Abstract
1. Changes in [Ca2+]i and pHi, mitochondrial membrane potential (psi m) and mitochondrial [NADH] have been measured independently using fluorescent techniques in single isolated guinea-pig ventricular myocytes subjected to Ca2+ overload. 2. The changes in NADH autofluorescence on the inhibition or uncoupling of respiration are consistent with the signal emanating from the mitochondrial NADH. 3. Removal of Ca2+ and Mg2+ from the bathing Tyrode solution induced a modest fall in both [Ca2+]i and pHi, a small slowly developing depolarization of psi m and an initial fall followed by a rise in mitochondrial [NADH]. 4. In myocytes that maintained an intact sarcolemma, return to Ca(2+)-containing fluid elicited a strong but brief intracellular acidification, a rise in [Ca2+]i which generally recovered more slowly to stabilize above the initial level in Tyrode solution, a steep fall in mitochondrial [NADH] and a brief transient recovery followed by a large sustained depolarization of psi m. NADH autofluorescence and mitochondrial depolarization often reached values that were not further increased by uncoupling respiration although recovery of NADH was elicited by inhibitors of respiration. 5. These changes were reduced when the Ca2+ overload was less severe as evidenced by a reduced hypercontracture upon Ca2+ repletion. A similar reduction could be routinely achieved by elevation of [Mg2+]o during the period of Ca2+ depletion. 