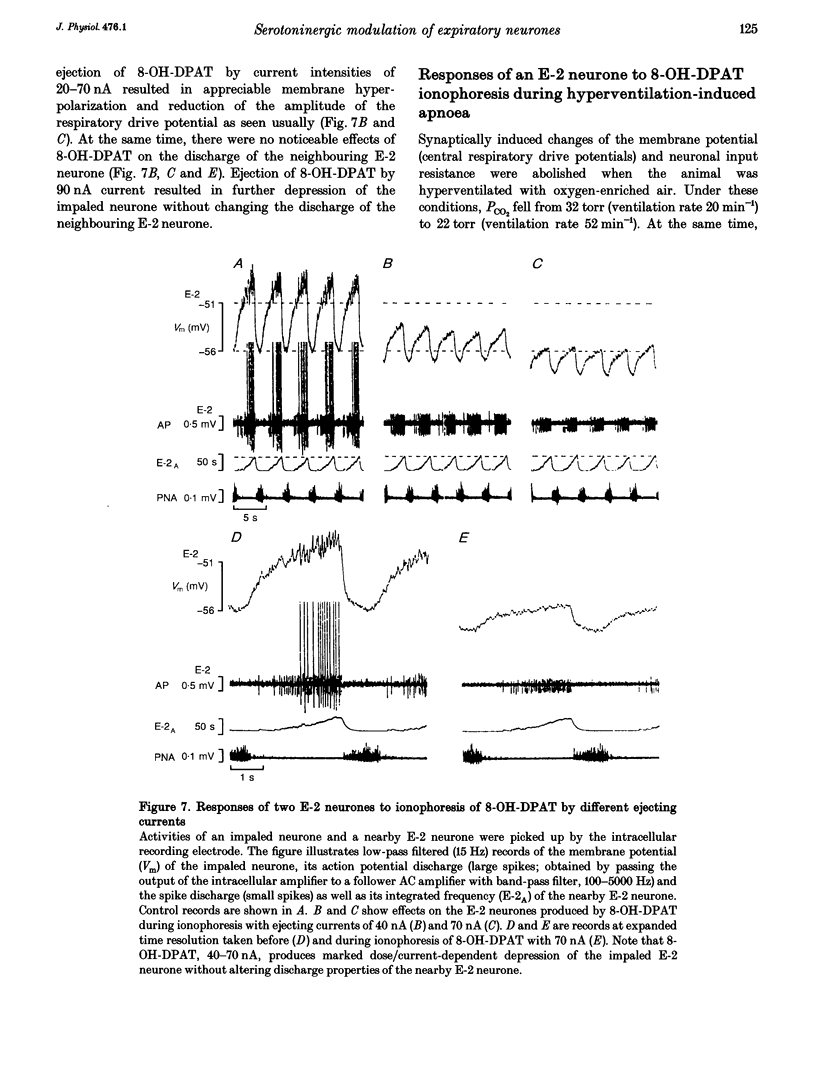
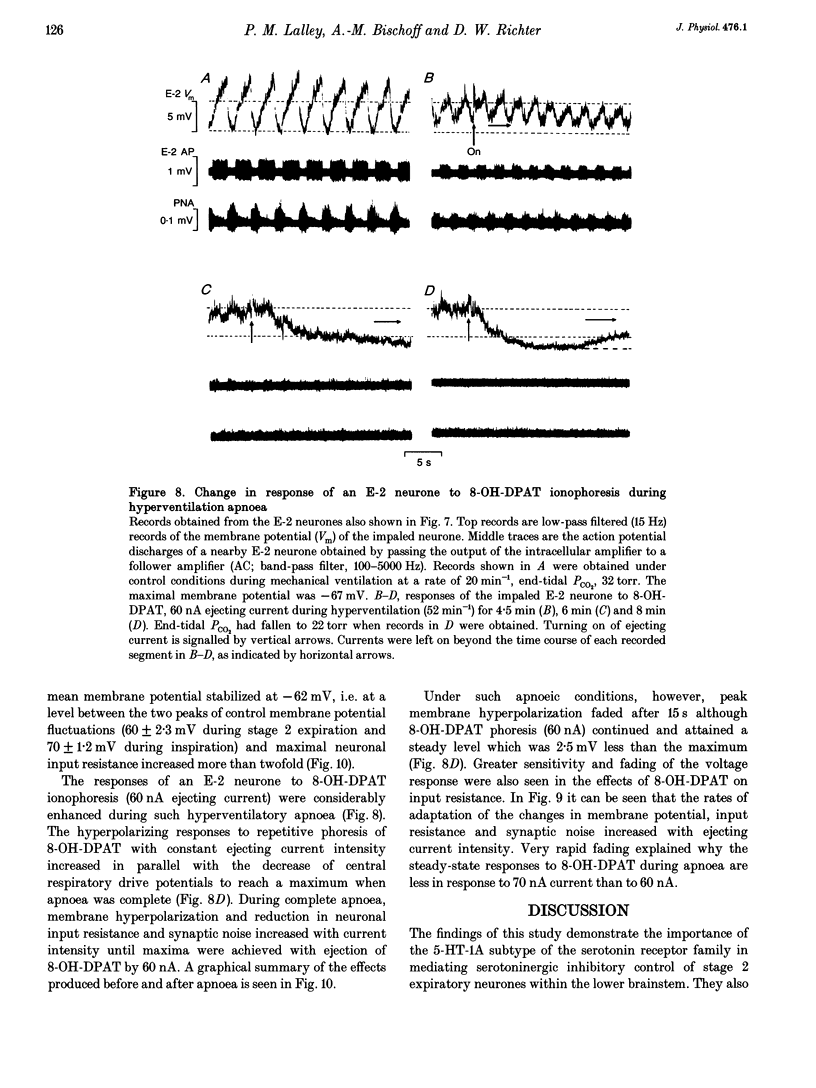
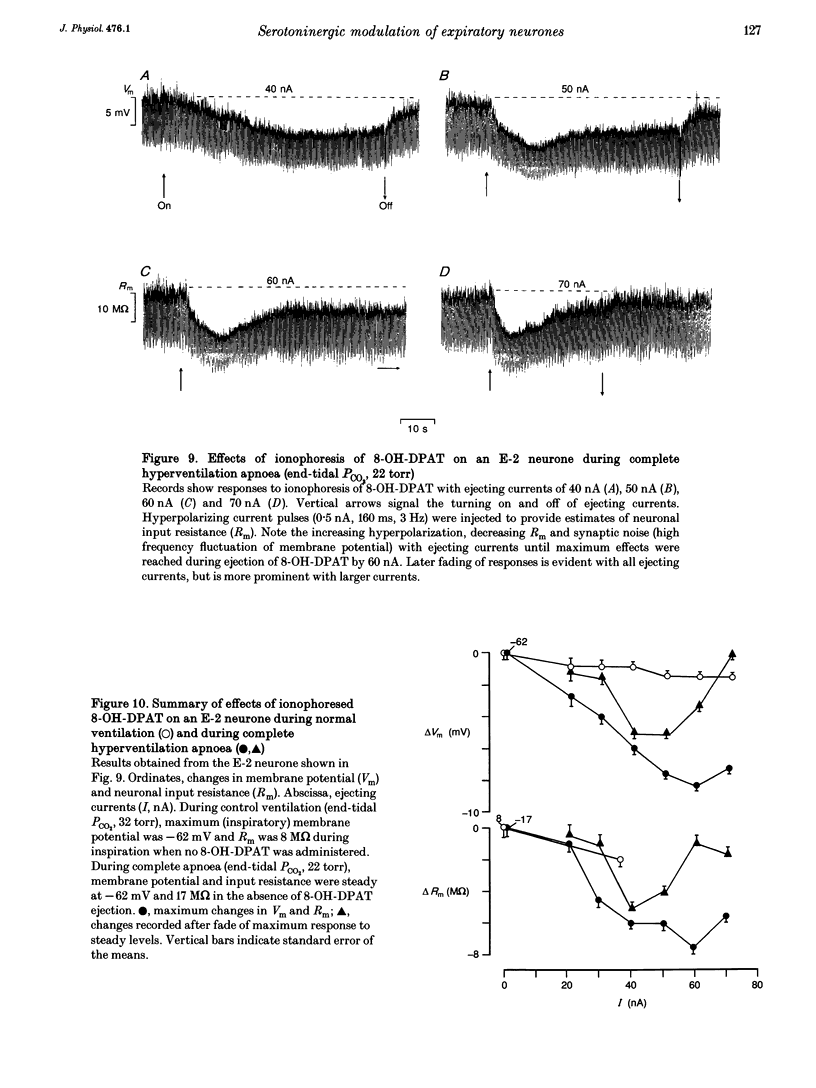
The involvement of the 5-HT-1A receptor in serotoninergic responses of stage 2 expiratory (E-2) neurones was investigated in pentobarbitone-anaesthetized, mechanically ventilated cats. The specific agonist of the 5-HT-1A receptor, 8-hydroxy-diproplaminotetralin (8-OH-DPAT), administered systemically or by ionophoresis directly on to the neurones, had a clear depressant effect. Administration of 8-OH-DPAT at doses of 10-50 micrograms kg-1 (I.V.) increased the membrane hyperpolarizations of E-2 neurones during the inspiratory and postinspiratory phases, and shortened their duration of activity in association with shortening of phrenic nerve activity. Discharges of E-2 neurones were also less intense. At doses of 50-90 micrograms kg-1, 8-OH-DPAT reduced or abolished inspiratory hyperpolarizations, and reduced expiratory depolarizations of membrane potential and discharge in parallel with inhibition of phrenic nerve discharges. The effects of the larger doses were reversed by I.V. injection of NAN-190, an antagonist at the 5-HT-1A receptor. Dose-dependent effects on the membrane potential and discharge of E-2 neurones, but not on phrenic nerve activity, were also seen by ionophoretic administration of 8-OH-DPAT on to E-2 neurones. At low currents, ejection of 8-OH-DPAT hyperpolarized the neurones without affecting the duration of inspiratory hyperpolarization and expiratory depolarization. This hyperpolarization depressed the intensity and the duration of expiratory discharges. Ejection with larger currents hyperpolarized the E-2 neurones further, and depressed expiratory depolarization leading to blockade of expiratory discharges. The effects on membrane potential were accompanied by decreased neuronal input resistance. This depressed the excitability of E-2 neurones as tested by discharge evoked by intracellular current injection. The amplitudes of action potentials decreased in parallel with the changes in input resistance. The effects were attributed to a postsynaptic effect of 8-OH-DPAT leading to a gradually developing inhibition by activation of 5-HT-1A receptors. Hyperventilatory apnoea depressed on-going