Abstract
In this study, we found that the E6-associated protein (E6-AP/UBE3A) directly interacts with and coactivates the transcriptional activity of the human progesterone receptor (PR) in a hormone-dependent manner. E6-AP also coactivates the hormone-dependent transcriptional activities of the other members of the nuclear hormone receptor superfamily. Previously, it was shown that E6-AP serves the role of a ubiquitin-protein ligase (E3) in the presence of the E6 protein from human papillomavirus types 16 and 18. Our data show that the ubiquitin-protein ligase function of E6-AP is dispensable for its ability to coactivate nuclear hormone receptors, showing that E6-AP possesses two separable independent functions, as both a coactivator and a ubiquitin-protein ligase. Disruption of the maternal copy of E6-AP is correlated with Angelman syndrome (AS), a genetic neurological disorder characterized by severe mental retardation, seizures, speech impairment, and other symptoms. However, the exact mechanism by which the defective E6-AP gene causes AS remains unknown. To correlate the E6-AP coactivator function and ubiquitin-protein ligase functions with the AS phenotype, we expressed mutant forms of E6-AP isolated from AS patients and assessed the ability of each of these mutant proteins to coactivate PR or provide ubiquitin-protein ligase activity. This analysis revealed that in the majority of the AS patients examined, the ubiquitin-protein ligase function of E6-AP was defective whereas the coactivator function was intact. This finding suggests that the AS phenotype results from a defect in the ubiquitin-proteosome protein degradation pathway.
Steroids, thyroid hormones, vitamin D, and retinoids regulate diverse biological processes including growth, development, and homeostasis through their cognate nuclear hormone receptors, which make up a superfamily of structurally related intracellular ligand-activated transcription factors (18, 34, 40, 47). Nuclear hormone receptors contain common structural motifs which include a poorly conserved amino-terminal activation function (activation factor 1 [AF-1]) that affects transcription efficiency, a central DNA-binding domain, which mediates receptor binding to specific DNA enhancer sequences and determines target gene specificity, and a carboxy-terminal hormone-binding domain. The latter domain contains AF-2, a region which mediates the hormone-dependent activation function of receptors (40). When bound to hormone, these receptors undergo a conformational change, dissociation from heat shock proteins, receptor dimerization, phosphorylation, DNA binding at an enhancer element of the target gene, interaction with coactivators, and subsequent recruitment of basal transcription factors to form a stable preinitiation complex. These events are followed by either up-regulation or down-regulation of target gene transcription (40).
Nuclear hormone receptor coactivators represent a growing class of proteins which interact with receptors in a ligand-specific manner and serve to enhance their transcriptional activities (33). Prior to their identification, coactivators were predicted to exist based on experiments which showed that different receptors compete for a limiting pool of factors required for optimal transcription. Stimulation of one receptor resulted in transrepression of another receptor, indicating the depletion of a common coactivator pool (6, 10, 31, 39). Among the coactivators cloned to date are steroid receptor coactivator 1 (SRC-1) (33), TIF2 (GRIP1) (17, 51), p/CIP (ACTR/RAC3/AIB1/TRAM-1) (2, 9, 28, 46, 48), and ARA70 (54). Coactivators were originally envisioned to serve a bridging role, linking the receptor to the basal transcription machinery (36, 45). Recently, they were shown to possess enzymatic activities which contribute to their ability to enhance receptor-mediated transcription; SRC-1, p300/CBP, and RAC3/ACTR/AIB1 possess histone acetyltransferase activity (HAT) (2, 9, 28, 32, 41). Ligand-activated receptors are thought to bring these HAT activity-containing coactivators to the chromatin surrounding the receptor, disrupting the local repressive chromatin structure by acetylating histones and possibly other chromatin-associated factors (41). Because of their ability to enhance receptor-mediated gene expression, coactivators are thought to play an important role in regulating the magnitude of the biological response to steroids, vitamin D, and retinoids in different tissues or individuals. The level of coactivator expression may contribute to variations in hormone responsiveness seen in the population, and disruption in coactivator expression could lead to the pathological hyper- or hyposensitivity to steroid hormones. Recently, it was shown that disruption of the SRC-1 locus in mice resulted in an attenuated response to steroid hormones, a finding consistent with this hypothesis (53).
In this report, we describe the cloning and characterization of E6-associated protein (E6-AP) (21), a protein linked to Angelman syndrome (AS) (26, 30, 42), as a progesterone receptor (PR)-interacting protein. E6-AP was previously identified as a protein of 100 kDa, present in both the cytoplasm and the nucleus (14). E6-AP mediates the interaction of human papillomavirus type 16 and 18 E6 proteins with p53, a growth-suppressive and tumor-suppressive protein (14, 22). Initial in vitro studies suggested that the E6–E6-AP complex specifically interacts with p53 and promotes the degradation of p53 via the ubiquitin-proteasome degradation pathway, but recent in vivo studies show that E6-AP can directly interact with p53 and promote its degradation even in the absence of the papillomavirus E6 protein (11, 20, 38). E6-AP is a member of a family of proteins, known as E3 ubiquitin-protein ligases, which have been proposed to play a role in defining the substrate specificity of the ubiquitin-proteasome degradation system. Protein ubiquitination also involves two other classes of enzymes, namely, E1 ubiquitin-activating enzymes and E2 ubiquitin-conjugating enzymes, which activate ubiquitin moieties and transfer them to target proteins and E3, respectively (19). The carboxyl-terminal 350 amino acids (aa) of E6-AP constitute a hect (homologous to the E6-AP carboxy terminus) domain which is conserved among many E3 ubiquitin-protein ligases and E6-AP-related proteins (19). The extreme carboxyl-terminal 100-aa segment contains the catalytic region of E6-AP, which transfers ubiquitin to the protein targeted for degradation (19). The E6-binding domain consists of an 18-aa region located within the central portion of the E6-AP protein (22).
Recently, it was shown that a genetic disorder, AS, is caused by the absence of a functional maternal copy of the E6-AP gene (26, 30, 42). AS is a neurological disorder characterized by severe mental retardation, seizures, speech impairment, and other symptoms (5). However, the exact mechanism by which the defective E6-AP gene causes AS remains unknown. Our analysis of mutant E6-AP proteins from AS patients revealed that the ubiquitin-protein ligase function of E6-AP was defective, whereas the coactivator function was intact, in the majority of AS patients examined. In this report, we also show that the ubiquitin ligase activity of E6-AP is not required for the coactivation function of E6-AP. Furthermore, our data indicate that the catalytic function located within the hect domain of E6-AP is not necessary for the ability of E6-AP to interact with and coactivate steroid hormone receptor function. These findings suggest that E6-AP possesses two independent functions, as both a coactivator and a ubiquitin-protein ligase.