6. These results suggest that the well-established depletion of energy-rich phosphates that occurs on Ca2+ overload is due to the combined effects of the failure of the citric acid cycle to provide sufficient mitochondrial NADH for the respiratory chain and an uncoupling of respiration from ATP production due to depolarization of psi m. The former effect could result from the depletion of sarcoplasmic amino acids and the latter from increased Ca2+ cycling across the mitochondrial wall provoked by the elevated [Na+]i and [Ca2+]i.
Full text
PDF


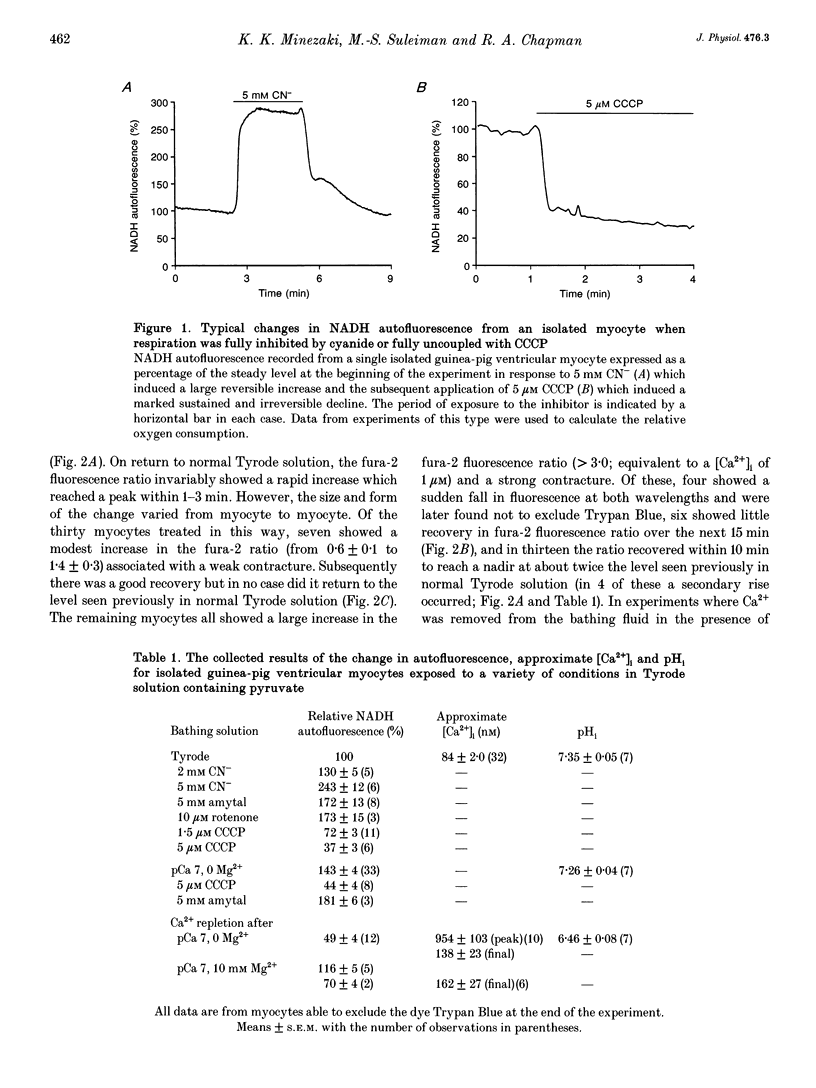
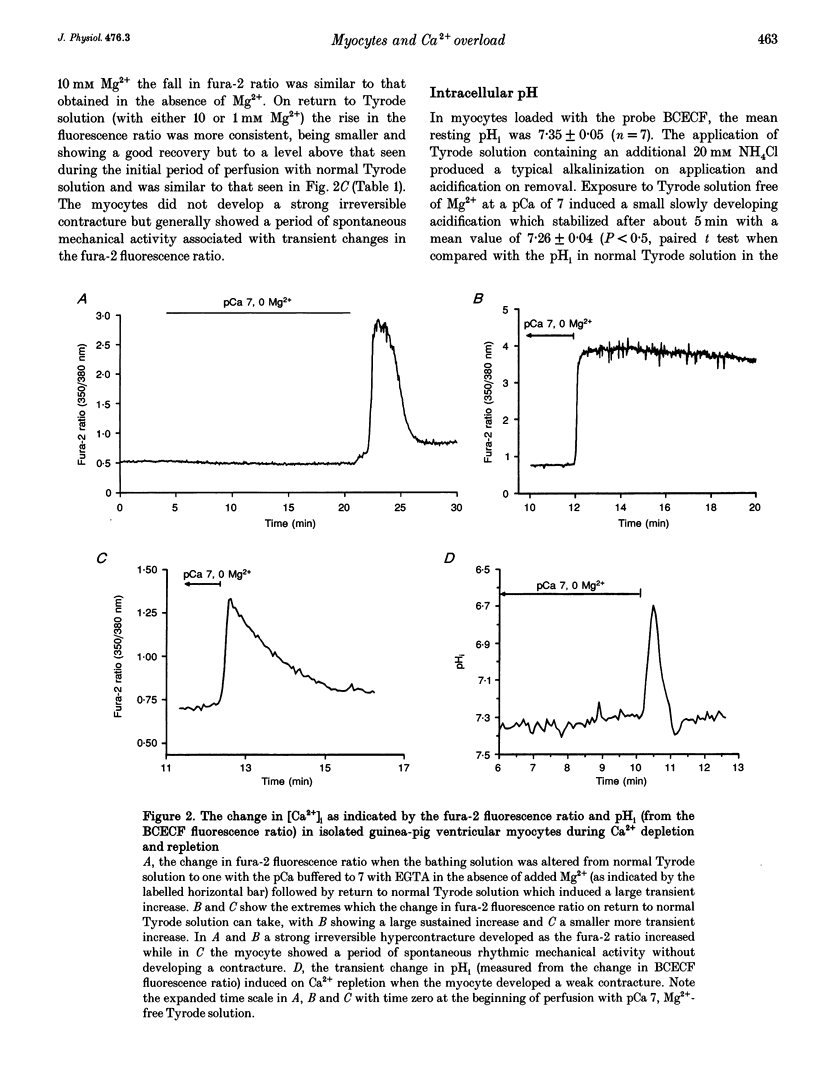
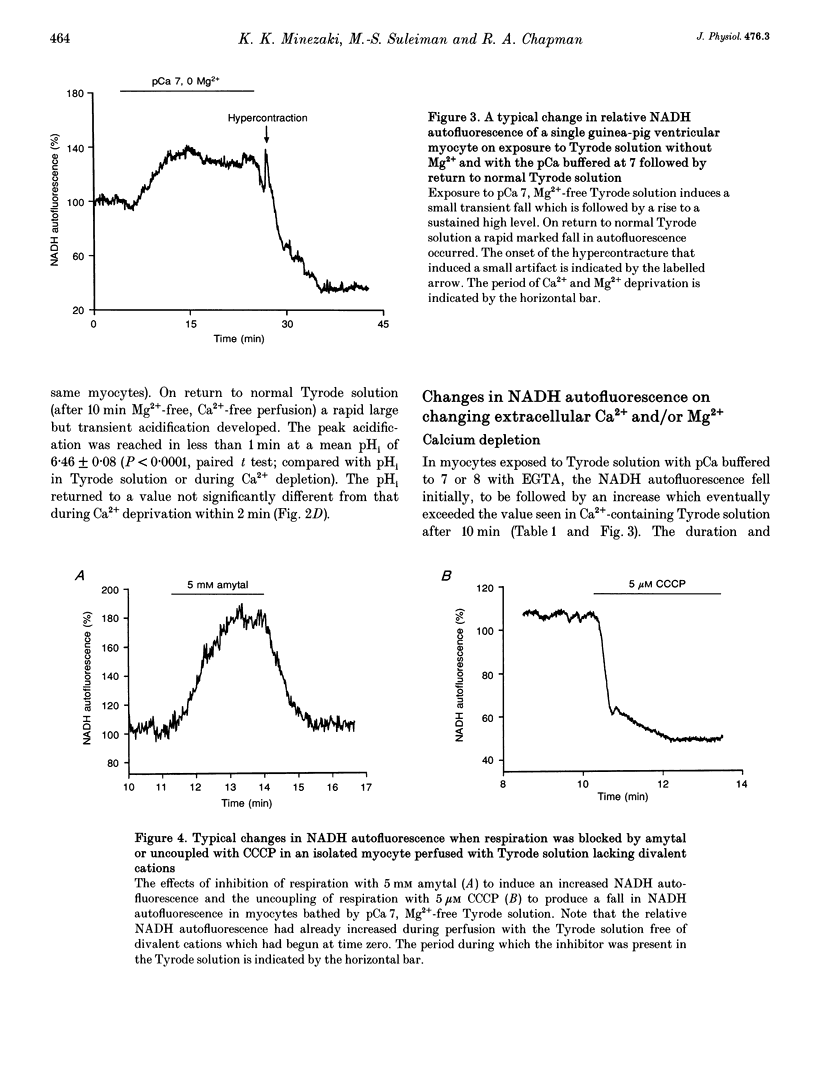
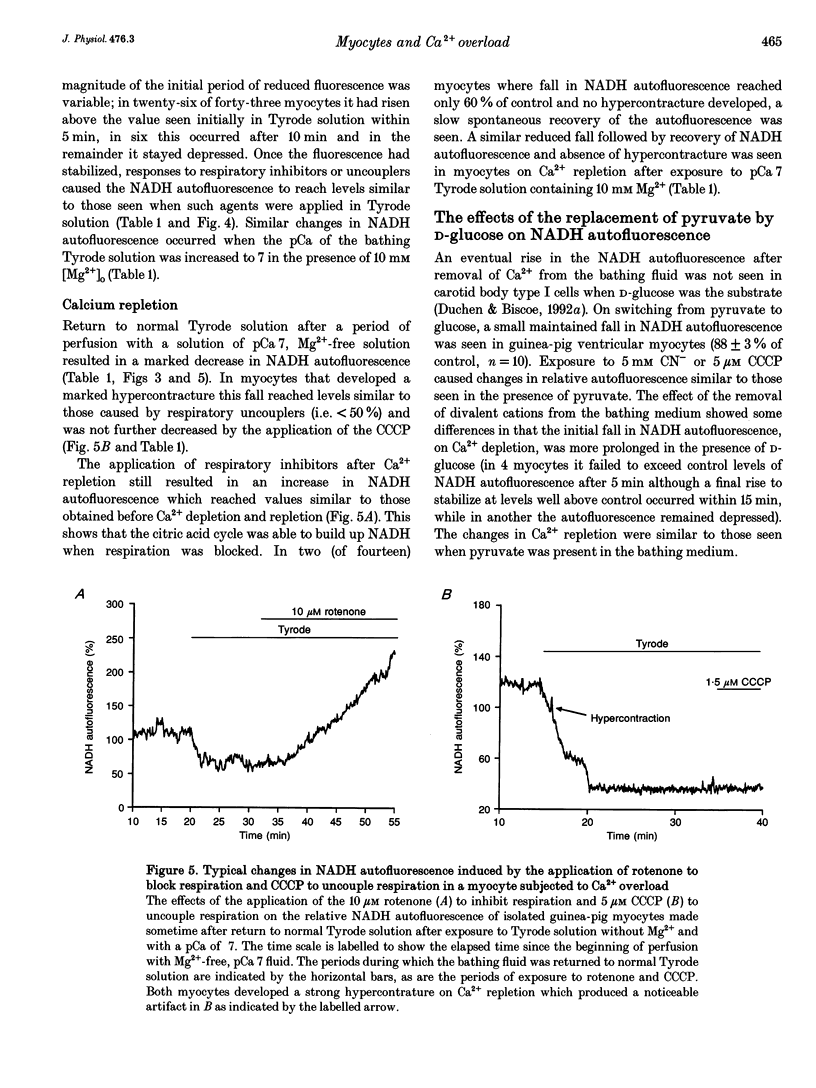
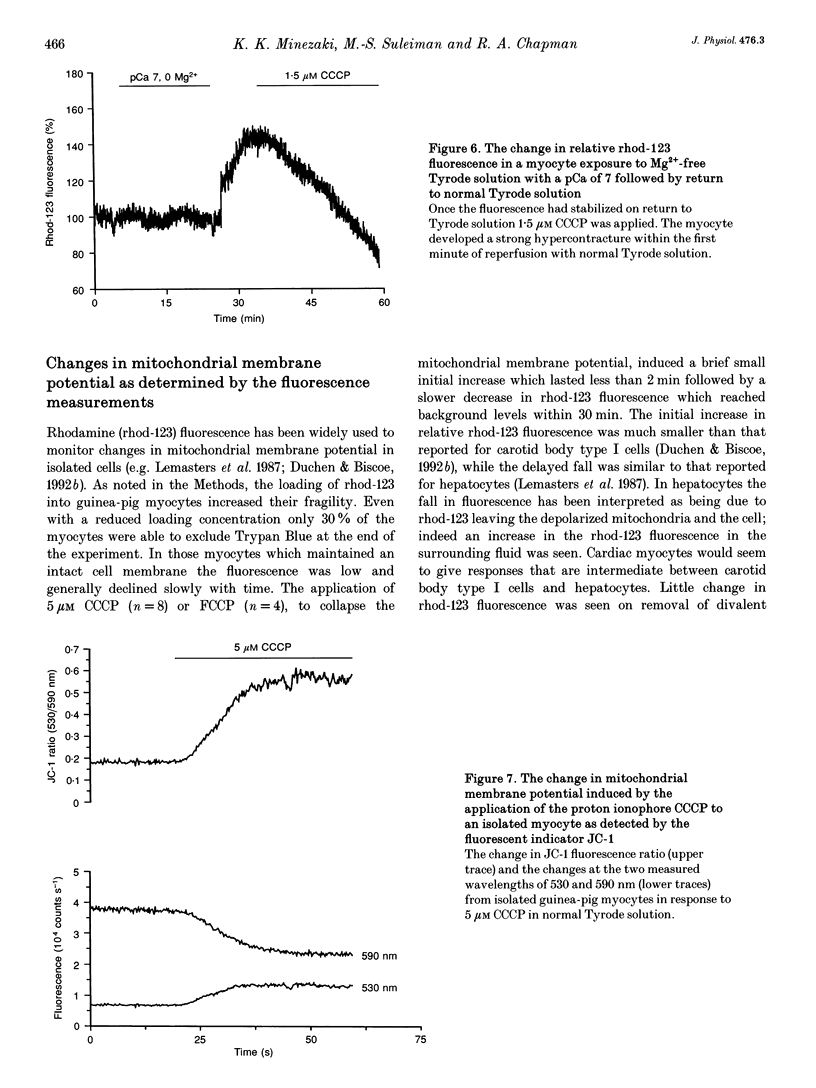




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Morris P. G., Orchard C. H., Pirolo J. S. A nuclear magnetic resonance study of metabolism in the ferret heart during hypoxia and inhibition of glycolysis. J Physiol. 1985 Apr;361:185–204. doi: 10.1113/jphysiol.1985.sp015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban R. S. Regulation of oxidative phosphorylation in the mammalian cell. Am J Physiol. 1990 Mar;258(3 Pt 1):C377–C389. doi: 10.1152/ajpcell.1990.258.3.C377. [DOI] [PubMed] [Google Scholar]