synaptic activity and unmasked the effect of ionophoretically applied 8-OH-DPAT. The responses of the E-2 neurone were enhanced, as evidenced by increased membrane hyperpolarization and greater reduction of input resistance. Both responses faded appreciably, indicating receptor desensitization. The degree and rate of apparent desensitization depended on the dose/ejecting current. The greater sensitivity and faster desensitization to 8-OH-DPAT were attributed to the hyperventilatory alkalinization of the extracellular fluid, which might influence agonist binding to 5HT-1A receptors and/or receptor properties.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade R., Nicoll R. A. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J Physiol. 1987 Dec;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Neurophysiological actions of 5-hydroxytryptamine in the vertebrate nervous system. Prog Neurobiol. 1990;35(6):451–468. doi: 10.1016/0301-0082(90)90031-b. [DOI] [PubMed] [Google Scholar]
- Arita H., Ochiishi M. Opposing effects of 5-hydroxytryptamine on two types of medullary inspiratory neurons with distinct firing patterns. J Neurophysiol. 1991 Jul;66(1):285–292. doi: 10.1152/jn.1991.66.1.285. [DOI] [PubMed] [Google Scholar]
- Armijo J. A., Mediavilla A., Flórez J. Inhibition of the activity of the respiratory and vasomotor centers by centrally administered 5-hydroxytryptamine in cats. Rev Esp Fisiol. 1979 Jun;35(2):219–227. [PubMed] [Google Scholar]
- Bainton C. R., Kirkwood P. A. The effect of carbon dioxide on the tonic and the rhythmic discharges of expiratory bulbospinal neurones. J Physiol. 1979 Nov;296:291–314. doi: 10.1113/jphysiol.1979.sp013006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne D., Richter D. W. The non-uniform character of expiratory synaptic activity in expiratory bulbospinal neurones of the cat. J Physiol. 1986 Jan;370:433–456. doi: 10.1113/jphysiol.1986.sp015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A. J., Bayliss D. A., Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett. 1992 Aug 31;143(1-2):164–168. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- Böhmer G., Dinse H. R., Fallert M., Sommer T. J. Microelectrophoretic application of antagonists of putative neurotransmitters onto various types of bulbar respiratory neurons. Arch Ital Biol. 1979 Jan;117(1):13–22. [PubMed] [Google Scholar]