MATERIALS AND METHODS
Plasmid construction.
The bait plasmid for the yeast two-hybrid system (pAS1-PRLBD) (33), mammalian expression plasmids for PR-B (1), estrogen receptor (ER) (7), and androgen receptor (AR) (44), E2F reporter plasmid UAS4-TATA-LUC (LUC denotes luciferase) (41), and E2F-, Sp1-, and CREB-responsive reporters (33) have been described previously. To construct the glucocorticoid receptor (GR) expression vector, the pSTCGR vector was digested with BamHI and then the BamHI fragment containing the GR cDNA was cloned into the corresponding sites of plasmid pCR3.1 (Invitrogen). pPRE/GRE.E1b.LUC and pERE.E1b.LUC were constructed by inserting PvuII-SmaI fragments of pPRE/GRE.E1b.CAT and pERE.E1b.CAT into the SmaI site of pGL3-basic (Promega). To construct mammalian expression plasmids for wild-type E6-AP (aa 1 to 851), 76-kDa E6-AP (aa 170 to 851), and C833S (change of cysteine 833 to serine) mutant E6-AP (aa 1 to 851), the BamHI-HindIII fragments of pGEM E6-AP (100 kDa), pGEM-E6-AP (76 kDa), and pGEM E6-AP(C833-S) were cloned into the corresponding sites of plasmid pBK.RSV (Stratagene). The C-terminal fragment of E6-AP (aa 680 to 851), the truncated mutant E6-AP (aa 1 to 449), and the 98-kDa (aa 1 to 834) form of E6-AP, found in AS (25, 29, 41), and the 99-kDa (aa 1 to 845), 86-kDa (aa 1 to 714), 47-kDa (aa 450 to 851), and 28-kDa (aa 1 to 240) forms of E6-AP were amplified by PCR with the following primer pairs: 5′-GCGGATCCACCATGAGGAATTCGGCACGAGATCTAAAGGAA-3′ (upper strand) and 5′-CGGAATTCAAGCTTGTTTTACAGCATGCCAAATCC-3′ (lower strand); 5′-GCGGATCCACCATGGAAGCCTGCACGAATGAGTTTTGTGCT-3′ (upper strand) and 5′-CCCAAGCTTGTTTTATGTTTCTACTTTGAAAAAAGTATA-3′ (lower strand); 5′-GCGGATCCACCATGAGGAATTCGGCACGAGATCTAAAGGAA-3′ (upper strand) and 5′-CCCAAGCTTGTTTTAAAGTTTTTCTTTGCTTGAGTATTC-3′ (lower strand); 5′-GCGGATCCACCATGAGGAATTCGGCACGAGATCTAAAGGAA-3′ (upper strand) and 5′-CCCAAGCTTGTTTTAGGCATACGTGATGGCCTTCAACAA-3′ (lower strand); 5′-GCGGATCCACCATGAGGAATTCGGCACGAGATCTAAAGGAA-3′ (upper strand) and 5′-CCCAAGCTTGTTTTACATATGAAAACCTCTCCGAAAAGC (lower strand); 5′-GCGGATCCACCATGTACAGTGAACGAAGAATCACTGTT-3′ (upper strand) and 5′-CGGAATTCGCGGCCGCGTTTTACAGCATGCCAAATCC-3′ (lower strand); and 5′-GCGGATCCACCATGGAAGCCTGCACGAATGAGTTTTGTGCT-3′ (upper strand) and 5′-GAATTCAAGCTTGTTTTACAAATATACAAGTGCATTGAG-3′ (lower strand). The PCR product was digested with BamHI-HindIII and cloned into the corresponding sites of plasmid pBK.RSV. Then the BamHI-NotI fragments of plasmid pBK.RSV-E6-AP were subcloned into the corresponding sites of plasmid pCR3.1 (Invitrogen). To construct the I804K and F782Δ mutant forms of E6-AP, we used site-directed mutagenesis to create the mutations in pCR3.1 E6-AP. To reconstitute the 104-kDa 1-885Δstop mutation in E6-AP, the BsaAI-HindIII fragment of E6-AP was amplified by PCR with the primers 5′-GTTGAAGGCCATCACGTATGCCAAAGG-3′ (lower strand) and 5′-GAATTCAAGCTTGTTTTAGTACTGGGACACTATCACCACCA-3′ (lower strand), using AS patient DNA as a template. Then this BsaAI-HindIII fragment was cloned into the corresponding sites of pGEM E6-AP. To reconstitute this mutation in the mammalian expression plasmid, the BamHI-HindIII fragment of E6-AP was cloned into the corresponding sites of plasmid pBK.RSV. The BamHI-NotI fragment of pBK.RSV-E6-AP was subcloned into the BamHI-NotI sites of pCR3.1. To reconstitute the full-length E6-AP gene in a yeast two-hybrid plasmid, HindIII-digested (and filled) pGEM E6-AP (100 kDa) was redigested with BamHI. The resulting BamHI-HindIII (filled) fragment was inserted into the BamHI-EcoRI (filled) sites of pGAD10 (Clontech). To reconstitute the PR-A gene in the yeast plasmid pAS1, the NcoI-SalI fragment of PR-A was ligated into the corresponding sites of the vector. To fuse E6-AP with the VP16 activation domain and GAL DNA-binding domain (DBD) (residues 1 to 147), the BamHI-HindIII fragment of full-length E6-AP and several deletion fragments of E6-AP were subcloned in frame into plasmids pABVP16 and pABGAL (3, 4). To fuse E6-AP with glutathione S-transferase (GST), the BamHI-NotI fragments of full-length E6-AP and various mutant forms of E6-AP were subcloned in frame with GST into plasmid pGEX4T (Pharmacia).
In vivo interaction assays.
The yeast two-hybrid and mammalian two-hybrid interaction assays were performed as described previously (12, 41).
In vitro interaction assay.
For the in vitro interaction assay, PR-B was expressed as a His-tagged protein in a baculovirus expression system in the presence or absence of progesterone and purified by using a nickel affinity column (Pharmacia). GST-tagged E6-AP was expressed in Escherichia coli and purified on glutathione-Sepharose beads. The purified and glutathione-bound E6-AP was incubated with the purified PR in NETN buffer (50 mM NaCl, 1 mM EDTA, 20 mM Tris [pH 8.0], 0.5% Nonidet P-40) overnight at 4°C, after which the beads were washed five times with NETN buffer. E6-AP-bound PR was eluted and separated on a sodium dodecyl sulfate–7.5% polyacrylamide gel and then analyzed by Western blotting using an antibody which specifically recognizes PR.
Transfections.
