Abstract
Objective
Bariatric surgery effectively treats non-alcoholic fatty liver disease (NAFLD). The glutamate-serine-glycine (GSG) index has emerged as a non-invasive diagnostic marker for NAFLD, but its ability to monitor treatment response remains unclear. This study investigates the GSG index’s ability to monitor NAFLD’s response to bariatric surgery.
Methodology
Ten NAFLD participants were studied at baseline and 6 months post-bariatric surgery. Blood samples were collected for serum biomarkers and metabolomic profiling. Hepatic steatosis [proton density fat fraction (PDFF)] and fibroinflammation (cT1) were quantified with multiparametric magnetic resonance imaging (mpMRI), and hepatic stiffness with magnetic resonance elastography (MRE). Amino acids and acylcarnitines were measured with mass spectrometry. Statistical analyses included paired Student’s t-test, Wilcoxon-signed rank test, and Pearson’s correlation.
Results
Eight participants provided complete data. At baseline, all had hepatic steatosis (BMI 39.3 ± 5.6 kg/m2, PDFF ≥5%). Post-surgery reductions in PDFF (from 12.4 ± 6.7% to 6.2 ± 2.8%, p = 0.013) and cT1 (from 823.3 ± 85.4 ms to 757.5 ± 41.6 ms, p = 0.039) were significant, along with the GSG index (from 0.272 ± 0.03 to 0.157 ± 0.05, p = 0.001).
Conclusion
The GSG index can potentially be developed as a marker for monitoring the response of patients with NAFLD to bariatric surgery.
Keywords: non-alcoholic fatty liver disease, amino acids, metabolomics, bariatric surgeryx
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) refers to the presence of hepatic steatosis in the absence of other causes of secondary hepatic fat accumulation, such as alcohol consumption. NAFLD encompasses a spectrum of liver pathologies, ranging from benign steatosis in non-alcoholic fatty liver (NAFL) to non-alcoholic steatohepatitis (NASH), characterized by inflammation that can lead to fibrosis and cirrhosis. The incidence of NAFLD in Asia is rising and is projected to increase up to 20% within this decade.1
Weight reduction remains the primary treatment modality for NAFLD, and bariatric surgery is more effective than lifestyle interventions in combination with the best medical treatment.2 However, bariatric surgery may be associated with worsening hepatic fibrosis, cirrhosis, and liver failure. Rapid weight loss occurs after bariatric surgery, leading to the mobilization of lipids from peripheral depots and a large influx of free fatty acids (FFAs), which could cause hepatotoxicity.3 For these reasons, post-bariatric surgery patients need to be monitored for improvements in NAFLD and worsening of hepatic fibrosis.
The glutamate-serine-glycine (GSG) index has been investigated as a novel marker for the severity of NAFLD. This index involves the measurement of glutamate, serine, and glycine, which are precursors of GSH, and is calculated as the ratio of Glutamate/(Serine + Glycine).4 The GSG index has been studied in both adult and paediatric NAFLD populations. It correlates with the degree of hepatic steatosis and hepatic aminotransaminase levels, independent of traditional risk factors such as adiposity.4,5 However, the ability of this index to monitor the response of NAFLD to treatment has yet to be evaluated.
The primary objective of this study is to investigate the ability of the GSG index to monitor the improvement in the severity of NAFLD following bariatric surgery. NAFLD status will be evaluated using multiparametric magnetic resonance imaging (mpMRI) and magnetic resonance elastography (MRE), which measures the degree of hepatic steatosis, fibroinflammation, and fibrosis. We hypothesize that the GSG index decreases in the first 6 months post-bariatric surgery, and the post-surgery change in the GSG index correlates with improvement in hepatic fibroinflammation. Apart from dysregulated amino acid pathways, lipid metabolism is also altered in NAFLD resulting in the accumulation of various intermediates of incomplete lipid oxidation such as acylcarnitines.6-8 Altered serum acylcarnitine profiles are associated with NAFLD,9 and this study also aims to explore post-surgery changes in serum acylcarnitines in patients with NAFLD.
METHODOLOGY
Study design and participants
This prospective observational study was conducted at Singapore General Hospital. Ethics approval was obtained from the SingHealth Institutional Review Board (CIRB Ref: 2019/2179), and informed consent was obtained from participants. Participants were eligible for the study if they: (i) were between 21 and 65 years of age, (ii) had a BMI of ≥ 32.5 kg/m2 with obesity-related complications. Participants were ineligible for the study if they: (i) consumed excessive alcohol (defined as >1 unit/day for females and >2 units/ day for males); (ii) had chronic liver disorders other than NAFLD; (iii) took medications that may induce hepatic steatosis; (iv) had contraindications to MRI. All eligible participants under the care of the study team members were invited to participate. Sample size calculation and sampling strategies were not employed due to the exploratory nature of this study.
Participants were assessed at baseline and 6 months post-surgery. Blood samples were collected for serum biochemical analyses and comprehensive metabolomic profiling. mpMRI and MRE were conducted to assess the severity of NAFLD.
Biochemical analyses and metabolomic profiling
Liver function tests, lipid profiles, and serum creatinine, glucose, and insulin levels were analysed with Abbott Architect i200 (Abbott Diagnostics). HbA1c levels were measured with Roche Cobas c501 (Roche Diagnostics). Amino acids and acylcarnitines were measured by liquid chromatography-mass spectrometry (LC-MS). To extract the amino acids, 30 μL of sample was dried and derivatized using phenyl isothiocyanate by incubating at room temperature for 1 hour. The sample was then reconstituted in 5 mM ammonium acetate in methanol and centrifuged to obtain the precipitate. To separate the individual amino acid components, the precipitated protein pellets were diluted with water and analysed on a Waters Acquity UPLC BEH C18 column (1.7 μm, 2.1x50 mm) using a Waters Acquity I-Class liquid chromatography system coupled to a Waters Xevo TQ-XS mass spectrometer (Waters). The liquid chromatography run was performed with 0.2% formic acid in water as mobile phase A and 0.2% formic acid in acetonitrile as mobile phase B. The gradient started at 5% B, before increasing to 12% B at 1.5 minutes, 17.5% B at 2.7 minutes, 50% B at 4 minutes, and 100% B at 4.5 - 5.0 minutes, before returning to 5% B at 5.1 - 5.8 minutes. The flow rate was maintained at 0.8 mL/min except at 4.7 - 5.1 minutes where it was increased to 1.0 mL/min. Temperature of the column was fixed at 50 °C, and the the injection volume at 5 μL. Compounds were ionized in positive mode using electrospray ionization. Data processing was carried out with the Waters TargetLynx software v4.2 (Waters).
