Abstract
The c-Mer receptor tyrosine kinase (RTK) is most closely related to chicken c-Eyk and belongs to the Axl RTK subfamily. Although not detected in normal lymphocytes, c-Mer is expressed in B- and T-cell leukemia cell lines, suggesting an association with lymphoid malignancies. To gain an understanding of the role of this receptor in lymphoid cells, we expressed in murine interleukin-3 (IL-3)-dependent Ba/F3 pro-B-lymphocyte cells a constitutively active receptor, CDMer, formed from the CD8 extracellular domain and the c-Mer intracellular domain. Cells transfected with a plasmid encoding the CDMer receptor became IL-3 independent. When tyrosine (Y)-to-phenylalanine (F) mutations were introduced into c-Mer, only the Y867 change significantly reduced the IL-3-independent cell proliferation. The Y867 residue in the CDMer receptor mediated the binding of Grb2, which recruited the p85 phosphatidylinositol 3-kinase (PI 3-kinase). Despite the difference in promotion of proliferation, both the CDMer and mutant F867 receptors activated Erk in transfected cells. On the other hand, we found that both transcriptional activation of NF-κB and activation of PI 3-kinase were significantly suppressed with the F867 mutant receptor, suggesting that the activation of antiapoptotic pathways is the major mechanism for the observed phenotypic difference. Consistent with this notion, apoptosis induced by IL-3 withdrawal was strongly prevented by CDMer but not by the F867 mutant receptor.
The human c-Mer receptor tyrosine kinase (RTK) has been identified by screening a B-lymphoblastoid expression library with antiphosphotyrosine antibodies (22), and mouse c-Mer was described as a homologue of human c-Mer (21). We also independently isolated c-Mer in the search for the mammalian homologue of avian c-Eyk by screening a mouse embryo library (50). Previously, the proto-oncogene c-eyk, which encodes the c-Eyk RTK (25), was identified as the cellular counterpart of a sarcoma-inducing oncogene of an avian retrovirus (26).
Both c-Mer and c-Eyk have the same overall structure, consisting of an extracellular region comprising two immunoglobulin-like and two fibronectin type III repeats, a transmembrane region, and an intracellular region containing the kinase domain. This particular structure led to their assignment to the Axl/Ufo subfamily of RTKs (35). The amino acid sequence identity between c-Eyk and mouse c-Mer (69% in the intracellular domain) is lower than that between the mouse and human c-Mer proteins (86%) but higher than that between c-Mer and other members of the Axl RTK family (e.g., 57% identity with mouse Axl), suggesting that c-Mer might be a homologue of c-Eyk (21). The ligand for c-Eyk is unknown, and only very recently were data obtained indicating that Gas6 is a ligand for c-Mer (10, 33). Gas6 has been previously identified as the ligand for Axl (45) and Rse (18), both members of the Axl RTK family. The physiological roles of the Axl family receptors are not known. Recently, it has been shown that Gas6-Axl signaling through the phosphatidylinositol 3-kinase (PI 3-kinase) and Src-dependent pathways was required for the prevention of apoptosis in cells expressing this receptor (20). The signaling pathway of c-Eyk was studied by utilizing a CD8–c-Eyk fusion system that is constitutively activated through dimerization (49). This study indicated that activated c-Eyk specifically stimulates the Jak-Stat pathway, with little effect on the mitogen-activated protein (MAP) kinase or c-Jun N-terminal kinase (JNK) pathway. Upon constitutive activation, it also induces rat cell transformation, which appears to be correlated with Stat activation. A study of c-Mer signaling and transformation, using colony-stimulating factor 1 stimulation of an Fms-Mer chimeric receptor transfected into NIH 3T3 fibroblasts, pointed out that fibroblasts proliferate and can be transformed upon stimulation (30). Although Stat activation was not investigated, the authors showed that phospholipase C-γ (PLC-γ), PI 3-kinase/p70 S6 kinase, Grb2, Shc, Raf-1, and MAP kinase are downstream components of the c-Mer transduction pathways. This suggested that c-Eyk and c-Mer signal through different downstream effectors.
The expression of the c-Mer mRNA occurs mainly in monocytes and tissues of epithelial and reproductive origin (22). Although it is not detected in normal peripheral lymphocytes (22) or in thymocytes (21), c-Mer is expressed in B- and T-cell leukemia cell lines, suggesting an association of its expression with lymphoid malignancies (22). In this study, to gain an understanding of the signaling of c-Mer in hematopoietic cells, we expressed a constitutively active receptor, CDMer, formed from the extracellular region of CD8 and the intracellular region of c-Mer, in hematopoietic cells that do not express endogenous c-Mer. These cells, which require interleukin-3 (IL-3) for growth, were rendered IL-3 independent when stably transfected with the CDMer receptor. To determine which region in the c-Mer intracellular domain conveys signals for proliferation, we altered four tyrosine (Y) residues, three of which are conserved among the members of the Axl RTK family, to phenylalanine (F). A Y867-to-F mutation in the Grb2 binding site of the receptor, but not other Y-to-F mutations, reduced significantly the ability of transfected cells to proliferate in the absence of IL-3. While looking for the pathway responsible for the difference in proliferation, we found that nuclear factor κB (NF-κB) was strongly activated in cells expressing CDMer and significantly less activated in cells expressing the F867 mutant receptor. We show here that constitutively active c-Mer induces NF-κB activation, which is correlated with the proliferative and antiapoptotic effects of this receptor in hematopoietic cells.
MATERIALS AND METHODS
Generation of CDMer and mutant receptors.
The full-length mouse c-Mer (994 amino acids) was cloned from a 16-day-old mouse embryo cDNA library. The retroviral expression vector pLXSN (31), in which the cloning site was modified by the insertion of the EcoRI site between the XhoI and BamHI sites, was used for cloning a chimeric receptor, CDMer (687 amino acids), formed from the intracellular part of c-Mer (amino acids 521 to 994, inserted between the EcoRI and BamHI sites) and the extracellular and transmembrane domains of the human CD8α receptor (amino acids 1 to 209). The CD8 component was inserted in the XhoI site of pLXSN as a SalI digest of the vector BSSK (a gift of A. August). Similarly, chimeric receptors containing mutations in the intracellular region of c-Mer were obtained. A truncated receptor, MerΔ (552 amino acids), lacking the entire carboxy-terminal region of c-Mer was obtained by inserting a stop codon after the kinase domain. Mutations in the intracellular region of c-Mer were introduced by PCR with primers containing the specific mutations (23). Mutation of the ATP-binding lysine (L) 614 to methionine (M) resulted in the kinase-negative mutant KN, and Y-to-F changes resulted in the mutants F544, F825, F867, F924, 2F (positions 867 and 924), and 3F (positions 544, 867, and 924). All of the PCR amplifications were performed in 30 cycles with the proof-reading Pfu DNA polymerase (Stratagene). The nucleotide sequence of the intracellular region for each of these constructs was determined to ensure that the expected mutations were present and that no additional mutations were introduced by PCR.
Cell lines and retroviral infection.
Murine IL-3-dependent Ba/F3 cells, a pro-B-lymphocyte cell line (37), grown in RPMI 1640 supplemented with 10% fetal calf serum (FCS) and 0.5% mouse IL-3-containing supernatant from the IL-3-overproducing X63 derivative cell line (27), were used for generating cell lines stably expressing the various receptors. Bosc23 retrovirus-packaging cells (38), maintained in Dulbecco modified Eagle’s medium with 10% FCS, were transfected in duplicate, using Lipofectamine (Gibco BRL) and 10 μg each of the plasmids encoding the various receptors. After 30 h, the transfected Bosc23 cells were treated for 3 h with mitomycin C (10 μg/ml) to arrest cell growth, washed three times with phosphate-buffered saline (PBS), and subsequently cocultured for 48 h with 106 Ba/F3 cells in the presence of IL-3 and Polybrene (4 μg/ml; Sigma). Infected Ba/F3 cells were transferred to new culture dishes and grown in selection medium containing G418 (1 mg/ml; Calbiochem). Stably transfected Ba/F3 cells were obtained after approximately 8 days of selection and further maintained in medium containing 0.5 mg of G418/ml.