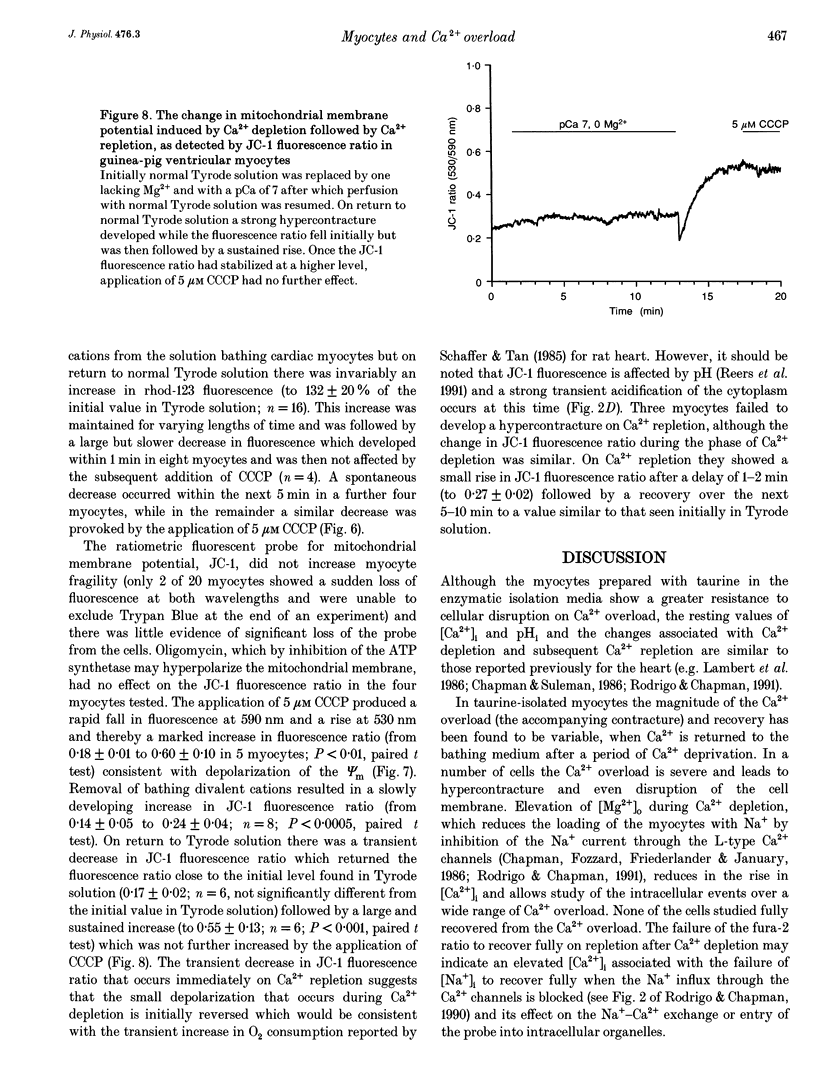
- Bionk A. B., Ruigrok T. J., Maas A. H., Zimmerman A. N. Changes in high-energy phosphate compounds of isolated rat hearts during Ca2+-free perfusion and reperfusion with Ca2+. J Mol Cell Cardiol. 1976 Dec;8(12):973–979. doi: 10.1016/0022-2828(76)90078-x. [DOI] [PubMed] [Google Scholar]
- Bowers K. C., Allshire A. P., Cobbold P. H. Bioluminescent measurement in single cardiomyocytes of sudden cytosolic ATP depletion coincident with rigor. J Mol Cell Cardiol. 1992 Mar;24(3):213–218. doi: 10.1016/0022-2828(92)93159-h. [DOI] [PubMed] [Google Scholar]
- Bulkley B. H., Nunnally R. L., Hollis D. P. "Calcium paradox" and the effect of varied temperature on its development: a phosphorus nuclear magnetic resonance and morphologic study. Lab Invest. 1978 Aug;39(2):133–140. [PubMed] [Google Scholar]
- CHANCE B., BALTSCHEFFSKY H. Respiratory enzymes in oxidative phosphorylation. VII. Binding of intramitochondrial reduced pyridine nucleotide. J Biol Chem. 1958 Sep;233(3):736–739. [PubMed] [Google Scholar]
- Chance B., Schoener B., Oshino R., Itshak F., Nakase Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals. J Biol Chem. 1979 Jun 10;254(11):4764–4771. [PubMed] [Google Scholar]
- Chapman R. A., Fozzard H. A., Friedlander I. R., January C. T. Effects of Ca2+/Mg2+ removal on aiNa, aiK, and tension in cardiac Purkinje fibers. Am J Physiol. 1986 Dec;251(6 Pt 1):C920–C927. doi: 10.1152/ajpcell.1986.251.6.C920. [DOI] [PubMed] [Google Scholar]