For the quantitative analysis of acylcarnitines species, samples were enriched with 10 μL of a deuterium-labelled mixture of acylcarnitines and diluted with 400 μL of methanol. Following centrifugation at 13,000 rpm for 5 minutes at 4°C, the supernatant was collected for analysis. Methanol extracts were erivatised with 3M hydrochloric acid in methanol (Sigma Aldrich) and reconstituted with 80% methanol for LC-MS analysis. Compounds were ionized in positive mode using electrospray ionization. Data acquisition and analysis were conducted using Agilent MassHunter Workstation B.06.00 Software (Agilent Technologies).
Multiparametric MRI
Non-contrast T1, T2*, and proton density fat fraction (PDFF) were acquired using the LiverMultiScan® protocol (Perspectum Ltd., Oxford, UK).10,11 PDFF measures hepatic steatosis, and iron-corrected T1 mapping (cT1) indicates hepatic fibroinflammatory disease activity. Four transverse slices positioned at the porta hepatis were captured using a shortened modified look-locker inversion (shMOLLI) and a multiecho-spoiled gradient-echo sequence to quantify liver T1 and iron (T2*) fat (PDFF), respectively. During image analysis, cT1 and PDFF maps of the liver were delineated into whole liver segmentation maps using a semiautomatic method. Three 15-mm diameter circular regions of interest were placed on the transverse T2* maps for each slice, covering a representative sample of the liver, to calculate average T2* values for T1 correction. Non-parenchymal structures such as bile ducts and large blood vessels as well as image artifacts were excluded from image analysis.
Magnetic resonance elastography
MRE measures liver stiffness and was performed using a 2-dimensional MRE protocol and interpreted by abdominal radiologists.12,13
Body composition
Lean body mass (LBM), fat-free mass (FFM), and fat mass (FM) were measured using dual-energy X-ray absorptiometry (Hologic Discovery Wi densitometer, Hologic, Inc., Massachusetts, USA).
Statistical analysis
The distribution of quantitative data was assessed with the Shapiro-Wilk test. Variables with a normal distribution were expressed as mean ± standard deviation and those with a non-normal distribution as median (interquartile range). The statistical significance of the post-surgery changes in clinical parameters, mpMRI and MRE parameters, serum amino acid and acylcarnitine profiles, and the GSG index were analysed either with the paired Student’s t-test (for data following a normal distribution) or the Wilcoxon signed-rank test (for data following a nonnormal distribution). Correlations between post-surgery changes in the GSG index with cT1, PDFF, and MRE were examined using Pearson’s correlation. Multiple variable analysis was not performed due to the small sample size and exploratory nature of this study. P-value <0.05 was considered statistically significant.
RESULTS
Baseline characteristics
Two participants did not provide data at all time points and were excluded from the analysis. The characteristics of participants at baseline (5 males, 3 females) are summarized in Table 1. Participants had a mean age of 44.6 ± 9.4 years and a body mass index (BMI) of 39.3 ± 5.6 kg/m2. All had hepatic steatosis at baseline, defined as PDFF ≥ 5%.
Table 1.
Baseline and post-surgery characteristics and clinical parameters
| Baseline | Post-surgery | p-value | |
|---|---|---|---|
| Age | 44.6 ± 9.4 | - | - |
| Weight (kg) | 106.9 ± 12.1 | 87.0 ± 12.4 | <0.001* |
| BMI (kg/m2) | 39.3 ± 5.6 | 32.0 ± 5.0 | <0.001* |
| Fat mass (kg) | 49.5 ± 14.3 | 31.0 ± 9.2 | 0.002* |
| Fat-free mass (kg) | 57.4 ± 16.2 | 56.0 ± 10.8 | 0.723* |
| Fat mass (%) | 46.5 ± 12.8 | 35.6 ± 9.7 | 0.012* |
| Hip circumference (cm) | 127.1 ± 13.8 | 105.6 ± 13.4 | 0.004* |
| Waist circumference (cm) | 117.7 ± 11.8 | 103.1 ± 11.0 | <0.001* |
| HbA1c (%) | 6.9 ± 1.0 | 5.9 ± 0.8 | 0.009* |
| Glucose (mmol/L) | 6.3 (2.0) | 5.3 (1.0) | 0.375** |
| Insulin (mU/L) | 16.8 ± 9.3 | 7.0 ± 4.2 | 0.018* |
| Creatinine | 69.8 ± 19.0 | 66.4 ± 15.7 | 0.397* |
| Total cholesterol (mmol/L) | 4.0 ± 1.2 | 4.7 ± 0.6 | 0.175* |
| HDL (mmol/L) | 1.1 ± 0.3 | 1.3 ± 0.3 | 0.037* |
| Triglycerides (mmol/L) | 1.4 (0.9) | 1.2 (0.2) | 0.742** |
| LDL (mmol/L) | 2.2 ± 1.0 | 2.8 ± 0.5 | 0.154* |
| Total protein (U/L) | 77.1 ± 4.9 | 71.4 ± 3.8 | 0.010* |
| ALT (U/L) | 26.5 (55.5) | 21.0 (7.0) | 0.0391** |
| AST (U/L) | 38.9 ± 18.9 | 25.1 ± 5.6 | 0.095* |
| ALP (U/L) | 80.6 ± 12.7 | 76.6 ± 10.1 | 0.101* |
| GGT (U/L) | 39.5 (12.5) | 24.0 (9.5) | 0.008** |
| Bilirubin (U/L) | 9.5 (3.5) | 13.0 (6.5) | 0.175** |
| Albumin (U/L) | 43.6 ± 2.3 | 40.6 ± 2.3 | 0.011* |
| PDFF (%) | 12.4 ± 6.7 | 6.2 ± 2.8 | 0.013* |
| cT1 (ms) | 823.3 ± 85.4 | 757.5 ± 41.6 | 0.039* |
| MRE LSM (kPa) | 2.3 (0.2) | 2.2 (0.3) | 0.813** |
Data presented as mean ± SD or median (IQR)
SD: standard deviation; IQR: interquartile range; ALT: alanine transaminase; AST: aspartate transaminase; ALP: alkaline phosphatase; GGT: gamma-glutamyl transferase; HDL: high-density lipoprotein; LDL: low-density lipoprotein; BMI: body mass index; PDFF: proton density fat fraction; cT1: iron-corrected T1; MRE: magnetic resonance elastography; LSM: liver stiffness measurement. Bolded p values indicate statistical significance (p <0.05).