Cytofluorometric analysis of cells.
The levels of expression of the stably transfected receptors were periodically determined by cytofluorometric analysis. Two anti-CD8 primary antibodies were used, with similar results: the monoclonal antibody OKT8 (Ortho) and the fluorescein (FITC)-conjugated antibody 3B5 (Caltag). Cells (106) were incubated for 30 min with the primary antibody and then washed three times with cold PBS containing 5% FCS and 0.02% sodium azide. When the primary antibody was directly labeled with FITC, a matching-isotype FITC-conjugated control (Caltag) was used.
Apoptosis of cells deprived of IL-3 for various periods of time (from 9 to 16 h) was measured by using FITC-conjugated annexin V (PharMingen) (46) and propidium iodide staining as directed by the manufacturer. Fluorescence was detected with a FACScan flow cytometer (Becton Dickinson), and 10,000 to 20,000 cells were acquired and analyzed with the Cell-Quest software.
Proliferation assay and inhibition of growth by the use of inhibitors.
The proliferation of cells transfected with plasmids encoding the various receptors in the absence of IL-3 was assessed with the colorimetric CellTiter 96 aqueous nonradioactive cell proliferation assay system (Promega). Cells were washed twice in RPMI 1640 supplemented with 5% FCS, counted with a hemocytometer after treatment with trypan blue, and dispensed in 96-well plates at a density of 2 × 104 or 1 × 105/well. Cells were cultured in RPMI 1640 with 10% FCS either in the absence of IL-3 or with IL-3 at optimal concentration for various periods of time. The cells were subsequently incubated for 4 h with the tetrazolium reagents provided in the CellTiter 96 kit in accordance with the instructions of the manufacturer. The absorbance at 490 nm, measuring the amount of the tetrazolium reagent [3-(4,5)-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt (MTS) that is reduced in direct proportion to the number of living cells, was recorded in an enzyme-linked immunosorbent assay plate reader.
Inhibitors of different signaling pathways were used in a proliferation assay. A proliferation dose-response curve was produced for IL-3-deprived, CDMer-transfected cells, using SB 203580 (Calbiochem), which specifically inhibits the p38 MAP kinase (12), and wortmannin and LY 294002 (Calbiochem), which inhibit the PI 3-kinase. To compare the levels of proliferation of cells expressing the various receptors, 50% inhibitory doses were used. Wortmannin was used in doses of 0.8 and 1.6 μM, previously reported to inhibit the phosphorylation of the S6 kinase that is downstream of the PI 3-kinase but not that of the MAP kinase in Ba/F3 cells (28). PD 098059 (Calbiochem), which inhibits MAP kinase kinase MEK1 (13), was used at 40 μM, the highest concentration at which, in our hands, the compound did not precipitate in the medium.
Immunoprecipitation, in vitro binding assay, and immunoblotting.
Cells were deprived of IL-3 by washing them twice and then culturing them overnight in medium without IL-3. IL-3-deprived or nondeprived cells, washed once in cold PBS containing 1 mM sodium orthovanadate, were lysed in lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 10 mM EDTA, 10% glycerol, 1% Triton X-100, 1 mM sodium orthovanadate, 0.1 mM sodium molybdate, 1 mM phenylmethylsulfonyl fluoride, 21 μg of aprotinin/ml 5 μg of leupeptin/ml). Proteins were immunoprecipitated by incubating the lysate with specific antibodies for 2 h or overnight and further collected on protein A/G-agarose beads (Santa Cruz Biotechnology) for 1 h at 4°C. The antibodies against c-Mer were kindly provided by H.-J. Kung (30), and those used for immunoprecipitating p85 PI 3-kinase were from L.-H. Wang. Other antibodies were purchased from Transduction Laboratories (anti-Grb2, anti-Shc, and antiphosphotyrosine [RC20]), Santa Cruz Biotechnology (anti-Grb2, anti-p85 PI 3-kinase, anti-Sos, anti-Erk1, and anti-Erk2), Upstate Biotechnology (antiphosphotyrosine [4G10]), and Sigma (anti-activated Erk). For in vitro association experiments, 10-μg quantities of glutathione S-transferase (GST) fusion proteins prepared by standard procedures were incubated with 3-mg quantities of cell lysates, and the complexes were collected by using glutathione-agarose beads (Molecular Probes). The immune complexes were washed three times in lysis buffer, denatured by boiling for 10 min in double-strength sample buffer (0.125 M Tris-HCl [pH 6.8], 4% sodium dodecyl sulfate (SDS), 10% 2-mercaptoethanol, 20% glycerol, 0.004% bromophenol blue), and resolved by SDS-polyacrylamide gel electrophoresis (PAGE). Proteins were transferred onto Immobilon membranes (Millipore). The membranes were blocked with 3% bovine serum albumin (Sigma) in TBS-T buffer (10 mM Tris-HCl [pH 8], 150 mM NaCl, 0.1% Tween 20) overnight at 4°C and then probed with specific antibodies. After incubation with specific horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories), the complexes were visualized with enhanced chemiluminescence solutions (NEN).
MAP kinase assay.
Cells deprived of IL-3 overnight were stimulated with 0.5% IL-3 for 10 min. Stimulated and unstimulated cells were lysed in kinase lysis buffer (10 mM Tris-HCl [pH 7.5], 50 mM NaCl, 30 mM sodium pyrophosphate, 50 mM sodium fluoride, 20 mM β-glycerophosphate, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100), and Erk1 and -2 were immunoprecipitated from the lysates. In vitro kinase reactions were carried out in 20-μl volumes of kinase reaction buffer containing 30 mM HEPES [pH 7.1], 10 mM MgCl2, 1 mM dithiothreitol, 50 μM ATP, 2.5 μCi of [γ-32P]ATP, and 1 μg of myelin basic protein (Sigma) as substrate. Following incubation for 30 min at 30°C, the reactions were terminated by addition of 5× sample buffer and proteins were resolved by SDS-PAGE.
Luciferase assays.
Transfection of Ba/F3 cells (2.5 × 106) with 10-μg quantities of the different luciferase reporter plasmids was done by electroporation at 960 μF and 300 V in culture medium at room temperature. After 12 h of recovery in IL-3-containing complete medium, the cells were washed twice, resuspended in RPMI supplemented with only 0.5% FCS, and divided into two portions; half of the cells were starved for 12 h, and half were starved for 6 h and subsequently stimulated with IL-3 for an additional 6 h. Cytoplasmic extracts and luciferase assays were performed in accordance with the Promega protocol. The NF-κB–luciferase reporter construct contains four repeats of the NF-κB-responsive element in a Rous sarcoma virus long terminal repeat minimal promoter (41) cloned in the vector pGV-B2 (Toyo Ink) (1). The minimal promoter lacking the NF-κB repeats was also engineered in the pGV-B2 luciferase reporter vector. The inhibition of the NF-κB activity was assayed by cotransfecting 10 μg of the inhibitor of κB (IκB-α), cloned in the vector pRc/CMV (24), and 7 μg of the NF-κB reporter plasmid into Ba/F3 cells.
RESULTS
Expression of CDMer and mutant receptors in IL-3-dependent cells.