- Chapman R. A. The effect of oximes on the dihydropyridine-sensitive Ca current of isolated guinea-pig ventricular myocytes. Pflugers Arch. 1993 Jan;422(4):325–331. doi: 10.1007/BF00374287. [DOI] [PubMed] [Google Scholar]
- Chapman R. A., Tunstall J. The calcium paradox of the heart. Prog Biophys Mol Biol. 1987;50(2):67–96. doi: 10.1016/0079-6107(87)90004-6. [DOI] [PubMed] [Google Scholar]
- Cobbold P. H., Bourne P. K. Aequorin measurements of free calcium in single heart cells. 1984 Nov 29-Dec 5Nature. 312(5993):444–446. doi: 10.1038/312444a0. [DOI] [PubMed] [Google Scholar]
- Cox D. A., Matlib M. A. A role for the mitochondrial Na(+)-Ca2+ exchanger in the regulation of oxidative phosphorylation in isolated heart mitochondria. J Biol Chem. 1993 Jan 15;268(2):938–947. [PubMed] [Google Scholar]
- Das A. M., Harris D. A. Defects in regulation of mitochondrial ATP synthase in cardiomyocytes from spontaneously hypertensive rats. Am J Physiol. 1990 Oct;259(4 Pt 2):H1264–H1269. doi: 10.1152/ajpheart.1990.259.4.H1264. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. On the role of the calcium transport cycle in heart and other mammalian mitochondria. FEBS Lett. 1980 Sep 22;119(1):1–8. doi: 10.1016/0014-5793(80)80986-0. [DOI] [PubMed] [Google Scholar]