p values obtained by paired Student’s t-test
p values obtained by Wilcoxon signed-rank test
Effects of bariatric surgery on clinical, mpMRI and MRE parameters
All participants underwent laparoscopic sleeve gastrectomy and had their post-surgery follow-up at 21.9 ± 1.9 weeks. There were significant reductions in weight, BMI, fat mass, fat mass percentage, hip and waist circumference, HbA1c, insulin, ALT, GGT, albumin, total protein, PDFF, and cT1. Serum HDL levels increased significantly post-surgery. However, the decrease in MRE liver stiffness measurements (LSM) was not significant.
Effects of bariatric surgery on serum acylcarnitine profiles
Changes in serum acylcarnitine profiles are listed in Table 2. Short-chain acylcarnitines including C2 (12768.7 (12969.3) nM vs. 10032.5 (4857.2) nM, p = 0.023), C5 (122.8 ± 19.1 nM vs. 67.6 ± 23.0 nM, p <0.001), and C5:1 (12.6 ± 3.5 nM vs. 7.9 ± 4.9 nM, p = 0.041) decreased post-surgery. Medium-chain acylcarnitines including C6 (72.1 ± 18.7 nM vs. 60.8 ± 13.2 nM, p = 0.042), C6-OH (48.6 ± 12.7 nM vs. 35.8 ± 7.2 nM, p = 0.019) and C12-OH (4.8 ± 1.6 nM vs. 3.1 ± 1.2 nM, p = 0.019), and long-chain acylcarnitines including C18:2 (96.5 ± 22.6 nM vs. 84.0 ± 21.6 nM, p = 0.048) and C18:3 (8.2 ± 2.7 nM vs. 5.5 ± 1.8 nM, p = 0.001) also decreased. On the other hand, serum levels of C18:2-OH (7.0 ± 3.6 nM vs. 10.5 ± 3.9 nM, p = 0.048) and C22 (2.7 ± 0.6 nM vs. 3.3 ± 0.9 nM, p = 0.014) increased.
Table 2.
Baseline and post-surgery serum acylcarnitine profiles (nM)
| Baseline | Post-surgery | p-value | |
|---|---|---|---|
| C2:0 | 12768.7 (12969.3) | 10032.5 (4857.2) | 0.023** |
| C3:0 | 470.9 (149.7) | 313.4 (70.1) | 0.148** |
| C4:0 | 282.8 ± 66.1 | 235.9 ± 93.4 | 0.254* |
| C5:1 | 12.6 ± 3.5 | 7.9 ± 4.9 | 0.041* |
| C5:0 | 122.8 ± 19.1 | 67.6 ± 23.0 | <0.001* |
| C4-OH | 52.9 (163.8) | 16.2 (31.4) | 0.078** |
| C6 | 72.1 ± 18.7 | 60.8 ± 13.2 | 0.042* |
| C5-OH/C3-DC | 28.1 ± 9.4 | 20.9 ± 5.5 | 0.067* |
| C4-DC,C6-OH | 48.6 ± 12.7 | 35.8 ± 7.2 | 0.019* |
| C8:1 | 176.7 ± 75.0 | 115.0 ± 61.4 | 0.091* |
| C8 | 150.7 ± 60.8 | 148.4 ± 35.6 | 0.859* |
| C5-DC | 52.5 ± 10.9 | 53.0 ± 11.8 | 0.897* |
| C8:1-OH/C6:1-DC | 36.9 ± 12.6 | 33.8 ± 7.2 | 0.595* |
| C8-OH/C6-DC | 86.1 ± 39.4 | 69.3 ± 22.0 | 0.222* |
| C10:3 | 34.9 (49.1) | 21.6 (11.6) | 0.078** |
| C10:2 | 15.0 ± 6.1 | 13.1 ± 5.9 | 0.589* |
| C10:1 | 126.6 (24.1) | 103.2 (46.3) | 0.844** |
| C10 | 241.8 ± 99.4 | 251.6 ± 54.5 | 0.698* |
| C7-DC | 20.8 ± 4.4 | 20.9 ± 5.1 | 0.630* |
| C8:1-DC | 18.3 ± 6.7 | 22.5 ± 14.6 | 0.309* |
| C8-DC | 47.0 ± 26.3 | 33.4 ± 11.1 | 0.083* |
| C12:2 | 13.2 ± 5.0 | 15.2 ± 9.5 | 0.512* |
| C12:1 | 107.2 ± 43.7 | 92.9 ± 33.3 | 0.424* |
| C12 | 90.9 ± 39.9 | 74.7 ± 20.2 | 0.169* |
| C12:2-OH/C10:2-DC | 6.7 ± 3.4 | 5.5 ± 1.9 | 0.419* |
| C12:1-OH | 14.1 (7.7) | 11.9 (5.1) | 0.742** |
| C12-OH/C10-DC | 4.8 ± 1.6 | 3.1 ± 1.2 | 0.019* |
| C14:3 | 4.9 ± 1.6 | 3.9 ± 1.8 | 0.238* |
| C14:2 | 32.1 (14.2) | 26.9 (14.0) | 0.229** |
| C14:1 | 72.3 (57.0) | 66.0 (19.5) | 0.461** |
| C14 | 34.5 ± 11.4 | 27.6 ± 9.2 | 0.203* |
| C14:3-OH/C12:3-DC | 1.2 ± 0.5 | 1.2 ± 0.6 | 0.942* |
| C14:2-OH | 5.7 ± 2.1 | 4.6 ± 2.0 | 0.096* |
| C14:1-OH | 16.5 ± 4.8 | 13.3 ± 3.7 | 0.113* |
| C14-OH/C12-DC | 12.5 ± 4.1 | 9.7 ± 3.5 | 0.247* |
| C16:3 | 7.9 ± 2.8 | 6.2 ± 2.9 | 0.082* |
| C16:2 | 8.2 (2.2) | 7.5 (1.6) | 0.313** |
| C16:1 | 26.2 (21.3) | 26.2 (7.9) | 0.250** |
| C16 | 148.3 ± 24.9 | 138.7 ± 31.7 | 0.415* |
| C16:3-OH/C14:3-DC | 1.6 ± 0.8 | 1.3 ± 0.6 | 0.340* |
| C16:2-OH | 6.8 ± 1.4 | 5.1 ± 1.5 | 0.051* |
| C16:1-OH/C14:1-DC | 7.9 (3.0) | 7.3 (1.5) | 0.313** |