- Champagnat J., Denavit-Saubié M., Henry J. L., Leviel V. Catecholaminergic depressant effects on bulbar respiratory mechanisms. Brain Res. 1979 Jan 5;160(1):57–68. doi: 10.1016/0006-8993(79)90600-0. [DOI] [PubMed] [Google Scholar]
- Champagnat J., Denavit-Saubié M., Moyanova S., Rondouin G. Involvement of amino acids in periodic inhibitions of bulbar respiratory neurones. Brain Res. 1982 Apr 15;237(2):351–365. doi: 10.1016/0006-8993(82)90447-4. [DOI] [PubMed] [Google Scholar]
- Chesler M., Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992 Oct;15(10):396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Connelly C. A., Ellenberger H. H., Feldman J. L. Are there serotonergic projections from raphe and retrotrapezoid nuclei to the ventral respiratory group in the rat? Neurosci Lett. 1989 Oct 23;105(1-2):34–40. doi: 10.1016/0304-3940(89)90007-4. [DOI] [PubMed] [Google Scholar]
- Glennon R. A., Naiman N. A., Pierson M. E., Titeler M., Lyon R. A., Weisberg E. NAN-190: an arylpiperazine analog that antagonizes the stimulus effects of the 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT). Eur J Pharmacol. 1988 Sep 23;154(3):339–341. doi: 10.1016/0014-2999(88)90212-9. [DOI] [PubMed] [Google Scholar]
- Haji A., Remmers J. E., Connelly C., Takeda R. Effects of glycine and GABA on bulbar respiratory neurons of cat. J Neurophysiol. 1990 May;63(5):955–965. doi: 10.1152/jn.1990.63.5.955. [DOI] [PubMed] [Google Scholar]
- Holtman J. R., Jr, Anastasi N. C., Norman W. P., Dretchen K. L. Effect of electrical and chemical stimulation of the raphe obscurus on phrenic nerve activity in the cat. Brain Res. 1986 Jan 8;362(2):214–220. doi: 10.1016/0006-8993(86)90446-4. [DOI] [PubMed] [Google Scholar]
- Holtman J. R., Jr, Dick T. E., Berger A. J. Involvement of serotonin in the excitation of phrenic motoneurons evoked by stimulation of the raphe obscurus. J Neurosci. 1986 Apr;6(4):1185–1193. doi: 10.1523/JNEUROSCI.06-04-01185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman J. R., Jr Immunohistochemical localization of serotonin- and substance P-containing fibers around respiratory muscle motoneurons in the nucleus ambiguus of the cat. Neuroscience. 1988 Jul;26(1):169–178. doi: 10.1016/0306-4522(88)90135-2. [DOI] [PubMed] [Google Scholar]