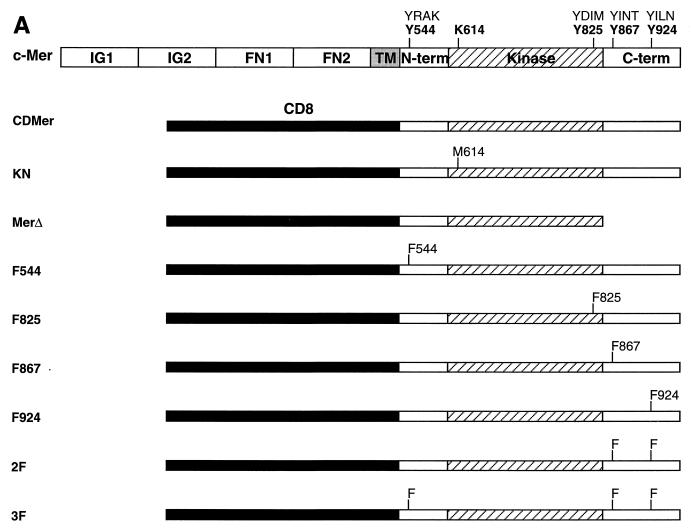
To dissect the signal transduction pathway of the c-Mer tyrosine kinase receptor, Y-to-F mutations were engineered in a constitutively active chimeric molecule, CDMer, formed from the extracellular domain of CD8α and the intracellular domain of the mouse c-Mer receptor. The extracellular domain of CD8α induces ligand-independent dimerization and, hence, constitutive activation of the dimeric receptor by forming intermolecular disulfide bonds (49). The intracellular part of c-Mer contains 16 tyrosine residues: 13 in the kinase domain, 1 in the N-terminal region, and 2 highly conserved residues among the members of the Axl RTK subfamily in the C-terminal region (Fig. 1A). One tyrosine in the kinase domain that is part of the consensus binding motif for the p85 PI 3-kinase (42) and all three tyrosines from the extrakinase regions were mutated, resulting in the single mutants F544, F825, F867, and F924 and the double and the triple mutants 2F and 3F, respectively (Fig. 1A). A deletion of the C-terminal part of c-Mer resulted in the MerΔ truncated receptor. The kinase-negative receptor KN was obtained via a K614-to-M mutation in the kinase ATP-binding site.
FIG. 1.
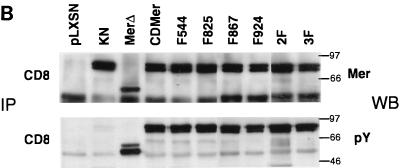
Expression of CDMer and mutant receptors. (A) Schematic drawing of c-Mer and the chimeric receptors used in this study. The transmembrane (TM) and extracellular regions of c-Mer, the latter consisting of two immunoglobulin-like (IG1 and IG2) and two fibronectin type III (FN1 and FN2) repeats, are replaced by the extracellular and transmembrane regions of CD8 in the CDMer construct. The intracellular region of c-Mer is schematically divided into the kinase domain, flanked by N-terminal (N-term) and C-terminal (C-term) domains. The amino acids that were mutagenized in the intracellular region of c-Mer and their corresponding 4-residue motifs are indicated above the map of c-Mer. Amino acid positions correspond to the c-Mer sequence. (B) Expression and phosphorylation of CDMer and mutant receptors. Receptors were immunoprecipitated (IP) with anti-CD8 antibodies from lysates of stably transfected Ba/F3 cells and detected as doublets of 91 to 93 kDa. Filters were Western blotted (WB) with antiphosphotyrosine antibody (pY), stripped, and reprobed with anti-Mer antibody. Due to the constitutive dimerization mediated by the CD8 extracellular regions, the receptors autophosphorylate in the absence of ligand. Data for only one transfected cell population for each receptor are shown, and they are identical to those for a second, independently transfected population.
In the IL-3-dependent Ba/F3 cells, we did not detect endogenous expression of c-Mer by immunoblotting with anti-Mer antibody or by reverse transcription of extracted mRNA followed by PCR with specific primers (data not shown). In these cells, CDMer and mutant receptors were expressed at similar levels, as determined by immunoblotting following immunoprecipitation with an anti-CD8 specific antibody (Fig. 1B) or by fluorescence-activated cell sorter (FACS) analysis, except for the MerΔ receptor, which by FACS analysis had a lower expression level (data not shown). The receptors, immunoprecipitated with anti-CD8 antibody, migrated in SDS-PAGE gels as doublets of 91 to 93 kDa (Fig. 1B). The MerΔ truncated receptor had a lower molecular mass. We examined the phosphorylation of the mutant receptors by immunoblotting with an antiphosphotyrosine antibody and found that all of the receptors were similarly phosphorylated (Fig. 1B). This indicated that the substituted tyrosines neither impaired the c-Mer kinase’s ability to autophosphorylate nor were major sites of autophosphorylation. As expected, the KN receptor did not show autophosphorylation.
The kinase activity and Y867 residue of CDMer are required to induce IL-3-independent cell proliferation.
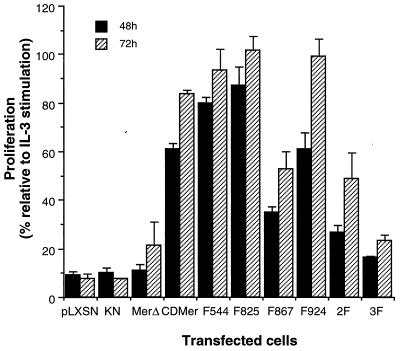
We analyzed the ability of Ba/F3 cells stably expressing CDMer or mutant receptors to proliferate in the absence of IL-3. Cells were plated in equal numbers in medium lacking IL-3, and their growth after 48 or 72 h was compared to that of matched cells grown in the presence of IL-3. As shown in Fig. 2, the cells expressing CDMer or one of three single mutants, F544, F825, or F924, maintained IL-3-independent growth. In contrast, IL-3-deprived cells transfected with the vector alone or with plasmids encoding the KN or MerΔ receptor exhibited minimal growth. Cells expressing the single-mutant F867, the double-mutant 2F, or the triple-mutant 3F receptor (the last two containing a mutation at Y867) displayed an intermediate phenotype in the proliferation assay. These data indicated that the constitutively active CDMer receptor could substitute signaling through the IL-3 receptor for cell proliferation and that Y867 is the major site for mediating this effect. The other mutation in the carboxyl tail (position 924) did not appear to impair cell growth, as shown for the single and double mutants. Cells with the triple-mutant receptor 3F, containing Y-to-F changes at all of the tyrosines of the extrakinase regions, exhibited a more profound retardation of growth than did those with the F867 receptor. Interestingly, the mutation at Y825, which lies in a PI 3-kinase consensus binding motif, promoted an increased proliferation of the cells. Cell proliferation was observed with all of the Y-to-F mutants, implying that other tyrosines in the kinase region or the presence of the tyrosine kinase activity of c-Mer might account for a basal proliferation level in cells deprived of IL-3.
FIG. 2.
Proliferation of cells expressing CDMer and mutant receptors. Cells were seeded in equal initial numbers (105/well) for five replicates of each transfected cell population in medium with and without IL-3. After 48 or 72 h, the absorbance at 490 nm, corresponding to the capacity of living cells to reduce MTS (tetrazolium reagent), of each culture was measured. Columns represent the percentage ratio of the mean absorbance of cells grown without IL-3 to the mean absorbance of the same cells grown in the presence of IL-3, and they reflect the ability of cells to grow in the absence of IL-3. Standard deviations, indicated by the error bars, were calculated from the percentage values obtained for two independently transfected populations of each construct. This experiment was repeated four times with similar results.
