Abstract
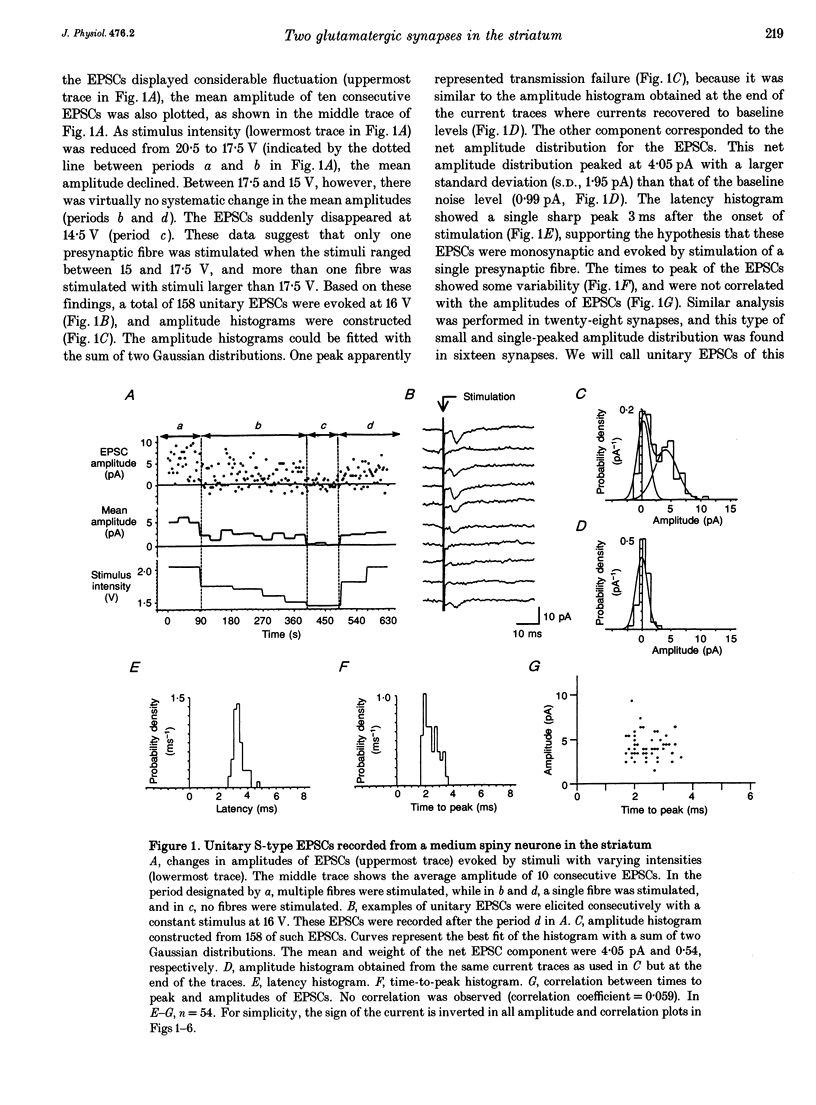
Excitatory postsynaptic currents (EPSCs) were recorded from the medium spiny neurones of neonatal rat striatal slices using the whole-cell patch clamp method. EPSCs were selectively elicited in the presence of picrotoxin with a glass stimulating pipette placed in the striatum. We found two distinct unitary EPSCs that were evoked by stimulation of single presynaptic fibres. The major type of EPSC, termed 'S-type', failed frequently and had a small mean amplitude (2.05 pA). They probably represented cortical afferents. The other type of unitary EPSC, the 'H-type', seldom failed and was 13 times larger than the S-type. Spontaneous EPSCs with amplitudes similar to those of H-type EPSCs could be induced. H-type EPSCs were mediated by both non-NMDA and NMDA receptors. The two types of EPSCs could be evoked in the same neurons. The intensity of stimulation for H-type EPSCs was higher than that for S-type EPSCs. H-type EPSCs could be polysynaptically activated, suggesting the presence of glutamatergic interneurones in the striatum that generated H-type EPSCs. H-type EPSCs displayed particularly long-lasting paired-pulse depression, while that displayed by the S-type EPSCs was short. The paired-pulse depression of both EPSCs was Ca2+ dependent and involved presynaptic mechanisms. We have demonstrated that the medium spiny neurones of neonatal rats receive two different glutamatergic input systems having different amplitudes, origins and paired-pulse depression, reminiscent of cerebellar Purkinje cells. This suggests that the two types of EPSCs also play distinctive roles in striatal neuronal circuitry.