| C16-OH | 13.2 ± 4.6 | 10.7 ± 2.3 | 0.098* |
| C18:3 | 8.2 ± 2.7 | 5.5 ± 1.8 | 0.001* |
| C18:2 | 96.5 ± 22.6 | 84.0 ± 21.6 | 0.048* |
| C18:1 | 181.4 ± 52.9 | 172.1 ± 26.6 | 0.643* |
| C18 | 38.9 ± 5.6 | 45.2 ± 9.3 | 0.062* |
| C18:3-OH/C16:3-DC | 4.4 ± 1.3 | 5.0 ± 2.0 | 0.524* |
| C18:2-OH/C16:2-DC | 7.0 ± 3.6 | 10.5 ± 3.9 | 0.048* |
| C18:1-OH/C16:1-DC | 9.0 ± 4.9 | 6.7 ± 2.8 | 0.165* |
| C18-OH/C16-DC | 9.0 ± 3.1 | 6.8 ± 2.2 | 0.194* |
| C20:4 | 5.6 ± 1.6 | 6.3 ± 2.7 | 0.495* |
| C20:3 | 6.0 (2.2) | 7.1 (2.3) | 0.863** |
| C20:2 | 5.5 ± 2.3 | 7.2 ± 1.3 | 0.102* |
| C20:1 | 7.6 ± 1.2 | 8.2 ± 2.2 | 0.510* |
| C20 | 4.4 ± 1.2 | 4.9 ± 1.7 | 0.340* |
| C20:3-OH/C18:3-DC | 2.5 (0.2) | 3.1 (1.6) | 0.945** |
| C20:2-OH/C18:2-DC | 2.1 ± 1.0 | 3.4 ± 1.5 | 0.118* |
| C20:1-OH/C18:1-DC | 9.0 ± 5.0 | 8.0 ± 2.7 | 0.513* |
| C20-OH/C18-DC | 8.7 ± 3.3 | 8.6 ± 2.5 | 0.970* |
| C22:5 | 2.4 ± 0.8 | 1.8 ± 0.9 | 0.223* |
| C22:4 | 1.5 ± 0.8 | 1.9 ± 1.2 | 0.316* |
| C22:3 | 0.9 ± 0.6 | 0.7 ± 0.4 | 0.529* |
| C22:2 | 0.9 (0.5) | 0.9 (0.7) | 1.000** |
| C22:1 | 2.1 ± 0.7 | 2.5 ± 0.8 | 0.410* |
| C22 | 2.7 ± 0.6 | 3.3 ± 0.9 | 0.014* |
| C24 | 21.4 ± 6.8 | 21.1 ± 7.5 | 0.947* |
| C26 | 30.5 ± 10.0 | 38.0 ± 7.4 | 0.104* |
| C28 | 2.2 ± 0.9 | 3.0 ± 0.8 | 0.187* |
Data presented as mean ± SD or median (IQR)
SD: standard deviation; IQR: interquartile range. Bolded p values indicate statistical significance (p <0.05).
p values obtained by paired Student’s t-test
p values obtained by Wilcoxon signed-rank test
Effects of bariatric surgery on serum amino acid profiles and the GSG index
Changes in the serum amino acid profiles and the GSG index are listed in Table 3. There was a significant reduction in the GSG index post-surgery (0.272 ± 0.03 vs. 0.157 ± 0.05, p = 0.001). Aspartic acid (15.7 ± 4.1 vs. 12.1 ± 3.0, p = 0.037), glutamic acid (83.0 ± 28.6 vs. 55.9 ± 16.1, p = 0.014), phenylalanine (79.0 ± 10.3 vs. 62.8 ± 8.4, p = 0.002), tyrosine (78.8 ± 17.3 vs. 64.2 ± 9.7, p = 0.004), leucine (192.4 ± 31.7 vs. 121.4 ± 23.7, p = 0.001), isoleucine (116.0 ± 16.6 vs. 79.5 ± 19.4, p = 0.002), valine (414.7 ± 42.2 vs. 267.0 ± 51.3, p <0.001), and proline (261.3 ± 63.7 vs. 213.2 ± 48.5, p = 0.005) also decreased, whereas levels of arginine (113.7 ± 18.3 vs. 126.4 ± 22.4, p = 0.012) increased.
Table 3.
Baseline and post-surgery serum amino acid profiles (umol/L) and the GSG index
| Baseline | Post-surgery | p-value* | |
|---|---|---|---|
| Glycine | 212.9 ± 47.5 | 249.6 ± 34.3 | 0.078 |
| Serine | 142.4 ± 33.3 | 134.6 ± 26.0 | 0.605 |
| Threonine | 111.7 ± 30.5 | 89.3 ± 30.3 | 0.137 |
| Alanine | 495.8 ± 122.7 | 442.4 ± 118.5 | 0.155 |
| Aspartic acid | 15.7 ± 4.1 | 12.1 ± 3.0 | 0.037 |
| Asparagine | 87.3 ± 12.5 | 78.0 ± 13.2 | 0.136 |
| Glutamic acid | 83.0 ± 28.6 | 55.9 ± 16.1 | 0.014 |
| Glutamine | 1276.4 ± 79.7 | 1302.2 ± 211.3 | 0.764 |
| Histidine | 90.8 ± 86.2 | 8.7 ± 6.1 | 0.278 |
| Phenylalanine | 79.0 ± 10.3 | 62.8 ± 8.4 | 0.002 |
| Tyrosine | 78.8 ± 17.3 | 64.2 ± 9.7 | 0.004 |
| Tryptophan | 58.2 ± 12.1 | 51.8 ± 6.8 | 0.153 |
| Leucine | 192.4 ± 31.7 | 121.4 ± 23.7 | 0.001 |
| Isoleucine | 116.0 ± 16.6 | 79.5 ± 19.4 | 0.002 |
| Valine | 414.7 ± 42.2 | 267.0 ± 51.3 | <0.001 |
| Methionine | 29.8 ± 5.6 | 27.0 ± 4.1 | 0.232 |
| Arginine | 113.7 ± 18.3 | 126.4 ± 22.4 | 0.012 |
| Ornithine | 83.3 ± 27.5 | 70.6 ± 11.5 | 0.085 |
| Citrulline | 32.5 ± 7.1 | 35.3 ± 7.6 | 0.267 |
| Proline | 261.3 ± 63.7 | 213.2 ± 48.5 | 0.005 |
| GSG index | 0.272 ± 0.03 | 0.157 ± 0.05 | 0.001 |
Data presented as mean ± SD
SD: standard deviation. Bolded p values indicate statistical significance (p <0.05).