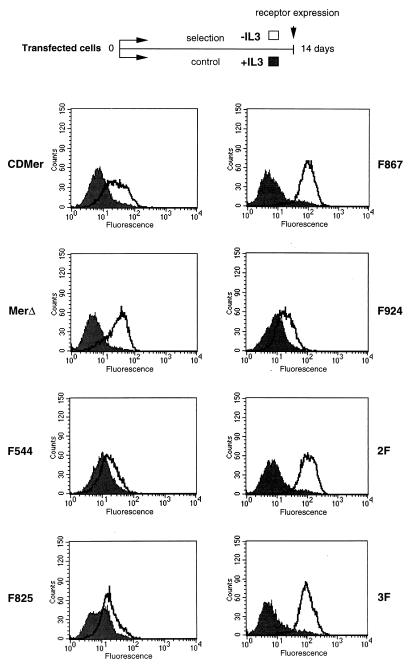
We next examined whether these cells could be maintained in culture in the absence of IL-3 for longer periods of time. Cells transfected with the vector or with the plasmid encoding the KN receptor mutant died after 3 days of incubation in medium lacking IL-3. This confirmed that the kinase activity was required for the proliferation phenotype. Consistent with their behavior after 3 days in the proliferation assay, CDMer, F544, F825, or F924 receptor-expressing cells did not die and could be propagated in the absence of IL-3. After 2 weeks of culture in medium without IL-3, these cells showed only a slight increase in the expression level of the receptor compared to cells cultured in medium containing IL-3 (Fig. 3). In the absence of IL-3, the majority of the cells expressing the MerΔ truncated-receptor mutant or, to a lesser extent, the cells expressing receptors with a mutation at Y867 died in the first 4 to 5 days of culture. However, after 7 days, cells growing independently of IL-3 could be selected. These selected surviving cells had significantly increased receptor expression levels compared to the corresponding control cells grown in the presence of IL-3 (Fig. 3). This experiment showed that the receptors containing nonmutated Y867 residues were able to sustain long-term IL-3-independent proliferation. The need for a large increase in the level of expression of receptors with a mutation at Y867 in order to maintain proliferation suggested that Y867 is a major determinant in the proliferation process.
FIG. 3.
Increased receptor expression after selection of cells in the absence of IL-3. Cells expressing the indicated receptors were maintained for 14 days in medium with (+) IL-3 (control cells) (filled-in curve) or without (−) IL-3 (selected surviving cells) (unfilled curve). The expression of the receptors was detected by FACScan analysis using an FITC-conjugated anti-CD8 antibody. A scheme of the selection process is shown above the receptor expression histograms.
The Y867 residue in the receptor mediates the binding of Grb2 and the recruitment of p85 PI 3-kinase complexed to a phosphorylated p95 protein.
Y867 is part of a Grb2 consensus binding motif (42). While receptors without a mutation at this site coimmunoprecipitated with Grb2, the Y867-to-F mutants lost the ability to bind Grb2 (Fig. 4A). The amount of Grb2 bound to the receptor was very low, less than 0.2% of the total Grb2 present in the lysate. We could not show direct binding of c-Mer to other endogenously expressed molecules, such as PLC-γ, the p85 subunit of PI 3-kinase, or protein tyrosine phosphatase 1C or 1D, in coprecipitation assays (data not shown).
FIG. 4.
Binding of Grb2 to the receptor and recruitment of p85 PI 3-kinase are dependent on Y867. (A) Binding of Grb2 to the receptors requires phosphorylation of Y867 in the Grb-2 consensus motif. Filters containing immunoprecipitates (IP), obtained with the indicated antibodies, of 3-mg portions of protein from lysates of IL-3-starved cells were Western blotted (WB) with the indicated antibodies. (B) Grb2 complex formation. Protein (3 mg) from lysates of IL-3-starved cells transfected with pLXSN vector or with a plasmid encoding CDMer or the F867 mutant receptor were incubated with GST-Grb2 fusion proteins. GST was fused to full-length (FL) Grb2 or to either of two Grb2 domains: the SH2 domain and the C-terminal SH3 domain (C-SH3). GST alone was used as a control. The filter was probed with antiphosphotyrosine antibody (pY), stripped, and reprobed with the indicated antibodies (WB). Immunoprecipitation of a 95-kDa phosphorylated protein (p95) with anti-p85 PI 3-kinase antibody (C) or with GST-p85 fusion proteins (prepared as for the GST-Grb2 fusion proteins) (D). This phosphorylated protein associated with p85 only in cells expressing receptors with Y867 in the Grb2 binding site.
Grb2 contains one SH2 domain flanked by two SH3 domains and functions as an adapter protein, coupling an activated receptor to many other signaling proteins (40). To determine which proteins associate with Grb2, we used GST-Grb2 fusion proteins in a pulldown assay (Fig. 4B). The p85 subunit of PI 3-kinase was specifically present in a complex with full-length Grb2 or with the C-terminal SH3 domain of Grb2 in CDMer lysates and not in the vector- or F867 mutant-transfected cell lysates. On the other hand, the Ras guanine nucleotide exchange factor Sos was associated with full-length Grb2 in all tested lysates. Considerably more tyrosine-phosphorylated proteins were associated with the GST-Grb2 fusion proteins in the CDMer cell lysate than in the F867 lysate (Fig. 4B).
In Ba/F3 cells, a protein of 95 kDa has been described to be tyrosine phosphorylated and associated with p85 PI 3-kinase after IL-3 stimulation (32), data that we also confirmed (not shown). We also detected phosphorylation of the p95 protein and its association with the p85 subunit of PI 3-kinase in cells expressing CDMer but not in cells expressing with the plasmid encoding the F867 mutant receptor (Fig. 4C). This association was mediated by the N-terminal SH2 domain of p85 PI 3-kinase (Fig. 4D). We failed to identify this p85-binding protein by immunoblotting with specific antibodies to 90- to 95-kDa proteins (CDMer, 80K-H, Gab1, FRS2, eps8, or Stat5). A 95-kDa phosphorylated protein was immunoprecipitated by the C-terminal SH3 domain of Grb2 in the CDMer cell lysate (Fig. 4B), and it is likely that it corresponds to the p95 protein binding to the PI 3-kinase (Fig. 4C and D). If this holds true, the p95 protein might be an adapter protein, bridging the binding of PI 3-kinase to Grb2 in CDMer cells. Similar to the p95 protein, a not-yet-identified 97-kDa adapter protein described in a recent report (17) was phosphorylated upon IL-2 treatment of T lymphocytes and associated with p85 PI 3-kinase through SH2 domains and with Grb2 through SH3 domains. Very recently, a p95 protein phosphorylated after stimulation with IL-3 in Ba/F3 cells and constitutively bound to Grb2 was identified as an adapter PH domain-containing molecule (33a), and it would be interesting to confirm that it corresponds to the p95 protein phosphorylated in CDMer cells. Taken together, these data suggest that Y867 is necessary for binding of Grb2 to the receptor and for the tyrosine phosphorylation of another protein, p95, that may be required to bring p85 PI 3-kinase into the complex.
The MAP kinase is activated in cells expressing either CDMer or the F867 mutant receptor, but PI 3-kinase is preferentially activated by CDMer.
We examined whether the difference in proliferation between the cells expressing CDMer and those expressing the F867 mutant receptor was a consequence of a defect in the activation of a signaling pathway by the mutant receptor. In Ba/F3 cells, IL-3 activates the MAP kinase pathway (44). To determine whether Erk is activated by c-Mer, a MAP kinase assay was performed with cells that were IL-3 starved for 12 h. There was an approximately fivefold activation of Erk in cells expressing CDMer or mutant receptors compared to control vector-transfected cells (Fig. 5A). This slight activation was also confirmed by immunoblotting of immunoprecipitated Erk with antibodies specific for the activated Erk (Fig. 5B). The lack of a significant difference in Erk activation between CDMer- and F867 mutant receptor-expressing cells suggested that CDMer might activate Erk by mechanisms independent of Y867.
FIG. 5.
MAP kinase activation. (A) MAP kinase assay. Proteins (400 μg) from lysates of transfected cells deprived of IL-3 for 12 h were immunoprecipitated with anti-Erk1 and anti-Erk2 antibodies and assayed for kinase activity, using myelin basic protein (MBP) as the substrate. As a positive control, 12-h IL-3-starved cells were stimulated for 10 min with IL-3 and assayed for kinase activity. The amounts of immunoprecipitated Erk1 and Erk2, shown below, were determined by subjecting the same filter to Western blotting (WB) with anti-Erk antibodies. (B) Phosphorylation of MAP kinase. Protein (1,300 μg) from lysates prepared as described for panel A were immunoprecipitated with anti-Erk1 and anti-Erk2 antibodies. Immunoprecipitates were resolved by SDS-PAGE and analyzed by Western blotting with antibodies specifically recognizing activated phosphorylated Erk.