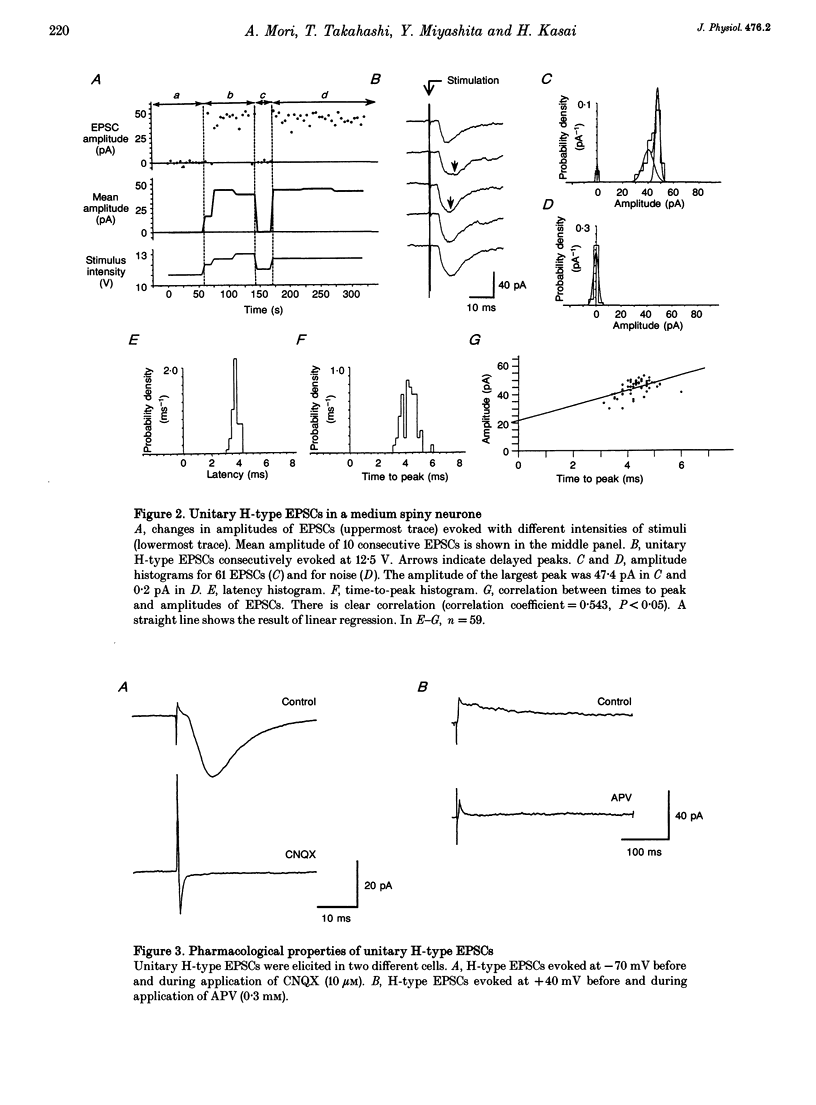
Full text
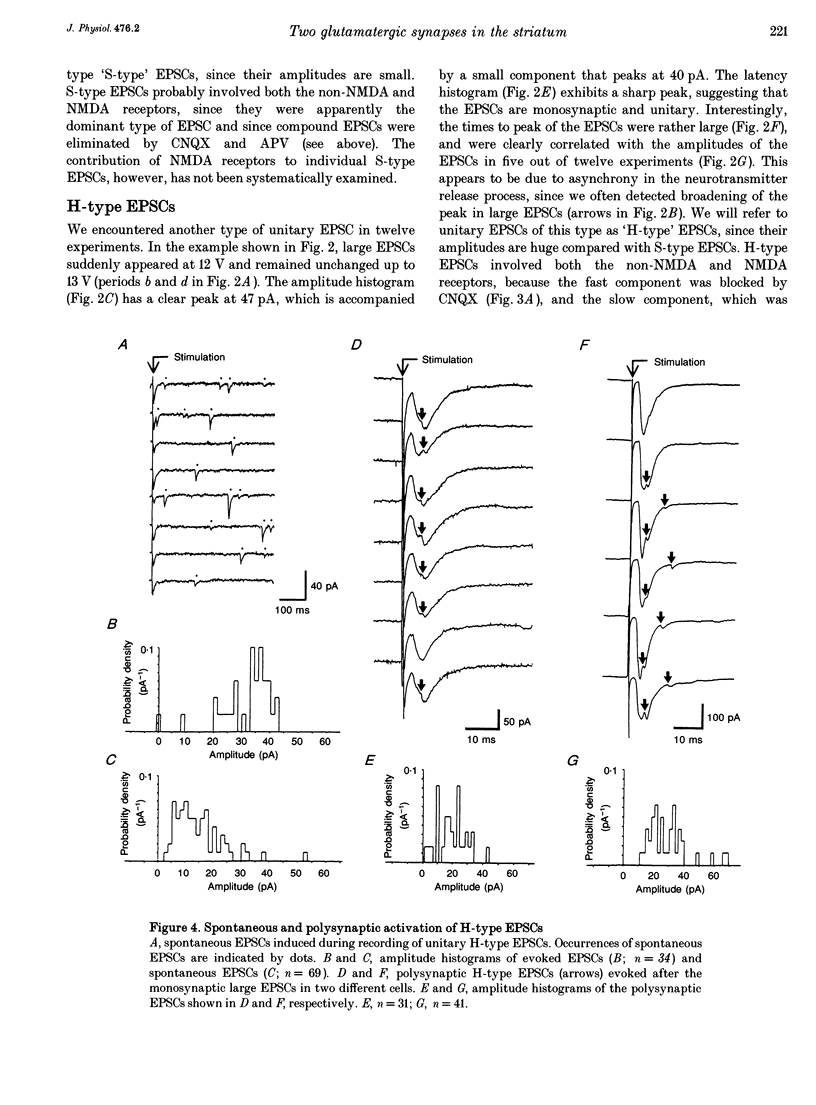
PDF

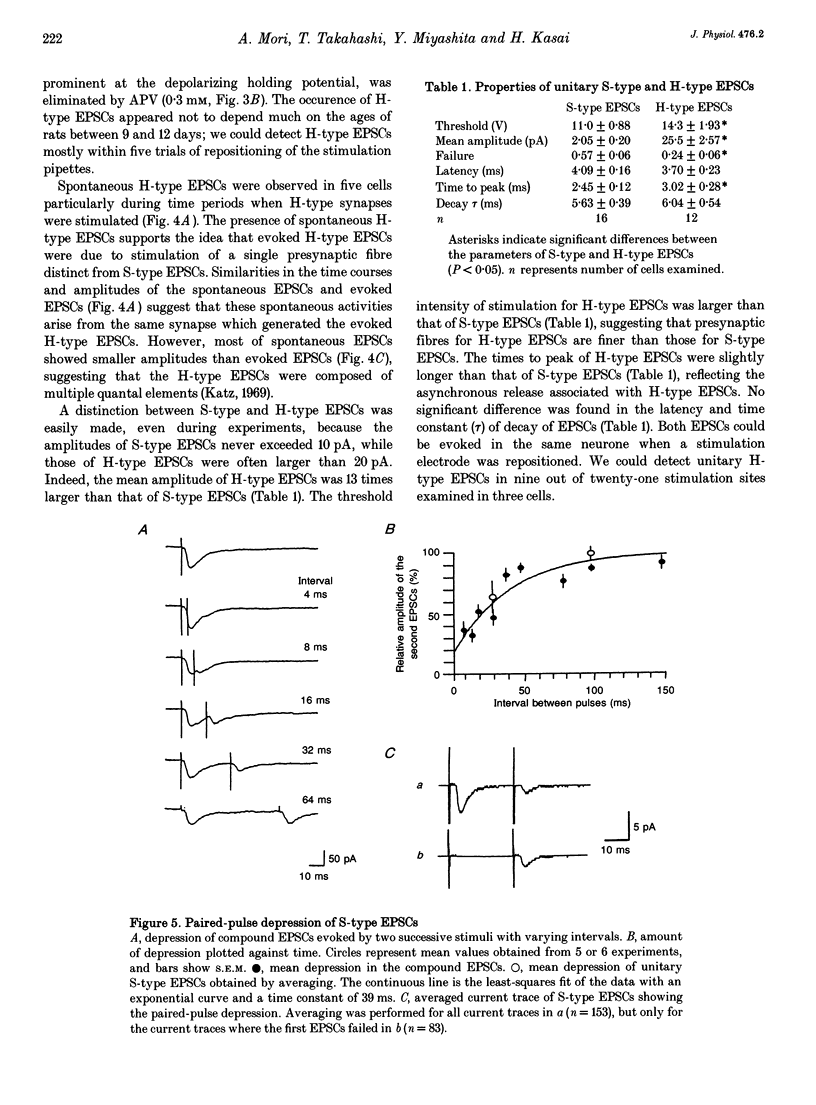



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
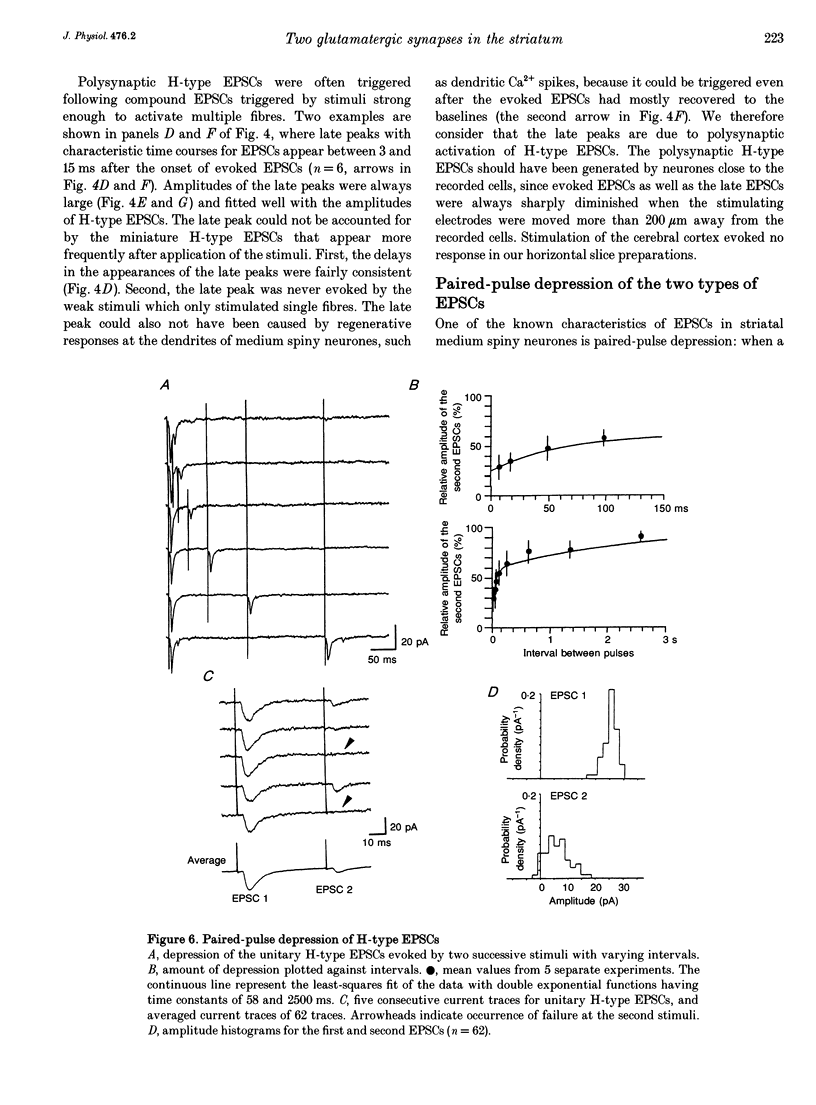
- Bolam J. P., Clarke D. J., Smith A. D., Somogyi P. A type of aspiny neuron in the rat neostriatum accumulates [3H]gamma-aminobutyric acid: combination of Golgi-staining, autoradiography, and electron microscopy. J Comp Neurol. 1983 Jan 10;213(2):121–134. doi: 10.1002/cne.902130202. [DOI] [PubMed] [Google Scholar]
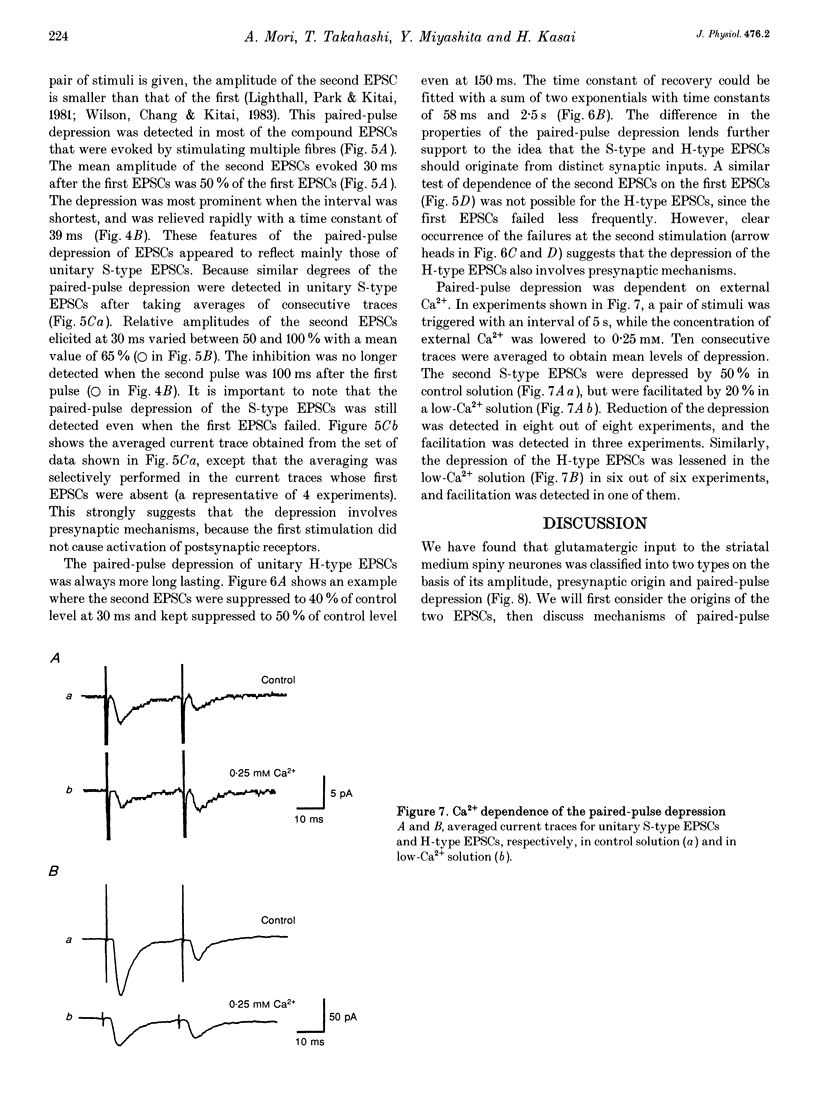
- Bolam J. P., Wainer B. H., Smith A. D. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience. 1984 Jul;12(3):711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
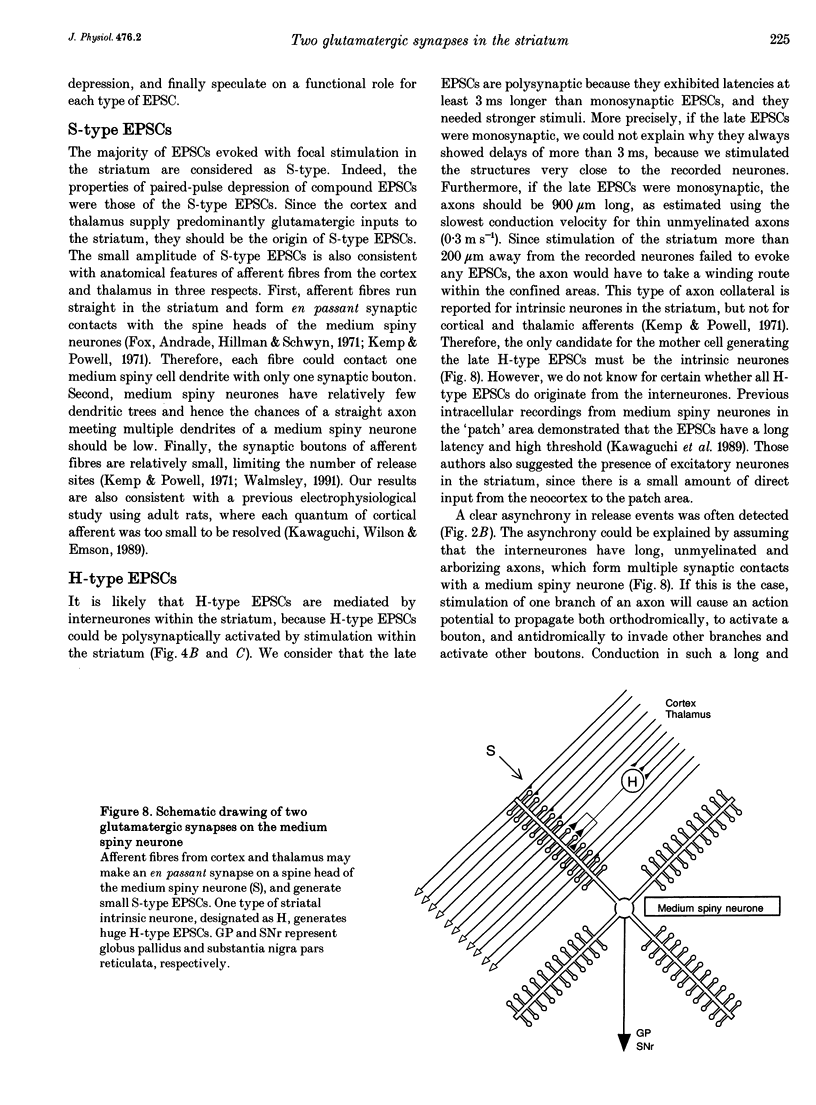
- Calabresi P., Maj R., Mercuri N. B., Bernardi G. Coactivation of D1 and D2 dopamine receptors is required for long-term synaptic depression in the striatum. Neurosci Lett. 1992 Aug 3;142(1):95–99. doi: 10.1016/0304-3940(92)90628-k. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Maj R., Pisani A., Mercuri N. B., Bernardi G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J Neurosci. 1992 Nov;12(11):4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P., Mercuri N. B., Bernardi G. Synaptic and intrinsic control of membrane excitability of neostriatal neurons. II. An in vitro analysis. J Neurophysiol. 1990 Apr;63(4):663–675. doi: 10.1152/jn.1990.63.4.663. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Mercuri N. B., Stefani A., Bernardi G. Synaptic and intrinsic control of membrane excitability of neostriatal neurons. I. An in vivo analysis. J Neurophysiol. 1990 Apr;63(4):651–662. doi: 10.1152/jn.1990.63.4.651. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Pisani A., Mercuri N. B., Bernardi G. Long-term Potentiation in the Striatum is Unmasked by Removing the Voltage-dependent Magnesium Block of NMDA Receptor Channels. Eur J Neurosci. 1992;4(10):929–935. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M., Aronin N. Ultrastructural features of immunoreactive somatostatin neurons in the rat caudate nucleus. J Neurosci. 1982 Sep;2(9):1267–1274. doi: 10.1523/JNEUROSCI.02-09-01267.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Fox C. A., Andrade A. N., Hillman D. E., Schwyn R. C. The spiny neurons in the primate striatum: a Golgi and electron microscopic study. J Hirnforsch. 1971;13(3):181–201. [PubMed] [Google Scholar]
- Gerfen C. R. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990 Jul;13(7):244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- Hovav G., Parnas H., Parnas I. Neurotransmitter release: facilitation and three-dimensional diffusion of intracellular calcium. Bull Math Biol. 1992 Sep;54(5):875–894. doi: 10.1007/BF02459934. [DOI] [PubMed] [Google Scholar]
- Jiang Z. G., North R. A. Membrane properties and synaptic responses of rat striatal neurones in vitro. J Physiol. 1991 Nov;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y., Wilson C. J., Emson P. C. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J Neurophysiol. 1989 Nov;62(5):1052–1068. doi: 10.1152/jn.1989.62.5.1052. [DOI] [PubMed] [Google Scholar]
- Kemp J. M., Powell T. P. The structure of the caudate nucleus of the cat: light and electron microscopy. Philos Trans R Soc Lond B Biol Sci. 1971 Sep 30;262(845):383–401. doi: 10.1098/rstb.1971.0102. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Llano I., Armstrong C. M. Synaptic currents in cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Pugh E. N., Jr G-protein cascades: gain and kinetics. Trends Neurosci. 1992 Aug;15(8):291–298. doi: 10.1016/0166-2236(92)90079-n. [DOI] [PubMed] [Google Scholar]
- Lighthall J. W., Park M. R., Kitai S. T. Inhibition in slices of rat neostriatum. Brain Res. 1981 May 11;212(1):182–187. doi: 10.1016/0006-8993(81)90048-2. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969 Jun;202(2):437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Nisenbaum E. S., Grace A. A., Berger T. W. Functionally distinct subpopulations of striatal neurons are differentially regulated by GABAergic and dopaminergic inputs--II. In vitro analysis. Neuroscience. 1992;48(3):579–593. doi: 10.1016/0306-4522(92)90403-o. [DOI] [PubMed] [Google Scholar]
- Nisenbaum E. S., Orr W. B., Berger T. W. Evidence for two functionally distinct subpopulations of neurons within the rat striatum. J Neurosci. 1988 Nov;8(11):4138–4150. doi: 10.1523/JNEUROSCI.08-11-04138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel W. H., Mugnaini E. Immunocytochemical studies of GABAergic neurons in rat basal ganglia and their relations to other neuronal systems. Neurosci Lett. 1984 Jun 29;47(3):233–238. doi: 10.1016/0304-3940(84)90519-6. [DOI] [PubMed] [Google Scholar]
- Takagi H., Somogyi P., Somogyi J., Smith A. D. Fine structural studies on a type of somatostatin-immunoreactive neuron and its synaptic connections in the rat neostriatum: a correlated light and electron microscopic study. J Comp Neurol. 1983 Feb 10;214(1):1–16. doi: 10.1002/cne.902140102. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Johansson O., Hökfelt T., Skirboll L., Elde R. P., Terenius L., Kimmel J., Goldstein M. NADPH-diaphorase: a selective histochemical marker for striatal neurons containing both somatostatin- and avian pancreatic polypeptide (APP)-like immunoreactivities. J Comp Neurol. 1983 Jul 1;217(3):252–263. doi: 10.1002/cne.902170303. [DOI] [PubMed] [Google Scholar]
- Walmsley B. Central synaptic transmission: studies at the connection between primary afferent fibres and dorsal spinocerebellar tract (DSCT) neurones in Clarke's column of the spinal cord. Prog Neurobiol. 1991;36(5):391–423. doi: 10.1016/0301-0082(91)90017-u. [DOI] [PubMed] [Google Scholar]
- Wilson C. J., Chang H. T., Kitai S. T. Disfacilitation and long-lasting inhibition of neostriatal neurons in the rat. Exp Brain Res. 1983;51(2):227–235. doi: 10.1007/BF00237198. [DOI] [PubMed] [Google Scholar]