- Jiang Z. H., Shen E. [Synaptic connection between the monoaminergic terminals and intercostal respiratory motoneurons in cats]. Sheng Li Xue Bao. 1985 Oct;37(5):479–485. [PubMed] [Google Scholar]
- Jordan L. M., Frederickson R. C., Phillis J. W., Lake N. Microelectrophoresis of 5-hydroxytryptamine: a clarification of its action on cerebral cortical neurones. Brain Res. 1972 May 26;40(2):552–558. doi: 10.1016/0006-8993(72)90161-8. [DOI] [PubMed] [Google Scholar]
- Kubin L., Tojima H., Davies R. O., Pack A. I. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992 May 25;139(2):243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- Lalley P. M. Effects of baclofen and gamma-aminobutyric acid on different types of medullary respiratory neurons. Brain Res. 1986 Jun 25;376(2):392–395. doi: 10.1016/0006-8993(86)90206-4. [DOI] [PubMed] [Google Scholar]
- Lalley P. M. Inhibition of phrenic and sympathetic vasomotor neurons in cats by the serotonin analog 5-methoxy-N,N-dimethyltryptamine. J Pharmacol Exp Ther. 1982 Jan;220(1):39–48. [PubMed] [Google Scholar]
- Lalley P. M. Responses of phrenic motoneurones of the cat to stimulation of medullary raphe nuclei. J Physiol. 1986 Nov;380:349–371. doi: 10.1113/jphysiol.1986.sp016290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley P. M. Serotoninergic and non-serotoninergic responses of phrenic motoneurones to raphe stimulation in the cat. J Physiol. 1986 Nov;380:373–385. doi: 10.1113/jphysiol.1986.sp016291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey B. G., Hernandez Y. M., Morris K. F., Shannon R. Functional connectivity between brain stem midline neurons with respiratory-modulated firing rates. J Neurophysiol. 1992 Apr;67(4):890–904. doi: 10.1152/jn.1992.67.4.890. [DOI] [PubMed] [Google Scholar]
- Loeschcke H. H. Central chemosensitivity and the reaction theory. J Physiol. 1982 Nov;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley B., Elde R. The ultrastructural localization of serotonin immunoreactivity within the nucleus of the solitary tract of the cat. J Neurosci. 1982 Oct;2(10):1499–1506. doi: 10.1523/JNEUROSCI.02-10-01499.