HeLa cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum. Twenty-four hours before transfection, 3 × 105 cells were plated per well in Falcon six-well dishes in DMEM containing 5% dextran-coated charcoal-stripped serum. Cells were transfected with the indicated DNAs by using Superfect reagent (Qiagen) or Lipofectamine (Gibco BRL) according to the manufacturer’s guidelines. Cells were washed, fed with DMEM containing 5% stripped serum, treated with various hormones, and harvested 24 h later. Cell extracts were assayed for luciferase activity, using the Promega luciferase assay system, and values were corrected for either protein concentration or β-galactosidase activity. Data are presented as means of triplicate values obtained from representative experiments.
Ubiquitin-protein ligase activity.
To study the ubiquitin-protein ligase activity of wild-type E6-AP and various mutant forms of E6-AP, wild-type E6-AP and various mutant forms of E6-AP (Table 1) were expressed and purified from E. coli as GST fusion proteins. The ubiquitin-protein ligase activities of these proteins were measured by using HHR23A as a target protein as described previously (27, 49).
TABLE 1.
Coactivation and ubiquitin-protein ligase activities of wild-type and mutant forms of E6-APa
| Form of E6-AP | Coactivationa | Ubiquitin-protein ligase activityb |
|---|---|---|
| Wild type (aa 1–851) | ++++ | + |
| Mutant | ||
| aa 450–851 | + | − |
| aa 680–851 | − | − |
| aa 1–845 | ++++ | − |
| aa 1–834c | ++++ | − |
| aa 1–714 | ++ | − |
| R417Xc | + | − |
| aa 1–240 | Not tested | − |
| C833S | ++++ | − |
| I804Kc | ++++ | + |
| F782Δc | ++++ | − |
| 1–885Δstopc | ++++ | Not tested |
HeLa cells were transfected with 0.1 μg of PR-B expression plasmid, 1 μg of pPRE/GRE.E1b.LUC, and 0.25 μg of expression plasmid for wild-type E6-AP or 0.25 μg of each of the indicated mutant forms of E6-AP. The cells were incubated with 10−7 M progesterone. Coactivation by each mutant form of E6-AP is presented as relative to coactivation by wild-type E6-AP, scored as ++++.
The wild-type and mutant forms of E6-AP were expressed and purified from E. coli as GST fusion proteins, and their ubiquitin-protein ligase activities were measured with HHR23A as a target protein. +, positive for ubiquitin-protein ligase activity; −, negative for ubiquitin-protein ligase activity.
Natural mutant form of E6-AP cloned from an AS patient.
RESULTS
Isolation and characterization of E6-AP as a PR-interacting protein.
To identify novel proteins which selectively modulate the transactivation functions of members of the nuclear receptor superfamily, we screened a HeLa cDNA library by using the ligand-binding domain of PR as a bait in a yeast two-hybrid screening assay. We isolated 13 colonies which strongly interacted with this domain of PR. These colonies contained cDNAs with identical sequences. A sequence similarity search in the GenBank database revealed that all colonies encoded the carboxy-terminal aa 680 to 851 of the E6-AP (see Fig. 2A).
FIG. 2.
(A) Schematic representation of E6-AP showing positions of the catalytic region (solid box), hect domain, transactivation domain, and PR-binding domains. Wild-type E6-AP is a 100-kDa (aa 1 to 851) protein; 76 kDa (aa 170 to 851) and 47 kDa (aa 450 to 851) represent E6-AP with a deletion at the N terminus. The 21-kDa (aa 680 to 851) form represents the carboxyl terminus of E6-AP identified in the yeast two-hybrid screen; 99 kDa (aa 1 to 845), 98 kDa (aa 1 to 834), 86 kDa (aa 1 to 714), 53 kDa (aa 1 to 449), and 28 kDa (aa 1 to 240) represent E6-AP C-terminal deletion mutants. C833S represents a cysteine 833-to-serine mutant form of E6-AP. The AS disease mutants are represented by 98 kDa (deletion of 17 aa from the C terminus) and 53 kDa (C-terminally truncated E6-AP) (aa 1 to 449); the I804K form of E6-AP contains lysine at position 804 instead of isoleucine, F782Δ contains a deletion of phenylalanine at position 782, and the 104-kDa 1-885Δstop form of E6-AP is a readthrough mutant. (B) Localization of the PR interaction site in E6-AP. To determine the PR interaction site on E6-AP, full-length E6-AP and various deletion fragments of E6-AP shown in panel A were fused in frame with the VP16 activation domain, and the ability of E6-AP to interact with PR was determined in a mammalian two-hybrid assay. HeLa cells were cotransfected with 0.3 μg of PR expression plasmid and 0.3 μg of pPRE/GRE.E1b.LUC in the absence (control) or presence of expression plasmid pABVP16-E6-AP (0.9 μg) (aa 1 to 851 [wild-type], 170 to 851, aa 680 to 851, 1 to 714, aa 1 to 449, and aa 1 to 240). The cells were treated with either vehicle only (□) or 10−7 M progesterone (■). Data are presented as relative light units per microgram of protein, and each bar depicts the average of at least three wells.
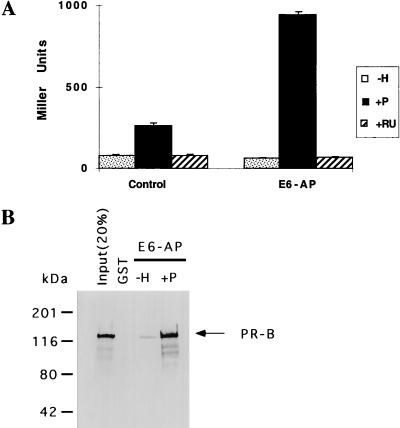
Full-length E6-AP interacts with the liganded form of PR both in vivo and in vitro. As shown in Fig. 1A, in a yeast two-hybrid assay, E6-AP interacts with PR in a progesterone-dependent manner. In the absence of ligand or in the presence of the antihormone compound RU486, we observed no significant level of interaction relative to the control between E6-AP and PR. To document further that the progesterone-dependent interaction observed in the yeast two-hybrid assay is due to a direct physical association of E6-AP and PR, we purified baculovirus-expressed His-tagged PR on a nickel affinity column and then incubated it with GST–E6-AP which was purified and subsequently bound to glutathione-Sepharose beads in the absence or presence of progesterone. As a control, purified GST was incubated with PR. After extensive washing, E6-AP-bound PR was analyzed by immunoblotting using a PR-specific antibody (Fig. 1B). A significant level of E6-AP interaction with PR was observed in the presence of progesterone but not in its absence.
FIG. 1.