p values obtained by paired Student’s t-test
Correlation between post-surgery changes in the GSG index, mpMRI and MRE parameters
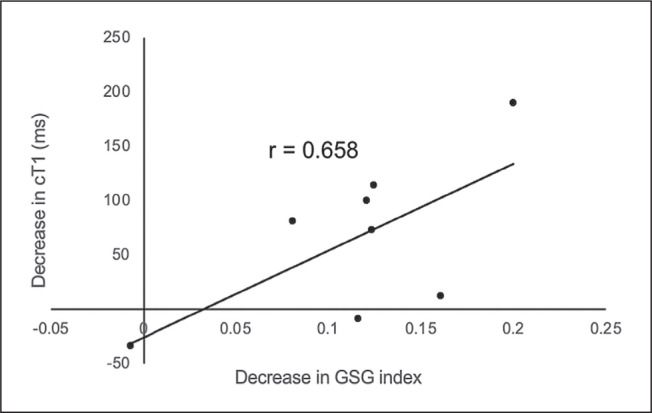
The post-surgery change in the GSG index and cT1 showed a correlation coefficient of 0.658, although statistical significance was not achieved (p = 0.076) (Figure 1). No statistically significant correlations were found between the post-surgery change in the GSG index and MRE LSM (r = 0.232, p = 0.580) or PDFF (r = -0.405, p = 0.320).
Figure 1.
Correlation between the post-surgery decrease in cT1 and the decrease in GSG index.
DISCUSSION
The study aimed to investigate the ability of the GSG index to monitor the effects of bariatric surgery in NAFLD patients. We demonstrated that the post-bariatric surgery improvement in hepatic steatosis and hepatic fibroinflammation was significant, along with the reduction in the GSG index.
Bariatric surgery effectively achieves sustained weight loss and can reverse risk factors contributing to NAFLD’s pathogenesis, such as dyslipidemia, insulin resistance, and inflammation.14,15 This concurs with our findings, which demonstrated reduced adiposity and improved lipid and glycemic control. Bariatric surgery also reduces hepatic steatosis, inflammation, and hepatocyte ballooning.16 Our study similarly showed post-surgery improvements in liver biochemistry, hepatic fat content, and fibroinflammation. However, there was no significant post-surgery change in hepatic fibrosis. This may be because none of the participants had significant fibrosis at baseline and the improvement in fibrosis is slower than steatosis and inflammation.17,18
Metabolomic profiling involves analyzing a broad range of metabolites from cellular processes and biochemical pathways, including amino acids, lipids, carbohydrates, nucleotides, organic acids, and various small molecules. Compared to general laboratory parameters, it reflects the current metabolic state of the body more accurately. It can be a sensitive and specific biomarker of NAFLD, and the progression of NAFLD has previously been associated with higher serum acylcarnitines levels.19 Acylcarnitines, especially medium- and long-chain species, activate proinflammatory signaling pathways that are involved in the pathogenesis and progression of NAFLD.20-22 Serum acylcarnitine levels also reflect fatty acid oxidation23 and altered fatty acid oxidation has been linked to hepatic steatosis and insulin resistance and is an important factor behind NAFLD.24-26 Though not demonstrated in this study, levels of unsaturated long-chain acylcarnitine species such as C14:1 and C18:1 were found to be increased with the progression of fibrosis.9 Likewise, this may also be attributed to the fact that none of the participants had significant fibrosis at baseline and that improvements in fibrosis are seen over longer periods of time.17,18
Liver biopsy is the gold standard for diagnosing and staging liver diseases, including NAFLD. However, biopsies are invasive with significant risks and complications and repeated biopsies to track changes in the severity of NAFLD are challenging to perform in clinical practice. Biopsy results are also subject to sampling error and interand intra-observer variability. Laboratory parameters such as blood concentrations of hepatic transaminases are non-invasive indicators of liver function. However, these indirect measurements cannot reliably predict the severity of liver disease.27 As such, advanced imaging methods such as mpMRI and MRE have been developed as non-invasive tools to diagnose and monitor the progression of NAFLD. We previously showed that improvements in the severity of NAFLD after bariatric surgery can be monitored with mpMRI and MRE.28 Although these imaging methods have a place in secondary and tertiary care settings, MRI can be costly and may not be widely available, limiting their accessibility for routine clinical care. Therefore, there is a clinical need to develop alternative methods to determine the severity of the condition and monitor the response of NAFLD to treatment in a way that is accurate, non-invasive, and accessible.
The liver participates in protein and amino acid metabolism. NAFLD and NASH are characterized by alterations in pathways involving several aspects of amino acid and lipid metabolism.29-31 One such pathway is the synthesis of glutathione (GSH),32,33 an important cellular redox buffer and defender against oxidative stress in hepatocytes.34 Increased oxidative stress in the liver is associated with liver damage and the progression of NAFLD to NASH. GSH levels have been shown to regulate hepatocyte cell death.35-37 Thus, measuring GSH levels may serve as a marker of NAFLD. Various methods for measuring GSH include enzymatic assays, high-performance liquid chromatography (HPLC), and mass spectrometry. However, HPLC techniques have poor detection limits for GSH.38 GSH is also sensitive to oxidation, and careful sample preparation is required for analyses, affecting measurement accuracy and reliability. GSH measurements are also not routinely available in clinical laboratories. Measurements of amino acids related to GSH biosynthesis can be a more reliable method for quantifying GSH status.