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Williamson A. Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8098–8102. doi: 10.1073/pnas.86.20.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon D. R., Lalley P. M. Inhibition of respiratory neural discharges by clonidine and 5-hydroxytryptophan. J Pharmacol Exp Ther. 1982 Sep;222(3):771–777. [PubMed] [Google Scholar]
- Millhorn D. E., Hökfelt T., Seroogy K., Verhofstad A. A. Extent of colocalization of serotonin and GABA in neurons of the ventral medulla oblongata in rat. Brain Res. 1988 Sep 27;461(1):169–174. doi: 10.1016/0006-8993(88)90736-6. [DOI] [PubMed] [Google Scholar]
- Millhorn D. E. Stimulation of raphe (obscurus) nucleus causes long-term potentiation of phrenic nerve activity in cat. J Physiol. 1986 Dec;381:169–179. doi: 10.1113/jphysiol.1986.sp016320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. A., Herbert D. A. The effect of carbon dioxide on the membrane potential of medullary respiratory neurons. Brain Res. 1974 Jul 26;75(2):345–349. doi: 10.1016/0006-8993(74)90759-8. [DOI] [PubMed] [Google Scholar]
- Morin D., Hennequin S., Monteau R., Hilaire G. Serotonergic influences on central respiratory activity: an in vitro study in the newborn rat. Brain Res. 1990 Dec 10;535(2):281–287. doi: 10.1016/0006-8993(90)91611-j. [DOI] [PubMed] [Google Scholar]
- Oliver A. P. Technical contribution. A simple rapid method for preparing parallel micropipette electrodes. Electroencephalogr Clin Neurophysiol. 1971 Sep;31(3):284–286. doi: 10.1016/0013-4694(71)90100-3. [DOI] [PubMed] [Google Scholar]
- Pasternack M., Bountra C., Voipio J., Kaila K. Influence of extracellular and intracellular pH on GABA-gated chloride conductance in crayfish muscle fibres. Neuroscience. 1992;47(4):921–929. doi: 10.1016/0306-4522(92)90040-9. [DOI] [PubMed] [Google Scholar]
- Richter D. W. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982 Oct;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Voss M. D., De Castro D., Lipski J., Pilowsky P. M., Jiang C. Serotonin immunoreactive boutons form close appositions with respiratory neurons of the dorsal respiratory group in the cat. J Comp Neurol. 1990 May 8;295(2):208–218. doi: 10.1002/cne.902950205. [DOI] [PubMed] [Google Scholar]