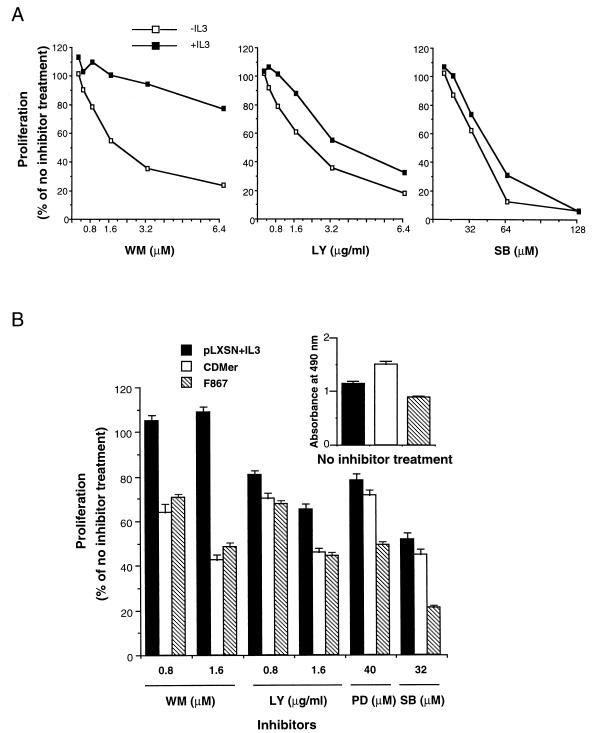
To assess the contribution of signaling pathways to the CDMer-dependent cell proliferation, inhibitors blocking the PI 3-kinase (wortmannin and LY 294002), MEK1 (PD 098059), or p38/MAP kinase (SB 203580) were used in a proliferation assay at approximately 50% inhibitory doses (dose-dependent proliferation curves are displayed in Fig. 6A). The proliferation of CDMer-expressing cells was affected by all inhibitors, suggesting that all of these pathways contribute to the proliferation effect induced by this receptor (Fig. 6B). The Erk pathway inhibitor PD 098059 had a stronger antiproliferative effect on F867 mutant receptor-expressing cells than on CDMer-expressing cells, although Erk was slightly less activated in the former (Fig. 5A). This suggested that the F867 mutant cells deprived of IL-3 were more sensitive to inhibitory stimuli than CDMer-expressing cells. This increased susceptibility to inhibition could be explained by the impaired viability of IL-3-deprived F867 mutant cells (Fig. 6B, upper histogram). In contrast to the MAP kinase inhibitors, which affected the proliferation of F867 mutant cells more strongly than they affected that of CDMer-expressing cells, the PI 3-kinase inhibitors affected both cell types to the same extent (Fig. 6B). This suggests that the contribution of the PI 3-kinase to proliferation is significantly smaller in the case of F867 mutant cells than it is in CDMer-expressing cells.
FIG. 6.
Proliferation of CDMer- or F867 mutant receptor-expressing cells in the presence of inhibitors. (A) Dose-dependent curves at 48 h for CDMer-expressing cells grown in the presence (+IL3) or in the absence (−IL3) of IL-3. (B) Cells expressing the CDMer or F867 mutant receptor were grown in the absence of IL-3, and vector-transfected cells, used as a control, were grown in the presence of IL-3 (pLXSN+IL3) in a 48-h proliferation assay (105 cells/well). The reference proliferation levels of these cells in the absence of inhibitors (with the vehicle dimethyl sulfoxide) are indicated by the absorbance values shown in the upper-right miniature histogram. Wortmannin (WM), LY 294002, PD 098059, and SB 203580 were used at the indicated concentrations. The columns represent the percentage ratio of the mean absorbance (from four replicates) of cells treated with inhibitor to that of cells grown without inhibitor. Error bars indicate the standard deviations of the ratio of the two means calculated from four replicates each. This experiment was repeated three times with similar results.
CDMer and F867 receptors induce differential NF-κB transcription activation and protection from apoptosis in the absence of IL-3.
Since the use of chemical inhibitors could not clearly distinguish between the proliferative responses of cells expressing the CDMer and F867 receptors, we next examined the activation of transcription factors by using Ba/F3 cells transfected with reporter plasmids containing specific responsive elements. Of the four promoters tested—serum-responsive element, NF-κB (1), Lyd9E for Stat1 (47), and β-casein for Stat5 (32)—only the assay for the NF-κB transcription factor resulted in significant differences. As shown in Fig. 7A, the NF-κB transcriptional activity in CDMer-expressing cells was at least 10-fold higher than that in vector-transfected cells and more than 3-fold higher than that in cells expressing the F867 mutant receptor. This difference correlates well with the proliferation phenotypes of these cells, suggesting that NF-κB might be an essential mediator for the biological effects induced by c-Mer. Cells with a high level of expression of the receptor, such as the CDMer-IL3 or F867-IL3 receptor-expressing cells selected in the absence of IL-3 (Fig. 3), demonstrated higher levels of NF-κB activation (Fig. 7B). The specificity of NF-κB activation was shown by transfecting IκB-α (24), which reduced the NF-κB transcriptional activity by at least 10-fold in all of the cell lines tested (Fig. 7B). Also, the reporter plasmid pGV-B2, containing only the minimal promoter lacking the NF-κB elements, was not activated in CDMer-expressing cells (Fig. 7B). We also analyzed whether the inhibitors used for the proliferation study affected NF-κB activation (Fig. 7C). Wortmannin and LY 294002, but not SB 203580, partially decreased the activation of NF-κB by CDMer, suggesting that there might be a link between the activation of PI 3-kinase and NF-κB.
FIG. 7.
NF-κB transcriptional activation by CDMer and F867 receptors. (A) Activation of NF-κB is dependent on Y867. Cells in equal numbers were transfected with 10 μg of NF-κB–luciferase reporter plasmid by electroporation, allowed to recover for 12 h in complete culture medium, and divided into two portions; the luciferase activity was measured after 12 h of IL-3 and serum starvation (black columns) or 6 h of starvation followed by 6 h of 1% IL-3 stimulation (hatched columns). This experiment was repeated five times with the two different transfected populations for each construct, with similar results being obtained every time. (B) Inhibition of NF-κB activation by IκB-α. Cells cotransfected with 10 μg of IκB-α or vector (v) alone and with 7 μg of NF-κB–luciferase reporter plasmid were grown for 18 h in complete medium, and the luciferase activities were measured after 6 h of serum and IL-3 starvation. As a control, the luciferase reporter plasmid pGV-B2 with the minimal promoter (MP) lacking the NF-κB-responsive elements was included. Two populations of cells, CDMer-IL3 and F867-IL3, that were selected after 2 weeks of growth in the absence of IL-3 (see Fig. 3, in which the unfilled curves indicate the level of receptor expression for these cells) were also tested for NF-κB transcriptional activation. This experiment was repeated twice with similar results. (C) NF-κB activation in the presence of inhibitors. CDMer cells transfected with 10 μg of NF-κB–luciferase reporter plasmid and grown for 18 h in complete medium were divided into three portions and either treated with wortmannin (WM), LY294002, or SB 203580 for 6 h in medium without serum and IL-3 or left untreated. This experiment was repeated at least twice. For wortmannin, concentrations of 0.1, 0.5, 1, and 2 μM were tested with similar results. For all experiments, columns represent means from three independently transfected replicates and error bars represent the standard deviations of these values.