(A) Interaction of PR with wild-type E6-AP in a yeast two-hybrid assay. The entire coding sequence of PR-A was fused in frame with the yeast GAL4 DBD, and the resultant GAL DBD–PR-A construct was coexpressed with either control vector or the GAL4-AD–E6-AP construct (GAL4 activation domain fused in frame with wild-type E6-AP) along with a reporter plasmid in yeast strain BJ2186. The transformants were propagated, and β-galactosidase activities from three independent colonies were determined. The yeast cells were treated with either vehicle alone (−H), 10−6 M progesterone (+P), or 10−6 M RU486 (+RU). Each bar depicts the average of three assays. (B) In vitro interaction of E6-AP with PR. Baculovirus-expressed purified PR was incubated with a purified GST–E6-AP fusion protein or with GST alone (control) bound to glutathione-Sepharose beads either in the absence or in the presence of 10−6 M progesterone. E6-AP-bound PR was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 7.5% gel followed by Western blot analysis using antibodies which specifically recognize PR.
Regions of E6-AP required for interaction with PR.
Since E6-AP interacts with PR in a hormone-dependent manner, we next defined the regions of E6-AP important for interaction with PR. For this purpose, we used an in vivo mammalian two-hybrid interaction assay system (41). In this assay, full-length E6-AP and various deletion fragments of E6-AP were fused to the VP16 activation domain (Fig. 2A), and the ability of each of the VP16–E6-AP hybrid proteins to interact with PR was determined in the absence or presence of progesterone. As shown in Fig. 2B, wild-type E6-AP, N-terminal deletion fragments (aa 170 to 851 and 680 to 851), and C-terminal deletion fragments (aa 1 to 714 and 1 to 449) of E6-AP were able to interact with PR, while the control vector lacking E6-AP cDNA and the N-terminal fragment (aa 1 to 240) of E6-AP did not interact, suggesting that at least two PR interaction sites are located within the E6-AP protein. One site is located within the C-terminal fragment (aa 680 to 851; 21 kDa) of E6-AP, the fragment originally isolated in the yeast two-hybrid screen. The second PR interaction site is located within aa 240 to 449 and overlaps the E6-binding site. Each of these regions of E6-AP interacts with both transcriptional activation factor 1 and transcriptional activation factor 2 of PR.
E6-AP as a coactivator for the nuclear hormone receptor superfamily.
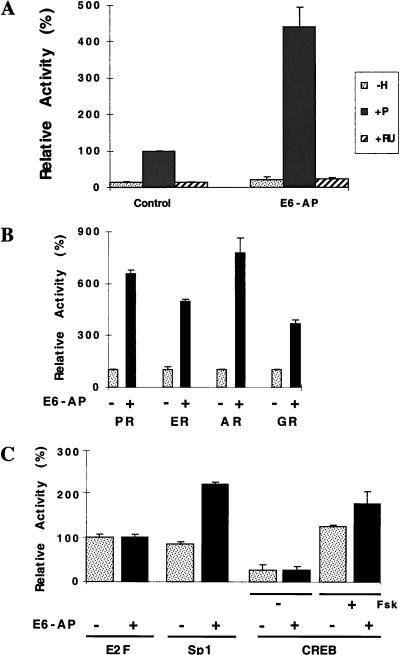
To investigate whether E6-AP may play a role in receptor-dependent activation of target gene expression, we performed transient cotransfection assays of HeLa cells. HeLa cells were transfected with expression vectors for PR and a reporter plasmid containing a progesterone response element with or without an expression vector for E6-AP. In the absence of ligand, PR had a minimal effect on reporter gene expression either in the absence or in the presence of E6-AP (Fig. 3A). Addition of the hormone yielded an 8-fold increase in PR activity in the absence of E6-AP; when E6-AP was coexpressed with PR, the activity of PR was further stimulated by ∼5-fold, a total of 40-fold over the basal level. In contrast, coexpression of E6-AP with PR had no significant effect on the transcription of the reporter gene when receptor was bound to the antihormone compound RU486 (Fig. 3A). These data are consistent with previously published data which indicate that RU486 induces a distinct conformational change in the receptor molecule that has reduced affinity for coactivators (1, 33, 50, 52). Since (i) HeLa cells are derived from a papillomavirus type 18-positive cervical carcinoma patient and thus express the E6 protein and (ii) E6-AP was originally cloned as an E6-interacting protein, it was necessary to rule out the possibility that the E6 protein influences the coactivation function of E6-AP. E6-AP was able to stimulate the hormone-dependent transcriptional activity of steroid hormone receptors in the E6-negative HepG2 and SK-N-SH cell lines (data not shown), suggesting that the coactivation observed in HeLa cells is not dependent on the E6 protein.
FIG. 3.
(A) E6-AP coactivates the transcriptional activity of PR-B. HeLa cells were transiently transfected with 0.2 μg of pPR-B expression plasmid and 1 μg of pPRE/GRE.E1b.LUC in the absence or presence of E6-AP expression plasmid pBK.RSV-E6-AP (0.250 μg). The cells were treated with either vehicle only (−H), 10−7 M progesterone (+P), or 10−7 M RU486 (+RU). Each bar depicts the average of at least three wells. Activity in the presence of hormone and in the absence of exogenous coactivator was defined as 100%, and data for the other bars were scaled accordingly. (B) E6-AP coactivates the hormone-dependent transcriptional activity of nuclear hormone receptors. HeLa cells were transfected with receptor expression plasmids for PR, ER, AR, and GR and their cognate hormone-responsive reporter plasmids in the absence and presence of E6-AP (0.250 μg). The cells were treated with appropriate hormones as follows: PR, progesterone (10−7 M); ER, estradiol (10−9 M); AR, R1881 (2.5 × 10−10 M); and GR, dexamethasone (10−7 M). The extent of coactivation by E6-AP on the hormone-dependent transcriptional activities of various receptors ranges from four- to eightfold. The level of coactivation by E6-AP is dependent on both cell type and cell passage number (data not shown). Each bar depicts the average of at least three wells. Activity in the presence of hormone and in the absence of exogenous coactivator was defined as 100%, and data for the other bars were scaled accordingly. (C) Effect of E6-AP expression on transcriptional activities of diverse transcription factors. HeLa cells were transfected with an E2F expression plasmid (0.05 μg) along with 2.5 μg of an E2F-responsive or Sp1-responsive reporter plasmid. To test the effect of E6-AP on the transcriptional activity of the CREB transcription factor, LMTK− cells were transfected with a CREB-responsive reporter plasmid (2.5 μg) in the absence or presence of E6-AP (0.25 μg). The CREB transcription factor was activated by treating cells with 10 μM forskolin (Fsk). Each bar depicts the average of at least three wells.