Previous studies have demonstrated the role of the GSG index as a marker of the severity of NAFLD, independent of traditional risk factors such as adiposity. The GSG index was previously found to be higher among NAFLD patients and positively correlated with levels of liver enzymes and the degree of hepatic steatosis.4,5 Our findings extend these earlier observations by demonstrating a reduction in the GSG index post-surgery along with reductions in hepatic steatosis and fibroinflammation. We also found a near statistically significant correlation between the changes in GSG index and hepatic fibroinflammation.
Glutamate and glycine, two amino acids that constitute the GSG index, are independent risk factors for hepatic fibrosis.39 Glycine functions as a rate-limiting substrate for the synthesis of GSH,33 and decreased glycine level is associated with altered liver metabolism in patients with hepatic fibrosis.39 It was also previously demonstrated that plasma glycine concentrations and de novo glycine synthesis increased after bariatric surgery, suggesting impaired glycine synthesis due to obesity-induced insulin resistance in NAFLD.40 On the other hand, increased levels of glutamate were associated with altered liver metabolism.39 A previous study also demonstrated an association between glutamate concentrations with GGT. Among other amino acids, glutamate was more strongly associated with the severity of hepatic fibrosis.4
Apart from amino acids that constitute the GSG index, branched-chain amino acids (BCAAs) such as leucine, isoleucine, and valine are increased in insulin-resistant states,4,41 which is a major phenotype in NASH.42 Higher levels of BCAAs are associated with an increased risk of NAFLD.43-45 BCAA oxidation reduces after bariatric surgery due to the slower breakdown of body proteins as the ability of insulin to suppress proteolysis is restored.46-48 Our study also showed that these amino acids decreased post-bariatric surgery.
Moreover, increased levels of aromatic amino acids such as tyrosine and phenylalanine have also been found to be associated with an increased severity of liver diseases.32,49-51 Phenylalanine is converted to tyrosine in the liver, which is further metabolized. NAFLD patients exhibit increased tyrosine concentrations, which is likely due to impaired hepatic metabolism.32,51 Our findings are corroborated by another study, which also found that levels of these amino acids are reduced post-bariatric surgery among NAFLD patients.48
This is the first study investigating the ability of the GSG index to monitor changes in hepatic steatosis, inflammation, and fibrosis following bariatric surgery. Metabolomics may provide a more sensitive understanding of the metabolic changes associated with NAFLD, and our study illustrates the potential of the GSG index as an accessible and noninvasive biomarker to diagnose NAFLD and monitor its response to various interventions.
Our study is exploratory in nature and has a small sample size with a relatively short duration of follow-up. Future studies with larger sample sizes and regression analysis with the addition of other variables will be needed to validate the effectiveness of the GSG index in monitoring NAFLD treatment response. Future work should also evaluate the utility of these biomarkers over longer followup periods. In addition, although liver biopsies were not performed to evaluate the response of NAFLD to bariatric surgery, MRI parameters such as PDFF and cT1 have been shown to correlate with histopathological findings.52 Our study also recruited NAFLD patients with morbid obesity undergoing bariatric surgery. The ability of the GSG index to monitor the response to other pharmacological or lifestyle interventions will, therefore, also need to be examined.
CONCLUSION
The post-surgery change in the GSG index is in the same direction as the improvements in hepatic steatosis and fibroinflammation. The GSG index can potentially be developed as a marker for monitoring the response of patients with NAFLD to bariatric surgery.
Funding Statement
Funding Source None.
Statement of Authorship
All authors certified fulfillment of ICMJE authorship criteria.
CRediT Author Statement
NYYT: Formal Analysis, Writing – original draft preparation, Writing – review and editing; ES: Writing – review and editing; LTEC: Investigation, Resources, Writing – review and editing; ASCL: Writing – review and editing; CHH: Writing – review and editing; AKHE: Writing – review and editing; WHC: Writing – review and editing; PCL: Writing – review and editing; MFT: Investigation, Resources; JPEC: Writing – review and editing; YMB: Writing – review and editing; GBBG: Writing – review and editing; JC: Resources, Writing – original draft preparation; Writing – review and editing; KVC: Writing – review and editing; SHYH: Writing – review and editing; JPK: Writing – review and editing; HCT: Conceptualization, Writing – review and editing, Supervision, Project administration, Funding acquisition
Author Disclosure
The authors declared no conflict of interest.
Data Availability Statement
Datasets analyzed in the study are under license and not publicly available for sharing.