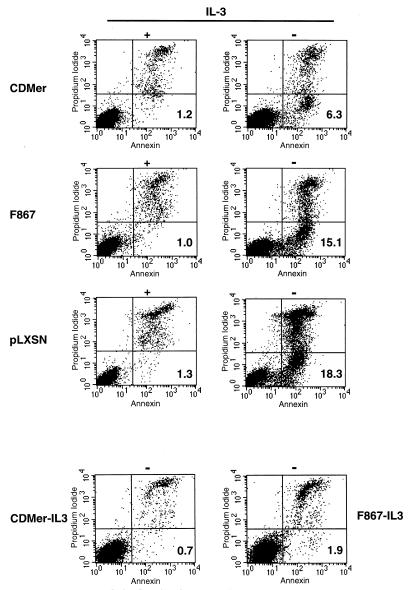
Since NF-κB activation has been shown to be linked to the regulation of programmed cell death in response to diverse stimuli (3), we examined apoptosis by labeling Ba/F3 cells with annexin V after IL-3 deprivation. By this method, early apoptotic cells are detected since annexin V binds to phosphatidylserine phospkolipids that are exposed on the external plasma membrane in an early apoptotic stage. All IL-3-deprived cells, whether transfected with a plasmid encoding CDMer or the F867 mutant receptor or with the vector alone, exhibited apoptosis compared with controls grown in IL-3 (Fig. 8). The number of cells undergoing early apoptosis within the CDMer-expressing population upon withdrawal of IL-3 was almost three times lower than that in the vector-transfected population. This indicates that CDMer protected cells from apoptosis, although not completely, at the given level of expression. Higher levels of CDMer expression completely prevented apoptosis, as observed for CDMer-IL3 cells (Fig. 8). Cells expressing the F867 mutant receptor had low antiapoptotic activity, but the F867-IL3 cells selected in the absence of IL-3 with a very high receptor expression level exhibited only residual apoptosis. These data correlated well with the levels of NF-κB transcriptional activity in these cells (shown in Fig. 7), suggesting that the activation of NF-κB might be essential for the survival of transfected cells deprived of IL-3.
FIG. 8.
Expression of CDMer protects cells from apoptosis induced by IL-3 withdrawal. Cells (2 × 106) transfected with vector (pLXSN) or with a plasmid encoding the CDMer or F867 mutant receptor were grown in medium with (+) or without (−) IL-3 for 12 h before being labeled with annexin V-FITC and propidium iodide. The CDMer-IL3 and F867-IL3 cells that were selected for 2 weeks in the absence of IL-3 (see Fig. 3, in which the unfilled curves indicate the level of receptor expression for these cells) were grown without IL-3. Numbers in the lower-right quadrants indicate percentages of early apoptotic cells labeled only with annexin V.
DISCUSSION
To date, the Axl RTK family comprises three members expressed in a wide variety of tissues (11). Unlike other RTKs, the roles of these receptors and of their ligands are largely unknown. Axl, the prototype member of the family, was shown to be activated by Gas6 (45). However, Gas6 was also shown to activate Rse (18) and, more recently, c-Mer (10). Upon stimulation with Gas6, Axl triggers antiapoptotic signals, and only high doses of ligand induce mitogenesis (5, 19). Although not much is known about its function in normal tissues, Axl has been reported to be overexpressed in some malignancies (9, 43). Based on sequence similarity, c-Mer is thought to be the mammalian homologue of chicken c-Eyk, which was discovered as the cellular counterpart of a retrovirus oncogene (25, 26). In our study, we chose to express c-Mer and study its role in early lymphoid Ba/F3 cells, since c-Mer is frequently expressed in lymphoid malignant cells (22). A constitutively active chimeric receptor containing the intracellular domain of c-Mer was able to support IL-3-independent growth of factor-dependent Ba/F3 cells. By mutational analysis of the c-Mer RTK, we were able to show that the mutation of Y867 in the carboxy terminus drastically affected the signaling and the phenotype of the cells, although other sites might also contribute to the activity of this receptor. Y867 is part of a Grb2 consensus binding site, YXNX (42), and its equivalent in the Axl receptor has also been studied in vivo and in vitro (8, 16). However, unlike c-Mer, this site in Axl is YXNM, which forms the consensus binding motif for Grb2 as well as for p85 PI 3-kinase. Thus, Axl was shown to bind directly to Grb2 and p85 through this site (8, 16), but it was also shown by overexpression experiments to bind to PLC-γ, c-Src, and Lck (8). We were not able to demonstrate direct binding of endogenous p85 to CDMer, but we could specifically detect it in complexes with Grb2 and a phosphorylated p95 molecule only in cells expressing CDMer and not in those expressing the F867 mutant receptor, suggesting an alternate pathway for activation of the PI 3-kinase. Moreover, it may be an important general mechanism of PI-3 kinase activation, since a methionine-to-alanine mutation in the +3 position of the consensus sequence in Axl did not affect the biological behavior induced by the receptor, although it did abolish the direct binding of p85 to the receptor (16). Similarly to our data, in which the Y-to-F point mutation of this site significantly reduced the proliferation and survival of the IL-3-deprived cells, deletion of the entire site in Axl extinguished the proliferation of another IL-3-dependent cell line (16). Therefore, it appears that although they are not identical, these equivalent sites play similar and essential roles in receptor signaling.
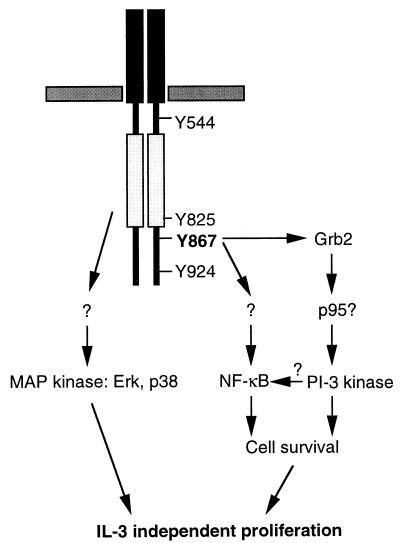
Overall, in IL-3-dependent cells, the constitutively active c-Mer receptor maintained growth in the absence of IL-3 by activating many signaling pathways (Fig. 9). Some of these, such as the MAP kinase and the PI 3-kinase pathways, were activated by both CDMer and IL-3, as illustrated in the proliferation assay using inhibitors. Others, like the Jak-Stat pathway or the one(s) leading to NF-κB activation, were more restricted to IL-3 (2) or CDMer signaling, respectively. Although the Jak-Stat pathway has been shown to be activated by c-Eyk, the chicken homologue of c-Mer (49), we did not find evidence of phosphorylation of Jak2 and Stat5 or activation of Stat5 in CDMer-expressing cells (data not shown). This discrepancy most likely arises from the low degree of amino acid identity between the two receptors in their carboxy termini (22%) and from the presence of three additional tyrosines in the intracellular extrakinase regions of c-Eyk that might be responsible for differences in signaling between these receptors. It is surprising that the two chicken receptors from the Axl family described to date, c-Eyk and Rek (7), have low degrees of overall amino acid identity with their putative mammalian homologues, c-Mer (56%) and Rse (68%), respectively, and that both are most highly divergent in the carboxy terminus. Therefore, it is still debatable whether they are orthologues of the mammalian receptors.
FIG. 9.
A model for signaling pathways of activated c-Mer in Ba/F3 cells.
According to the biological data obtained with CDMer and derivative receptors in Ba/F3 cells, the signals triggered by CDMer protected the cells from apoptosis and induced their proliferation. The antiapoptotic effect appeared to be mediated through the phosphotyrosine in the Grb2 binding site of the receptor, since its mutation to phenylalanine increased the number of cells undergoing apoptosis in the absence of IL-3 almost to the level of the vector-transfected cells. On the other hand, in the presence of the F867 mutant receptor, the proliferation of cells, although significantly affected, was not abolished. There remained a basal level of proliferation, apparently independent of Grb-2 binding, which was affected by the MAP kinase (Erk and p38) inhibitors. This is in agreement with the presence of Erk activity in the F867 mutant cells. It is not clear what signaling intermediates determine this Erk activation in F867 mutant cells, since the interaction of Grb2 with the mutated receptor is disrupted. In Ba/F3 cells, it has been shown that low levels of Ras activation are sufficient for Erk2 activation and that these levels can be achieved by a truncated granulocyte colony-stimulating factor receptor lacking all intracytoplasmic tyrosines (39). In our case, an intact kinase activity of the constitutively active CDMer and mutant receptors that was responsible for an increased total protein phosphorylation in cells (data not shown), together with intermolecular interactions at other sites, might mediate the activation of the Ras-MAP kinase pathway. Supporting this hypothesis, cells expressing the KN mutant receptor lacked total protein phosphorylation and Erk activation (data not shown) as well as a proliferative response.