Our data suggest that E6-AP stimulates the hormone-dependent transcriptional activity of PR by acting as a coactivator. To determine if E6-AP functions as a coactivator for members of the nuclear receptor superfamily, we examined the effect of E6-AP expression on the ligand-dependent transcriptional activities of different nuclear hormone receptors and on several other transcription factors (Fig. 3B and C). E6-AP significantly enhanced the hormone-dependent transcriptional activity of PR, ER, AR, and glucocorticoid receptor (GR). It also enhanced the transcriptional activity of retinoic acid (receptor alpha and thyroid hormone receptor (data not shown). E6-AP had minimal or no effect on the transcriptional activity of E2F and CREB. Coexpression of E6-AP had only a moderate effect on the activation function of Sp1 (Fig. 3C). These data suggest that E6-AP preferentially coactivates the hormone-dependent transcriptional activity of nuclear hormone receptors but is not uniquely specific for them as is the case for other coactivators such as SRC-1 (33).
E6-AP relieves squelching between ER and PR.
It has been shown that ER and PR share certain coactivators since hormone-bound ER can sequester limited pools of coactivators from PR, a phenomenon known as squelching (10, 31, 39). We examined whether coexpression of E6-AP was able to reverse this squelching phenomenon. The hormone-induced transcriptional activity mediated by PR was reduced by 91% upon coexpression of estradiol-bound ER (Fig. 4A; compare lanes 2 and 3). Addition of E6-AP reversed this squelching by as much as 9.6-fold (Fig. 4A; compare lanes 3 and 6) in a dose-dependent manner. At the highest concentration of E6-AP used in this reverse squelching experiment, PR activity was enhanced only 2.6-fold (compare lanes 2 and 7). However, in control cells which do not express ER, E6-AP enhanced the transcriptional activity of PR from four- to fivefold (compare lanes 2 and 8). These data suggest that E6-AP is a limiting factor which is necessary for efficient PR and ER transactivation. The fold coactivation by E6-AP is lower in this experiment than in that shown in Fig. 3B, due to differences in experimental conditions. In Fig. 4A (lane 7), the coactivation effect of E6-AP on the transcriptional activity of PR was observed in the presence of the ER expression plasmid, whereas in Fig. 3B, only a single receptor was transfected. As expected, no significant reverse squelching was observed (Fig. 4B; compare lanes 3 and 6) with the C-terminal fragment of E6-AP (aa 680 to 851) (Fig. 2A), which weakly interacts with ER (data not shown) and has no activation function (Fig. 5). However, this fragment did not possess dominant negative activity under our experimental conditions. Western blot analysis confirmed that the C-terminal fragment of E6-AP (aa 680 to 851) and full-length E6-AP are equally expressed (data not shown).
FIG. 4.
(A) E6-AP but not a C-terminal mutant lacking the activation domain reverses the transcriptional squelching between PR and ER. HeLa cells were transfected with 0.2 μg of PR expression plasmid, 0.3 μg of ER expression plasmid, 1.0 μg of pPRE/GRE.E1b.LUC, and increasing concentrations (0, 0.1, 0.5, and 1.0 μg) of wild-type E6-AP. Cells were then treated with progesterone (Prog) or progesterone and estradiol (E2) together (each at 10−8 M). Lane 8 represents control cells which were transfected with only PR and E6-AP expression plasmids. Each bar depicts the average of at least three wells. Activity in the presence of hormone and in the absence of exogenous coactivator was defined as 100%, and data for the other bars were scaled accordingly. (B) The C-terminal fragment of E6-AP (aa 680 to 851) was unable to reverse the transcriptional interference between PR and ER. HeLa cells were transfected with 0.2 μg of PR expression plasmid, 0.3 μg of ER expression plasmid, 1.0 μg of pPRE/GRE.E1b.LUC, and increasing concentrations (0, 0.1, 0.5, and 1.0 μg) of the C-terminal fragment of E6-AP. Cells were then treated with progesterone (Prog) or progesterone and estradiol (E2) together (10−8 M). Each bar depicts the average of at least three wells. Activity in the presence of hormone and in the absence of exogenous coactivator was defined as 100%, and data for the other bars were scaled accordingly.
FIG. 5.
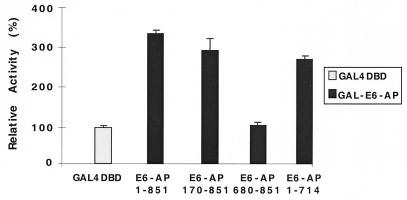
Transcriptional activity of the GAL4–E6-AP fusion protein. The indicated forms of E6-AP (Fig. 1A) were fused to the yeast GAL4 DBD. HeLa cells were then transfected with 0.5 μg of UAS4-TATA-luciferase reporter DNA and GAL4 DBD or GAL4–E6-AP expression plasmid (1.0 μg). Each bar depicts the average of at least three wells. Activation of the GAL4 DBD was define as 100%, and the activity of each GAL4–E6-AP fusion protein was adjusted accordingly.
E6-AP contains an intrinsic activation domain.
To ascertain whether E6-AP possesses an intrinsic, transferable activation domain, wild-type and deletion fragments of E6-AP were recruited to DNA by linking them to the GAL4 DBD. Wild-type E6-AP (aa 1 to 851) and the N-terminal (aa 170 to 851, 76 kDa) and C-terminal (aa 1 to 714, 86 kDa) deletion fragments stimulated the transcriptional activity of the reporter gene compared to that of the control vector containing only the GAL4 DBD (Fig. 5), while the 21-kDa fragment (aa 680 to 851) did not. This finding suggests that E6-AP itself contains a transcriptional activation domain located between aa 170 and 680.
E6-AP contains two independent, separable functions, coactivation and ubiquitin-ligase activity.
Since E6-AP is a ubiquitin-protein ligase, we examined whether the coactivation function of E6-AP is dependent on this enzymatic function. It has been shown that the conserved C833 residue in E6-AP forms a thioester bond with ubiquitin and is necessary for the transfer of ubiquitin to the protein targeted for ubiquitination. The mutation of C833 to A or S has been shown to eliminate the ubiquitin-protein ligase activity of E6-AP (19). In cotransfection experiments, an E6-AP bearing a C-to-S mutation at this critical site was still able to coactivate PR (Table 1) and ER (data not shown) to nearly the same extent as wild-type E6-AP. Furthermore, the C833S mutant form of E6-AP also can reverse squelch the hormone-dependent transcriptional activity of PR to a similar extent as wild-type E6-AP (data not shown). Our data suggest that the ubiquitin-protein ligase activity of E6-AP is not required for the coactivation function of E6-AP. To further confirm that the ubiquitin-proteasome pathway is not involved in the coactivation function of E6-AP, we analyzed a deletion mutant of E6-AP (aa 1 to 845) which lacks 6 aa at the carboxy terminus and has been shown to be defective for ubiquitin-protein ligase activity (19). Like the C833S mutant, this mutant also retains the ability to coactivate the hormone-dependent transcriptional activity of PR (Table 1), further confirming that the ubiquitin-protein ligase activity of E6-AP is not necessary for E6-AP to function as a coactivator. Our data indicate that E6-AP possesses two independent, separable functions, coactivation and ubiquitin-protein ligase activity.