References
- 1.Estes C, Chan HvLY, Chien RN, et al. Modelling NAFLD disease burden in four Asian regions-2019-2030. Aliment Pharmacol Ther. 2020;51(8):801–11. PMID: 32133676 PMCID: DOI: 10.1111/apt.15673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verrastro O, Panunzi S, Castagneto-Gissey L, et al. Bariatric-metabolic surgery versus lifestyle intervention plus best medical care in nonalcoholic steatohepatitis (BRAVES): A multicentre, open-label, randomised trial. Lancet. 2023;401(10390):1786–97. PMID: 37088093 DOI: 10.1016/S0140-6736(23)00634-7 [DOI] [PubMed] [Google Scholar]
- 3.Cerreto M, Santopaolo F, Gasbarrini A, Pompili M, Ponziani FR. Bariatric surgery and liver disease: General considerations and role of the gut-liver axis. Nutrients. 2021;13(8):2649. PMID: 34444807 PMCID: DOI: 10.3390/nu13082649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaggini M, Carli F, Rosso C, et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology. 2018;67(1):145–58. PMID: 28802074 DOI: 10.1002/hep.29465 [DOI] [PubMed] [Google Scholar]
- 5.Leonetti S, Herzog RI, Caprio S, Santoro N, Tricò D. Glutamate-serineglycine index: A novel potential biomarker in pediatric non-alcoholic fatty liver disease. Children (Basel). 2020;7(12):270. PMID: 33291552 PMCID: DOI: 10.3390/children7120270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibrahim SH, Kohli R, Gores GJ. Mechanisms of lipotoxicity in NAFLD and clinical implications. J Pediatr Gastroenterol Nutr. 2011;53(2):131–40. PMID: 21629127 PMCID: DOI: 10.1097/MPG.0b013e31822578db [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferramosca A, Zara V. Modulation of hepatic steatosis by dietary fatty acids. World J Gastroenterol. 2014;20(7):1746–55. PMID: 24587652 PMCID: DOI: 10.3748/wjg.v20.i7.1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obara N, Fukushima K, Ueno Y, et al. Possible involvement and the mechanisms of excess trans-fatty acid consumption in severe NAFLD in mice. J Hepatol. 2010;53(2):326–34. PMID: 20462650 DOI: 10.1016/j.jhep.2010.02.029 [DOI] [PubMed] [Google Scholar]
- 9.Enooku K, Nakagawa H, Fujiwara N, et al. Altered serum acylcarnitine profile is associated with the status of nonalcoholic fatty liver disease (NAFLD) and NAFLD-related hepatocellular carcinoma. Sci Rep. 2019;9(1):10663. PMID: 31337855 PMCID: DOI: 10.1038/s41598-019-47216-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mojtahed A, Kelly CJ, Herlihy AH, et al. Reference range of liver corrected T1 values in a population at low risk for fatty liver disease-a UK Biobank sub-study, with an appendix of interesting cases. Abdom Radiol (NY). 2019;44(1):72–84. PMID: 30032383 PMCID: DOI: 10.1007/s00261-018-1701-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachtiar V, Kelly MD, Wilman HR, et al. Repeatability and reproducibility of multiparametric magnetic resonance imaging of the liver. PLoS One. 2019;14(4):e0214921. PMID: 30970039 PMCID: DOI: 10.1371/journal.pone.0214921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin M, Talwalkar JA, Glaser KJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5(10):1207-13.e2. PMID: 17916548 PMCID: DOI: 10.1016/j.cgh.2007.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259(3):749–56. PMID: 21460032 PMCID: DOI: 10.1148/radiol.11101942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattar SG, Velcu LM, Rabinovitz M, et al. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann Surg. 2005;242(4):610–20. PMID: 16192822 PMCID: DOI: 10.1097/01.sla.0000179652.07502.3f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiner, R.A., Surgical treatment of non-alcoholic steatohepatitis and non-alcoholic fatty liver disease. Dig Dis. 2010;28(1):274–9. PMID: 20460923 DOI: 10.1159/000282102 [DOI] [PubMed] [Google Scholar]
- 16.Lee Y, Doumouras AG, Yu J, et al. Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;17(6):1040-60.e11. PMID: 30326299 DOI: 10.1016/j.cgh.2018.10.017 [DOI] [PubMed] [Google Scholar]
- 17.Eslam M, Sarin SK, Wong VW, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14(6):889–919. PMID: 33006093 DOI: 10.1007/s12072-020-10094-2 [DOI] [PubMed] [Google Scholar]
- 18.Heyens LJM, Busschots D, Koek GH, Robaeys G, Francque S. Liver Fibrosis in Non-alcoholic Fatty Liver Disease: From Liver Biopsy to Non-invasive Biomarkers in Diagnosis and Treatment. Front Med (Lausanne). 2021;8:615978. PMID: 33937277 PMCID: DOI: 10.3389/fmed.2021.615978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng KY, Watt MJ, Rensen S, et al. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. J Lipid Res. 2018;59(10):1977–86. PMID: 30042157 PMCID: DOI: 10.1194/jlr.M085613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutkowsky JM, Knotts TA, Ono-Moore KD, et al. Acylcarnitines activate proinflammatory signaling pathways. Am J Physiol Endocrinol Metab. 2014;306(12):E1378-87. PMID: 24760988 PMCID: DOI: 10.1152/ajpendo.00656.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sampey BP, Freemerman AJ, Zhang J, et al. Metabolomic profiling reveals mitochondrial-derived lipid biomarkers that drive obesityassociated inflammation. PLoS One. 2012;7(6):e38812. PMID: 22701716 PMCID: DOI: 10.1371/journal.pone.0038812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams SH, Hoppel CL, Lok KH, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. 2009;139(6):1073–81. PMID: 19369366 PMCID: DOI: 10.3945/jn.108.103754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wajner M, Amaral AU. Mitochondrial dysfunction in fatty acid oxidation disorders: Insights from human and animal studies. Biosci Rep. 2015;36(1):e00281. PMID: 26589966 PMCID: DOI: 10.1042/BSR20150240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura MT, Yudell BE, Loor JJ. Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res. 2014;53:124–44. PMID: 24362249 DOI: 10.1016/j.plipres.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 25.DiNicolantonio JJ, O’Keefe JH. Good fats versus bad fats: A comparison of fatty acids in the promotion of insulin resistance, inflammation, and obesity. Mo Med. 2017;114(4):303–7. PMID: 30228616 PMCID: [PMC free article] [PubMed] [Google Scholar]
- 26.Meex RCR, Watt MJ. Hepatokines: Linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. 2017;13(9):509–20. PMID: 28621339 DOI: 10.1038/nrendo.2017.56 [DOI] [PubMed] [Google Scholar]