As mentioned elsewhere, Axl transmits antiapoptotic signals upon Gas6 stimulation in mouse fibroblasts, and PI 3-kinase and c-Src were shown to be necessary in this process (20). We have shown here that constitutively active c-Mer protects hematopoietic cells from apoptotic death and activates NF-κB in these cells. NF-κB has been shown previously to be involved in protecting cells, including B lymphocytes, from apoptosis (4, 29, 48). In Ba/F3 cells, the NF-κB transcriptional activity was preferentially induced by the activated c-Mer receptor and, in perfect correlation with the antiapoptotic effect, was dependent on the tyrosine in the Grb-2 binding site of the receptor. NF-κB activation can be induced by a multitude of stimuli, including growth factors (6, 34, 36). It would be interesting to evaluate whether Gas6 induces NF-κB activation in cells expressing receptors from the Axl family. We are presently studying whether the activation of NF-κB by c-Mer occurs also in other cell types and, in addition, whether it is a common denominator for the antiapoptotic effects of other members of the Axl family. Preliminary data in studies using NIH 3T3 fibroblasts indicated that both constitutively active c-Mer and c-Eyk activate NF-κB.
The signaling events linking the c-Mer receptor to NF-κB activation are also under investigation. Mutation of the tyrosine at position 867 in c-Mer significantly decreased NF-κB activation, although it did not completely eliminate it. We have shown that this mutation disrupts the interaction of the receptor with Grb2, but similarly to the Axl receptor, in which this site is docking many other proteins (8), other interactions might have also been impaired for the F867 mutant receptor, although we were unable to detect them. Thus, we cannot infer from the mutational study alone that Grb2, and not another putative interactor, is required for NF-κB activation. In the preliminary study using inhibitors, the inactivation of the PI 3-kinase appeared to decrease to some extent the activation of NF-κB by CDMer. Since both PI 3-kinase and NF-κB have antiapoptotic effects, it would be interesting to define the downstream effectors of the PI 3-kinase pathway that contribute to the activation of NF-κB. No inhibition of CDMer-induced NF-κB activity resulted from treatment with the p38 inhibitor, eliminating a role for p38 in this process. The involvement of Erk in NF-κB activation is unlikely, since Erk was activated by the F867 receptor, which itself did not significantly activate NF-κB. However, a potential role remains for Ras and Raf1, which were previously implicated in NF-κB activation (14, 15).
In summary, we have shown that the activated c-Mer RTK triggers both antiapoptotic and proliferative signals in hematopoietic cells and that it specifically induces the transcriptional activation of NF-κB through a tyrosine residue (position 867) in the carboxy-terminal region of the receptor. These effects confer a growth advantage to hematopoietic cells expressing c-Mer, providing a clue to its observed expression in lymphoid malignancies.
ACKNOWLEDGMENTS
We are very grateful to Avery August, Hajime Karasuyama, Hsing-Jien Kung, Lu-Hai Wang, Shinya Tanaka, Klaus Okkenhaug, Lewis Cantley, Tsuyoshi Akagi, and Jean-Francois Peyron for kind gifts of reagents and to Tsuyoshi Akagi and Ray Birge for helpful discussions of the work and manuscript. We also thank Adelaide Aquaviva for secretarial work.
This study was supported by NIH grant CA44356 and by Council for Tobacco Research grant 4438 R1. M.M.G. is a recipient of a postdoctoral fellowship from the Medical Research Council of Canada.
REFERENCES
- 1.Akagi T, Ono H, Nyunoya H, Shimotohno K. Characterization of peripheral blood T-lymphocytes transduced with HTLV-I Tax mutants with different trans-activating phenotypes. Oncogene. 1997;14:2071–2078. doi: 10.1038/sj.onc.1201045. [DOI] [PubMed] [Google Scholar]
- 2.Azam M, Erdument-Bormage H, Krieder B L, Xia M, Quelle F, Basu R, Saris C, Tempst P, Ihle J N, Schindler C. Interleukin-3 signals through multiple isoforms of Stat5. EMBO J. 1995;14:1402–1411. doi: 10.1002/j.1460-2075.1995.tb07126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 4.Beg A A, Baltimore D. An essential role for NF-κB in preventing TNFα-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 5.Bellosta P, Zhang Q, Goff S P, Basilico C. Signaling through the ARK tyrosine kinase receptor protects from apoptosis in the absence of growth stimulation. Oncogene. 1997;15:2387–2397. doi: 10.1038/sj.onc.1201419. [DOI] [PubMed] [Google Scholar]
- 6.Bertrand F, Philippe C, Antoine P J, Baud L, Groyer A, Capeau J, Cherqui G. Insulin activates nuclear factor kappa B in mammalian cells through a Raf-1-mediated pathway. J Biol Chem. 1995;270:24435–24441. doi: 10.1074/jbc.270.41.24435. [DOI] [PubMed] [Google Scholar]
- 7.Biscardi J S, Denhez F, Buehler G F, Chestnutt D A, Baragona S C, O’Bryan J P, Der C J, Fiordalisi J J, Fults D W, Maness P F. rek, a gene expressed in retina and brain, encodes a receptor tyrosine kinase of the Axl/Tyro3 family. J Biol Chem. 1996;271:29049–29059. doi: 10.1074/jbc.271.46.29049. [DOI] [PubMed] [Google Scholar]
- 8.Braunger J, Schleithoff L, Schulz A S, Kessler H, Lammers R, Ullrich A, Bartram C R, Janssen J W. Intracellular signaling of the Ufo/Axl receptor tyrosine kinase is mediated mainly by a multi-substrate docking site. Oncogene. 1997;14:2619–2631. doi: 10.1038/sj.onc.1201123. [DOI] [PubMed] [Google Scholar]
- 9.Challier C, Uphoff C C, Janssen J W, Drexler H G. Differential expression of the ufo/axl oncogene in human leukemia-lymphoma cell lines. Leukemia. 1996;10:781–787. [PubMed] [Google Scholar]
- 10.Chen J, Carey K, Godowski P J. Identification of Gas6 as the ligand for Mer, a neural cell adhesion molecule related receptor tyrosine kinase implicated in cellular transformation. Oncogene. 1997;14:2033–2039. doi: 10.1038/sj.onc.1201039. [DOI] [PubMed] [Google Scholar]
- 11.Crosier K E, Crosier P S. New insights into the control of cell growth; the role of the Axl family. Pathology. 1997;29:131–135. doi: 10.1080/00313029700169744. [DOI] [PubMed] [Google Scholar]
- 12.Cuenda A, Rouse J, Doza Y N, Meier R, Cohen P, Gallagher T F, Young P R, Lee J C. SB203580 is a specific inhibitor of MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 13.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finco T S, Baldwin A S. κB site-dependent induction of gene expression by diverse inducers of nuclear factor κB requires Raf-1. J Biol Chem. 1993;268:17676–17679. [PubMed] [Google Scholar]
- 15.Finco T S, Westwick J K, Norris J L, Beg A A, Der C J, Baldwin A S., Jr Oncogenic Ha-Ras-induced signaling activates NF-κB transcriptional activity, which is required for cellular transformation. J Biol Chem. 1997;272:24113–24116. doi: 10.1074/jbc.272.39.24113. [DOI] [PubMed] [Google Scholar]
- 16.Fridell Y-W C, Jin Y, Quilliam L A, Burchert A, McCloskey P, Spizz G, Varnum B, Der C, Liu E T. Differential activation of the Ras/extracellular-signal-regulated protein kinase pathway is responsible for the biological consequences induced by the Axl receptor tyrosine kinase. Mol Cell Biol. 1996;16:135–145. doi: 10.1128/mcb.16.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gesbert F, Guenzi C, Bertoglio J. A new tyrosine-phosphorylated 97-kDa adaptor protein mediates interleukin-2-induced association of Shp-2 with p85-phosphatidylinositol 3-kinase in human T lymphocytes. J Biol Chem. 1998;273:18273–18281. doi: 10.1074/jbc.273.29.18273. [DOI] [PubMed] [Google Scholar]