The AS phenotype results from defects in the ubiquitin-protein ligase activity of E6-AP.
Recently, it was shown that a subset of AS patients express mutant forms of the E6-AP, rather than possessing the more common large-scale deletions of the 15q11-q13 region which contains E6-AP (26, 30, 42). To determine if the coactivator function of E6-AP is necessary for development of the AS phenotype, we generated several mutant E6-AP proteins corresponding to those found in these AS patients (Table 1). First, we tested the effect of an E6-AP mutant with a gross deletion in which the C-terminal half of the protein had been deleted due to a nonsense mutation at codon 417 (R417X). The ability of this AS mutant protein to coactivate PR is much less than that of wild-type E6-AP, but it can still interact with PR (Fig. 2B), indicating that a loss of coactivation is due to disruption of the activation domain located at aa 170 to 680. Furthermore, the loss of coactivation by the R417X mutant is not due to the loss of expression of mutant protein, since this mutant was able to interact with PR to the same extent as wild-type E6-AP in the mammalian cells used to assess coactivation (Fig. 2B). The R417X mutant of E6-AP was also unable to coactivate ER and AR (data not shown).
We then tested another mutant form of E6-AP which contains a small deletion in the hect domain due to a frameshift mutation which results in the truncation of the last 17 aa of the protein (aa 1 to 834) and the replacement of four different amino acids from the new reading frame. This mutant E6-AP was able to coactivate PR to the same extent as wild-type E6-AP (Table 1). Similarly, an artificial mutant which lacks 6 aa at the extreme C terminus of E6-AP (aa 1 to 845) was also able to act as a coactivator of PR activity (Table 1). We tested three other mutations for the ability to coactivate PR transcription: missense mutation I804K, in which isoleucine 804 was mutated to lysine; F782Δ, an internal in-frame deletion of phenylalanine 782; and 1-885 Δstop, a readthrough mutation which results in a longer mutant form of E6-AP. All three of these mutant forms of E6-AP were able to coactivate PR activity, suggesting that the coactivator function of E6-AP is not involved in the central nervous system phenotype of AS (Table 1).
To correlate the ubiquitin-protein ligase activity of E6-AP with AS, we tested the ubiquitin-ligase function of wild-type and AS mutant forms of E6-AP. Some AS mutant forms of E6-AP, such as the fragment comprising aa 1 to 834, R417X, and F782Δ, were unable to ubiquitinate a protein (HHR23A) implicated as a target of E6-AP ubiquitin-protein ligase activity in an in vitro ubiquitin assay (27, 49); the results suggest that loss of ubiquitin-protein ligase activity contributes to the AS phenotype in these patients. However, the AS missense mutant 804K was able to ubiquitinate the target protein HHR23A to an extent comparable to that of wild-type E6-AP (Table 1).
DISCUSSION
Nuclear hormone receptors are ligand-induced transcription factors. To activate transcription of target genes, these receptors undergo a complex multistep activation process (18, 34, 40, 47). These steps, though required for receptor function, are not sufficient to achieve optimal receptor function. Recently, it has been shown that coactivator proteins are necessary for maximal gene activation by the receptors (40). Coactivators enhance receptor function by acting as a bridge between DNA-bound receptor and basal transcription factors of the preinitiation complex or by providing HAT activity which disrupts the local repressive chromatin structure, contributing to increased transcriptional activity of the target gene (2, 9, 28, 36, 41, 45).
In this report, we demonstrate that E6-AP protein interacts only with the liganded form of PR, both in vivo and in vitro, and that it coactivates the transcriptional activity of the hormone-bound receptors. However, E6-AP fails to interact with PR in the presence of RU486, consistent with our previously published data indicating that coactivators do not interact efficiently with receptors in the presence of antihormone both in vitro and in vivo (1, 33, 50, 52). Like other cloned coactivators, E6-AP contains LXXLL motifs, which are thought to be important for receptor interaction (15, 16). Two of these motifs are located within the amino terminus of E6-AP whereas the third is located within the carboxy terminus, which supports our findings that E6-AP possesses receptor-interacting regions in both amino and carboxy termini.
The existence of coactivators in the signal transduction pathway of nuclear hormone receptors is supported by the finding that transcription activity of one receptor can be squelched by the overexpression of another receptor, indicating that both receptors compete for common factors. This observation led us to determine whether E6-AP is one of these limiting factors that can abrogate this squelching phenomenon (6, 10, 31, 39). Our study shows that overexpression of E6-AP in mammalian cells reverses the squelching effect of ER on PR transactivation in dose-dependent manner. These results further support the observation that E6-AP is a genuine coactivator for nuclear hormone receptors.
To date, several coactivators, e.g., SRC-1 (33), TIF2 (GRIP1) (17, 51), and p/CIP (ACTR/RAC3/AIB1/TRAM-1) (2, 9, 28, 46, 48), have been cloned. These coactivators contain intrinsic activation domains and enhance the transactivation of the nuclear hormone receptor superfamily. Most of the coactivators exhibit no receptor specificity and are able to coactivate a wide variety of nuclear hormone receptors (33). Like these other coactivators, E6-AP has an intrinsic activation domain and coactivates all nuclear hormone receptors tested.
E6-AP represents a unique class of coactivators because it exhibits ubiquitin-protein ligase activity. However, this ubiquitin-protein ligase activity is not part of the coactivator function of E6-AP. The data presented in this report indicate that E6-AP possesses two independent, separable functions, coactivation and ubiquitin-protein ligase activity. On the other hand, previously cloned coactivators such as SRC-1, p300/CBP, and RAC3/ACTR/AIB1 possess HAT activity and presumably manifest part of their in vivo coactivation function through this enzymatic activity (2, 9, 28, 32, 41). E6-AP possesses ubiquitin-protein ligase activity, instead of HAT activity, which is not a prerequisite for coactivation. This finding suggests that E6-AP works as a novel dual-function protein, orchestrating both steroid hormone receptor action and ubiquitin-proteasome-mediated degradation of p53. Another coactivator, TRIP230, has also been shown to be involved in cell cycle control by sequestering the hypophosphorylated form of the retinoblastoma protein (8).