- Duchen M. R., Biscoe T. J. Mitochondrial function in type I cells isolated from rabbit arterial chemoreceptors. J Physiol. 1992 May;450:13–31. doi: 10.1113/jphysiol.1992.sp019114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M. R., Biscoe T. J. Relative mitochondrial membrane potential and [Ca2+]i in type I cells isolated from the rabbit carotid body. J Physiol. 1992 May;450:33–61. doi: 10.1113/jphysiol.1992.sp019115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng J., Lynch R. M., Balaban R. S. Nicotinamide adenine dinucleotide fluorescence spectroscopy and imaging of isolated cardiac myocytes. Biophys J. 1989 Apr;55(4):621–630. doi: 10.1016/S0006-3495(89)82859-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassinen I. E. Mitochondrial respiratory control in the myocardium. Biochim Biophys Acta. 1986;853(2):135–151. doi: 10.1016/0304-4173(86)90008-x. [DOI] [PubMed] [Google Scholar]
- Lambert M. R., Johnson J. D., Lamka K. G., Brierley G. P., Altschuld R. A. Intracellular free Ca2+ and the hypercontracture of adult rat heart myocytes. Arch Biochem Biophys. 1986 Mar;245(2):426–435. doi: 10.1016/0003-9861(86)90234-1. [DOI] [PubMed] [Google Scholar]
- Lemasters J. J., DiGuiseppi J., Nieminen A. L., Herman B. Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature. 1987 Jan 1;325(6099):78–81. doi: 10.1038/325078a0. [DOI] [PubMed] [Google Scholar]