- 18.Godowski P J, Mark M R, Chen J, Sadick M D, Raab H, Hammonds R G. Reevaluation of the roles of protein S and Gas6 as ligands for the receptor tyrosine kinase Rse/Tyro3. Cell. 1995;82:355–358. doi: 10.1016/0092-8674(95)90424-7. [DOI] [PubMed] [Google Scholar]
- 19.Goruppi S, Ruaro E, Schneider C. Gas6, the ligand of Axl tyrosine kinase receptor, has mitogenic and survival activities for serum starved NIH3T3 fibroblasts. Oncogene. 1996;12:471–480. [PubMed] [Google Scholar]
- 20.Goruppi S, Ruaro E, Varnum B, Schneider C. Requirement of phosphatidylinositol 3-kinase-dependent pathway and Src for Gas6-Axl mitogenic and survival activities in NIH 3T3 fibroblasts. Mol Cell Biol. 1997;17:4442–4453. doi: 10.1128/mcb.17.8.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham D K, Bowman G W, Dawson T L, Stanford W L, Earp H S, Snodgrass H R. Cloning and developmental expression analysis of the murine c-mer tyrosine kinase. Oncogene. 1995;10:2349–2359. [PubMed] [Google Scholar]
- 22.Graham D K, Dawson T L, Mullaney D L, Snodgrass H R, Earp H S. Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell Growth Differ. 1994;5:647–657. [PubMed] [Google Scholar]
- 23.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 24.Imbert V, Rupec R A, Livolsi A, Pahl H L, Traenckner E B, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Bauerle P A, Peyron J-F. Tyrosine phosphorylation of IκB-α activates NF-κB without proteolytic degradation of IκB-α. Cell. 1996;86:787–796. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 25.Jia R, Hanafusa H. The proto-oncogene of v-eyk (v-ryk) is a novel receptor-type protein tyrosine kinase with extracellular Ig/GN-III domains. J Biol Chem. 1994;269:1839–1844. [PubMed] [Google Scholar]
- 26.Jia R, Mayer B J, Hanafusa T, Hanafusa H. A novel oncogene, v-ryk, encoding a truncated receptor tyrosine kinase is transduced into the RPL30 virus without loss of viral sequences. J Virol. 1992;66:5975–5987. doi: 10.1128/jvi.66.10.5975-5987.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karasuyama H, Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified expression vectors. Eur J Immunol. 1988;18:97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- 28.Kinoshita T, Shirouzu M, Kamiya A, Hashimoto K, Yokoyama S, Miyajima A. Raf/MAPK and rapamycin-sensitive pathways mediate the anti-apoptotic function of p21Ras in IL-3-dependent hematopoietic cells. Oncogene. 1997;15:619–627. doi: 10.1038/sj.onc.1201234. [DOI] [PubMed] [Google Scholar]
- 29.Lee H, Arsura M, Wu M, Duyao M, Buckler A J, Sonenshein G E. Role of Rel-related factors in control of c-myc gene transcription in receptor-mediated apoptosis of the murine B cell WEHI 231 line. J Exp Med. 1995;181:1169–1177. doi: 10.1084/jem.181.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ling L, Kung H-J. Mitogenic signals and transforming potential of Nyk, a newly identified neural cell adhesion molecule-related receptor tyrosine kinase. Mol Cell Biol. 1995;15:6582–6592. doi: 10.1128/mcb.15.12.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller A D, Rosman G J. Improved retroviral vectors for gene transfer and expression. BioTechniques. 1989;7:980–990. [PMC free article] [PubMed] [Google Scholar]
- 32.Mui A L-F, Wakao H, Kinoshita T, Kitamura T, Miyajima A. Suppression of interleukin-3-induced gene expression by a C-terminal truncated Stat5: role of Stat5 in proliferation. EMBO J. 1996;15:2425–2433. [PMC free article] [PubMed] [Google Scholar]
- 33.Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem. 1996;271:30022–30027. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- 33a.Neel, B. Personal communication.
- 34.Obata H, Biro S, Arima N, Kaieda H, Kihara T, Eto H, Miyata M, Tanaka H. NF-κB is induced in the nuclei of cultured rat aortic smooth muscle cells by stimulation of various growth factors. Biochem Biophys Res Commun. 1996;224:27–32. doi: 10.1006/bbrc.1996.0979. [DOI] [PubMed] [Google Scholar]
- 35.O’Bryan J P, Frye R A, Cogswell P C, Neubauer A, Kitch B, Prokop C, Espinosa III R, Le Beau M M, Earp H S, Liu E T. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olashaw N E, Kowalik T F, Huang E S, Pledger W J. Induction of NF-κB-like activity by platelet-derived growth factor in mouse fibroblasts. Mol Biol Cell. 1992;3:1131–1139. doi: 10.1091/mbc.3.10.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palacios R, Steinmetz M. IL-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985;41:727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- 38.Pear W, Nolan G, Scott M, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rausch O, Marshall C J. Tyrosine 763 of the murine granulocyte colony-stimulating factor receptor mediates Ras-dependent activation of the JNK/SAPK mitogen-activated protein kinase pathway. Mol Cell Biol. 1997;17:1170–1179. doi: 10.1128/mcb.17.3.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlessinger J. SH2/SH3 signaling proteins. Curr Opin Genet Dev. 1994;4:25–30. doi: 10.1016/0959-437x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 41.Shimotohno K, Takano M, Teruuchi T, Miwa M. Requirement of multiple copies of a 21-nucleotide sequence in the U3 regions of human T-cell leukemia virus type I and type II long terminal repeats for trans-acting activation of transcription. Proc Natl Acad Sci USA. 1986;83:8112–8116. doi: 10.1073/pnas.83.21.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Songyang Z, Shoelson S E, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J, Neel B G, Birge R B, Fajardo J E, Chou M M, Hanafusa H, Schaffhausen B, Cantley L C. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka K, Nagayama Y, Nakano T, Takamura N, Namba H, Fukada S, Kuma K, Yamashita S, Niwa M. Expression profile of receptor-type protein tyrosine kinase genes in the human thyroid. Endocrinology. 1998;139:852–858. doi: 10.1210/endo.139.3.5791. [DOI] [PubMed] [Google Scholar]
- 44.Terada K, Kaziro Y, Satoh T. Ras-dependent activation of c-Jun N-terminal kinase/stress-activated protein kinase in response to interleukin-3 stimulation in hematopoietic Ba/F3 cells. J Biol Chem. 1997;272:4544–4548. doi: 10.1074/jbc.272.7.4544. [DOI] [PubMed] [Google Scholar]
- 45.Varnum B C, Young C, Elliott G, Garcia A, Bartley T D, Fridell Y-W, Hunt R W, Trail G, Clogston C, Toso R J, Yanagihara D, Bennett L, Sylber M, Merewether L A, Tseng A, Escobar E, Liu E T, Yamano H K. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature. 1995;373:623–626. doi: 10.1038/373623a0. [DOI] [PubMed] [Google Scholar]
- 46.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Meth. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 47.Wen Z, Zhong Z, Darnell J E., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 48.Wu M, Lee H, Bellas R E, Schauer S L, Arsura M, Katz D, FitzGerald M J, Rothstein T L, Sherr D H, Sonenshein G E. Inhibition of NF-κB/Rel induces apoptosis of murine B cells. EMBO J. 1996;15:4682–4690. [PMC free article] [PubMed] [Google Scholar]
- 49.Zong C, Yan R, August A, Darnell J E, Jr, Hanafusa H. Unique signal transduction of Eyk: constitutive stimulation of the JAK-STAT pathway by an oncogenic receptor-type tyrosine kinase. EMBO J. 1996;15:4515–4525. [PMC free article] [PubMed] [Google Scholar]
- 50.Zong, C., and H. Hanafusa. Unpublished data.