- 27.Neuschwander-Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52(3):913–924. PMID: 20648476 PMCID: DOI: 10.1002/hep.23784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan HC, Shumbayawonda E, Beyer C, et al. Multiparametric magnetic resonance imaging and magnetic resonance elastography to evaluate the early effects of bariatric surgery on nonalcoholic fatty liver disease. Int J Biomed Imaging. 2023;2023:4228321. PMID: 37521027 PMCID: DOI: 10.1155/2023/4228321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caussy C, Hsu C, Singh S, et al. Serum bile acid patterns are associated with the presence of NAFLD in twins, and dose-dependent changes with increase in fibrosis stage in patients with biopsy-proven NAFLD. Aliment Pharmacol Ther. 2019;49(2):183–93. PMID: 30506692 PMCID: DOI: 10.1111/apt.15035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masoodi M, Gastaldelli A, Hyötyläinen T, et al. Metabolomics and lipidomics in NAFLD: Biomarkers and non-invasive diagnostic tests. Nat Rev Gastroenterol Hepatol. 2021;18(12):835–56. PMID: 34508238 DOI: 10.1038/s41575-021-00502-9 [DOI] [PubMed] [Google Scholar]
- 31.Nimer N, Choucair I, Wang Z, et al. Bile acids profile, histopathological indices and genetic variants for non-alcoholic fatty liver disease progression. Metabolism. 2021;116:154457. PMID: 33275980 PMCID: DOI: 10.1016/j.metabol.2020.154457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalhan SC, Guo L, Edmison J, et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism. 2011;60(3):404–13. PMID: 20423748 PMCID: DOI: 10.1016/j.metabol.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mardinoglu A, Bjornson E, Zhang C, et al. Personal model-assisted identification of NAD+ and glutathione metabolism as intervention target in NAFLD. Mol Syst Biol. 2017;13(3):916 DOI: 10.15252/msb.20167422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830(5): 3143–53. PMID: 22995213 PMCID: DOI: 10.1016/j.bbagen.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han D, Hanawa N, Saberi B, Kaplowitz N. Mechanisms of liver injury. III. Role of glutathione redox status in liver injury. Am J Physiol Gastrointest Liver Physiol. 2006;291(1):G1-7. PMID: 16500922 DOI: 10.1152/ajpgi.00001.2006 [DOI] [PubMed] [Google Scholar]
- 36.Yuan L, Kaplowitz N. Glutathione in liver diseases and hepatotoxicity. Mol Aspects Med. 2009;30(1-2):29–41. PMID: 18786561 DOI: 10.1016/j.mam.2008.08.003 [DOI] [PubMed] [Google Scholar]
- 37.Vairetti M, Ferrigno A, Bertone R, Richelmi P, Bertè F, Freitas I. Apoptosis vs. necrosis: Glutathione-mediated cell death during rewarming of rat hepatocytes. Biochim Biophys Acta. 2005;1740(3): 367–74. PMID: 15949704 DOI: 10.1016/j.bbadis.2004.11.022 [DOI] [PubMed] [Google Scholar]
- 38.Bayram B, Rimbach G, Frank J, Esatbeyoglu T. Rapid method for glutathione quantitation using high-performance liquid chromatography with coulometric electrochemical detection. J Agric Food Chem. 2014;62(2):402–8. PMID: 24328299 DOI: 10.1021/jf403857h [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa T, Iino C, Endo T, et al. Changed amino acids in NAFLD and liver fibrosis: A large cross-sectional study without influence of insulin resistance. Nutrients. 2020;12(5):1450. PMID: 32429590 PMCID: DOI: 10.3390/nu12051450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan HC, Hsu JW, Tai ES, et al. De novo glycine synthesis is reduced in adults with morbid obesity and increases following bariatric surgery. Front Endocrinol (Lausanne). 2022;13:900343. PMID: 35757406 PMCID: DOI: 10.3389/fendo.2022.900343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luzi L, Castellino P, DeFronzo RA. Insulin and hyperaminoacidemia regulate by a different mechanism leucine turnover and oxidation in obesity. Am J Physiol. 1996;270(2 Pt 1):E273-81. PMID: 8779949 DOI: 10.1152/ajpendo.1996.270.2.E273 [DOI] [PubMed] [Google Scholar]
- 42.Utzschneider KM, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006; 91(12):4753–61. PMID: 16968800 DOI: 10.1210/jc.2006-0587 [DOI] [PubMed] [Google Scholar]
- 43.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance [published correction appears in Cell Metab. 2009;9(4):311–26. PMID: 19356713 PMCID: DOI: 10.1016/j.cmet.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu J, Xie G, Jia W, Jia W. Insulin resistance and the metabolism of branched-chain amino acids. Front Med. 2013;7(1):53–9. PMID: 23385611 DOI: 10.1007/s11684-013-0255-5 [DOI] [PubMed] [Google Scholar]
- 45.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10(12): 723–36. PMID: 25287287 PMCID: DOI: 10.1038/nrendo.2014.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan HC, Hsu JW, Kovalik JP, et al. Branched-chain amino acid oxidation is elevated in adults with morbid obesity and decreases significantly after sleeve gastrectomy. J Nutr. 2020;150(12):3180–9. PMID: 33097955 DOI: 10.1093/jn/nxaa298 [DOI] [PubMed] [Google Scholar]
- 47.Yao J, Kovalik JP, Lai OF, et al. Comprehensive assessment of the effects of sleeve gastrectomy on glucose, lipid, and amino acid metabolism in Asian individuals with morbid obesity. Obes Surg. 2019;29(1): 149–58. PMID: 30191503 DOI: 10.1007/s11695-018-3487-2 [DOI] [PubMed] [Google Scholar]
- 48.Tan HC, Khoo CM, Tan MZ, et al. The effects of sleeve gastrectomy and gastric bypass on branched-chain amino acid metabolism 1 year after bariatric surgery. Obes Surg. 2016;26(8):1830–5. PMID: 26729279 DOI: 10.1007/s11695-015-2023-x [DOI] [PubMed] [Google Scholar]
- 49.Morgan MY, Marshall AW, Milsom JP, Sherlock S. Plasma aminoacid patterns in liver disease. Gut. 1982;23(5):362–70. PMID: 7076013 PMCID: DOI: 10.1136/gut.23.5.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Azuma Y, Maekawa M, Kuwabara Y, Nakajima T, Taniguchi K, Kanno T. Determination of branched-chain amino acids and tyrosine in serum of patients with various hepatic diseases, and its clinical usefulness. Clin Chem. 1989;35(7):1399–1403. PMID: 2758584 [PubMed] [Google Scholar]
- 51.Kawanaka M, Nishino K, Oka T, et al. Tyrosine levels are associated with insulin resistance in patients with nonalcoholic fatty liver disease. Hepat Med. 2015;7:29–35. PMID: 26082668 PMCID: DOI: 10.2147/HMER.S79100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dennis A, Kelly MD, Fernandes C, et al. Correlations between MRI biomarkers PDFF and cT1 with histopathological features of nonalcoholic steatohepatitis. Front Endocrinol (Lausanne). 2021;11:575843. PMID: 33584535 PMCID: DOI: 10.3389/fendo.2020.575843 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets analyzed in the study are under license and not publicly available for sharing.