Another potential coactivator identified in yeast and mammalian cells (RSP5/hRPF1) has been implicated as a coactivator of steroid hormone receptors that possesses a hect domain with 37% identity to that of E6-AP (24). UREB1, a DNA-binding protein which also contains a hect domain (32), is amino-terminally truncated (approximately 300 aa) compared to E6-AP and has no effect on the transactivation function of nuclear hormone receptors (data not shown), again suggesting that the hect domain alone is not sufficient for coactivation.
The results presented here for mutant E6-AP proteins identified in AS patients suggest that the coactivation function of E6-AP is not associated with the phenotypic manifestation of AS. However, our results do suggest that the AS phenotype results from a defect in the ubiquitin-protein ligase activity of E6-AP. Normally, only the maternal copy of E6-AP is expressed in certain regions of the brain, while the paternal copy is silent due to imprinting (37). However, it is still possible that gross or complete deletions of E6-AP (such as the R417X mutant) can result in defective steroid receptor coactivation in these regions of brain or other tissues where E6-AP is expressed in an imprinted manner. A more detailed analysis of the relationships among AS, E6-AP, and other nuclear hormone receptor-regulated processes awaits further investigation. Interestingly, haploinsufficiency of another nuclear hormone receptor coactivator, CREB-binding protein, is associated with Rubinstein-Taybi syndrome, a hereditary disease also characterized by diverse neurological defects (35).
In conclusion, our results demonstrate that E6-AP, a protein genetically linked to a human hereditary disease (AS), is a bona fide coactivator of nuclear hormone receptors. Although ubiquitin-proteasome pathway-mediated degradation of transcription factors recently has been shown to be important for transcriptional regulation (25, 29, 43), our experiments suggest that E6-AP’s ubiquitin-protein ligase activity is not sufficient to mediate the ability of E6-AP to coactivate nuclear hormone receptors. Nevertheless, it is possible that the ubiquitin-mediated degradation pathway(s) contributes to some aspects of nuclear hormone receptor function in vivo. E6-AP may modulate the transcriptional activity of nuclear hormone receptors by promoting the degradation of negative regulators of transcription such as corepressors. Consistent with this hypothesis, it has been shown that one of the nuclear receptor’s corepressors, N-CoR, can be degraded through the proteasome degradation pathway (55). It is also possible that subsequent to receptor activation of transcription, a mechanism is required to dissociate the preinitiation complex to allow reinitiation of transcription and elongation and ultimately to mediate the degradation of either the receptor or general transcription factors to exert tighter control of transcription. Further evidence of a link between the ubiquitin pathway and gene transcription has been suggested by a report that RSP5/RPF1 ubiquitinates the C-terminal domain of RNA polymerase II (23). Our report represents another example of a group of coactivators for nuclear receptors whose members contain distinct coactivation and enzymatic activities.
ACKNOWLEDGMENTS
We thank Andrew Denies and Sam Cho for technical support. We also thank Peter Howley and Sushant Kumar for the wild-type E6-AP, N-terminally truncated E6-AP,C833S mutant E6-AP, HHR23A, and ubiquitin reagents; Arthur Beaudet for AS mutant E6-AP cDNAs; and Austin Cooney and Neil McKenna for critical reading of the manuscript.
This work was supported by a grant from the NIH to B.W.O.
REFERENCES
- 1.Allan G F, Leng X, Tsai S Y, Weigel N L, Edwards D P, Tsai M J, O’Malley B W. Hormone and antihormone induce distinct conformational changes which are central to steroid receptor activation. J Biol Chem. 1992;267:19513–19520. [PubMed] [Google Scholar]
- 2.Anzick S L, Kononen J, Walker R L, Azorsa D O, Tanner M M, Guan X Y, Santer G, Kallioniemi O P, Trent J M, Meltzer P S. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 3.Baniahmad A, Ha I, Reinberg D, Tsai S Y, Tsai M-J, O’Malley B W. Interaction of human thyroid hormone receptor β with transcription factor TFIIB may mediate target gene derepression and activation by thyroid hormone. Proc Natl Acad Sci USA. 1993;90:8832–8836. doi: 10.1073/pnas.90.19.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baniahmad A, Leng X, Burris T P, Tsai S Y, Tsai M-J, O’Malley B W. The τ4 activation domain of the thyroid hormone receptor is required for release of a putative corepressor(s) necessary for transcriptional silencing. Mol Cell Biol. 1995;15:76–86. doi: 10.1128/mcb.15.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beuten J, Hennekam R C M, Van Roy B, Mangelschots K, Sutcliffe J S, Halley D J J, Hennekan F A M, Beaudet A R, Willems P J. Angelman syndrome in an inbred family. Hum Genet. 1996;97:294–298. doi: 10.1007/BF02185757. [DOI] [PubMed] [Google Scholar]
- 6.Bocquel M T, Kumar V, Stricker C, Chambon P, Gronemeyer H. The contribution of the N- and C-terminal regions of steroid receptors to activation of transcription is both receptor and cell specific. Nucleic Acids Res. 1989;17:2581–2594. doi: 10.1093/nar/17.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burris T P, Nawaz Z, Tsai M-J, O’Malley B W. A nuclear hormone receptor-associated protein that inhibits transactivation by the thyroid hormone and retinoic acid receptors. Proc Natl Acad Sci USA. 1995;922:9525–9529. doi: 10.1073/pnas.92.21.9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang K H, Chen Y, Chen T T, Chou W H, Chen P L, Ma Y Y, Feng T L Y, Leng X, Tsai M-J, O’Malley B W, Lee W H. A thyroid hormone receptor coactivator negatively regulated by the retinoblastoma protein. Proc Natl Acad Sci USA. 1997;94:9040–9045. doi: 10.1073/pnas.94.17.9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Lin R J, Schiltz R L, Chakravarti D, Nash A, Nagy L, Privalsky M L, Nakatani Y, Evans R M. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with p/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 10.Conneely O M, Kettelberger D M, Tsai M-J, O’Malley B W. Promoter specific activating domains of the chicken progesterone receptor. In: Roy A K, Clark J, editors. Gene regulation by steroid hormones IV. New York, N.Y: Springer-Verlag Press; 1989. pp. 220–223. [Google Scholar]
- 11.Daniel P R, Sanders C M, Maitland N J. Characterization of the interactions of human papillomavirus type 16 E6 with p53 and E6-associated protein in insect and human cells. J Gen Virol. 1998;79:489–499. doi: 10.1099/0022-1317-79-3-489. [DOI] [PubMed] [Google Scholar]