- McCormack J. G., Halestrap A. P., Denton R. M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990 Apr;70(2):391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- Nuutinen E. M. Subcellular origin of the surface fluorescence of reduced nicotinamide nucleotides in the isolated perfused rat heart. Basic Res Cardiol. 1984 Jan-Feb;79(1):49–58. doi: 10.1007/BF01935806. [DOI] [PubMed] [Google Scholar]
- Nuutinen M., Hassinen I. Plasma membrane phosphate transport and extracellular phosphate concentration in the regulation of cellular respiration in isolated perfused rat heart. Biochim Biophys Acta. 1981 Oct 12;637(3):481–489. doi: 10.1016/0005-2728(81)90054-2. [DOI] [PubMed] [Google Scholar]
- Reers M., Smith T. W., Chen L. B. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991 May 7;30(18):4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- Rodrigo G. C., Chapman R. A. A sodium-activated potassium current in intact ventricular myocytes isolated from the guinea-pig heart. Exp Physiol. 1990 Nov;75(6):839–842. doi: 10.1113/expphysiol.1990.sp003465. [DOI] [PubMed] [Google Scholar]
- Rodrigo G. C., Chapman R. A. The calcium paradox in isolated guinea-pig ventricular myocytes: effects of membrane potential and intracellular sodium. J Physiol. 1991 Mar;434:627–645. doi: 10.1113/jphysiol.1991.sp018490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer S. W., Tan B. H. Effect of calcium depletion and calcium paradox on myocardial energy metabolism. Can J Physiol Pharmacol. 1985 Nov;63(11):1384–1391. doi: 10.1139/y85-228. [DOI] [PubMed] [Google Scholar]
- Smiley S. T., Reers M., Mottola-Hartshorn C., Lin M., Chen A., Smith T. W., Steele G. D., Jr, Chen L. B. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleiman M. S., Chapman R. A. Changes in the principal free intracellular amino acids in the Langendorff perfused guinea pig heart during arrest with calcium-free or high potassium media. Cardiovasc Res. 1993 Oct;27(10):1810–1814. doi: 10.1093/cvr/27.10.1810. [DOI] [PubMed] [Google Scholar]
- Tani M., Neely J. R. Intermittent perfusion of ischemic myocardium. Possible mechanisms of protective effects on mechanical function in isolated rat heart. Circulation. 1990 Aug;82(2):536–548. doi: 10.1161/01.cir.82.2.536. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fay F. S. Intracellular calibration of the fluorescent calcium indicator Fura-2. Cell Calcium. 1990 Feb-Mar;11(2-3):75–83. doi: 10.1016/0143-4160(90)90061-x. [DOI] [PubMed] [Google Scholar]
- Wood E. H. Cardiovascular and pulmonary dynamics by quantitative imaging. Circ Res. 1976 Mar;38(3):131–139. doi: 10.1161/01.res.38.3.131. [DOI] [PubMed] [Google Scholar]