- 12.Durfee T, Becherer K, Chen P L, Yeh S H, Yang Y, Kilburn A E, Lee W H, Elledge S J. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- 13.Gu J, Dubner R, Fornace A J, Iadarola M J. UREB1, a tyrosine phosphorylated nuclear protein, inhibits p53 transactivation. Oncogene. 1995;11:2175–2178. [PubMed] [Google Scholar]
- 14.Hatakeyama S, Jensen J P, Weissman A M. Subcellular localization and ubiquitin-conjugating enzyme (E2) interactions of mammalian HECT family ubiquitin protein ligases. J Biol Chem. 1997;272:15085–15092. doi: 10.1074/jbc.272.24.15085. [DOI] [PubMed] [Google Scholar]
- 15.Heery D M, Kalkhoven E, Hoare S, Parker M G. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 16.Henttu P M, Kalkhoven E, Parker M G. AF-2 activity and recruitment of steroid receptor coactivator 1 to the estrogen receptor depend on a lysine residue conserved in nuclear receptors. Mol Cell Biol. 1997;17:1832–1839. doi: 10.1128/mcb.17.4.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong H, Kohli K, Trivedi A, Johnson D L, Stallcup M R. GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc Natl Acad Sci USA. 1996;93:4948–4952. doi: 10.1073/pnas.93.10.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horwitz K B, Jackson T A, Bain D L, Richer J K, Takimoto G S, Tung L. Nuclear receptor coactivators and corepressors. Mol Endocrinol. 1996;10:1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- 19.Huibregtse J M, Scheffner M, Beaudenon S, Howley P M. A family of proteins structurally and functionally related to the E6-AP ubiquitin-protein ligase. Proc Natl Acad Sci USA. 1995;92:2563–2567. doi: 10.1073/pnas.92.7.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huibregtse J M, Scheffner M, Howley P M. A cellular protein mediates association of human papillomavirus types 16 or 18. EMBO J. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huibregtse J M, Scheffner M, Howley P M. Cloning and expression of cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol Cell Biol. 1993;13:775–784. doi: 10.1128/mcb.13.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huibregtse J M, Scheffner M, Howley P M. Localization of the E6-AP regions that direct human papillomavirus E6-binding, association with p53, and ubiquitination of associated proteins. Mol Cell Biol. 1993;13:4918–4927. doi: 10.1128/mcb.13.8.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huibregtse J M, Yang J C, Beaudenon S L. The large subunit of RNA polymerase II is a substrate of the RSP5 ubiquitin-protein ligase. Proc Natl Acad Sci USA. 1997;94:3656–3661. doi: 10.1073/pnas.94.8.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imhof M O, McDonnell D P. Yeast RSP5 and its human homolog hRPF1 potentiate hormone-dependent activation of transcription by human progesterone and glucocorticoid receptors. Mol Cell Biol. 1996;166:2594–2605. doi: 10.1128/mcb.16.6.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim T K, Maniatis T. Regulation of interferon-γ-activated STAT1 by the ubiquitin-proteasome pathway. Science. 1995;273:1717–1719. doi: 10.1126/science.273.5282.1717. [DOI] [PubMed] [Google Scholar]
- 26.Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause angelman syndrome. Nat Genet. 1993;15:70–73. doi: 10.1038/ng0197-70. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S, Kao W H, Howley P M. Physical interaction between specific E2 and hect E3 enzymes determines functional cooperativity. J Biol Chem. 1997;272:13548–13554. doi: 10.1074/jbc.272.21.13548. [DOI] [PubMed] [Google Scholar]
- 28.Ii H, Gomes J, Chen D. RAC3, a steroid/nuclear receptor associated coactivator that is related to SRC-1 and TIF2. Proc Natl Acad USA. 1997;94:8479–8484. doi: 10.1073/pnas.94.16.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S, Li Y, Carthew R W, Lai Z C. Photoreceptor cell differentiation requires regulated proteolysis of the transcriptional repressor tramtrack. Cell. 1997;90:469–478. doi: 10.1016/s0092-8674(00)80507-3. [DOI] [PubMed] [Google Scholar]
- 30.Matsuura T, Sutcliff J S, Fang P, Galjaard R J, Jiang Y H, Benton C S, Rommens J M, Beaudet A L. De novo truncating mutations in E6-AP ubiquitin protein ligase gene (UBE3A) in angelman syndrome. Nat Genet. 1997;15:74–77. doi: 10.1038/ng0197-74. [DOI] [PubMed] [Google Scholar]
- 31.Meyer M E, Gronemeyer H, Turcotte B, Bocquel M T, Tasset D, Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989;57:433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- 32.Ogryzko V V, Schiltz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 33.Onate S A, Tsai S Y, Tsai M-J, O’Malley B W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 34.Perlmann T, Evans R M. Nuclear receptors in Sicily: all in the famiglia. Cell. 1997;90:391–397. doi: 10.1016/s0092-8674(00)80498-5. [DOI] [PubMed] [Google Scholar]
- 35.Petrij F, Giles R H, Dauwerse H G, Saris J J, Hennekam R C M, Masuno M, Tommerup N, van Ommen G B, Goodman R H, Peters D J M, Breuning M H. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature. 1995;376:348–351. doi: 10.1038/376348a0. [DOI] [PubMed] [Google Scholar]
- 36.Pugh B F, Tjian R. Diverse transcriptional function of the multisubunit eukaryotic TFIID complex. J Biol Chem. 1992;267:679–682. [PubMed] [Google Scholar]
- 37.Rougeulle C, Glatt H, Lalande M. The Angelman syndrome candidate gene, UBE3A/E6-AP is imprinted in brain. Nat Genet. 1997;17:14–15. doi: 10.1038/ng0997-14. [DOI] [PubMed] [Google Scholar]
- 38.Scheffner M, Huibregtse J M, Vierstra R D, Howley P M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 39.Shemshedini L, Ji J W, Brou C, Chambon P, Gronemeyer H. In vitro activity of the transcription activation functions of the progesterone receptor: evidence for intermediary factor. J Biol Chem. 1992;267:1834–1839. [PubMed] [Google Scholar]
- 40.Shibata H, Spencer T E, Onate S A, Jenster G, Tsai S Y, Tsai M-J, O’Malley B W. Role of coactivators and corepressors in the mechanism of steroid/thyroid receptor action. Recent Prog Hormone Res. 1997;52:141–165. [PubMed] [Google Scholar]
- 41.Spencer T, Jenster G, Burcin M M, Allis C D, Zhou J X, Mizzen C A, McKenna N J, Onate S A, Tsai S A, Tsai M-J, O’Malley B W. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 42.Sutcliff J S, Jiang Y H, Galjaad R J, Matsuura T, Fang P, Kubota T, Christian S L, Bressler J, Cattanach B, Ledbetter D H, Beaudet A L. The E6-AP ubiquitin protein ligase (UBE3A) gene is localized within a narrowed Angelman syndrome critical region. Genome Res. 1997;7:368–377. doi: 10.1101/gr.7.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang A H, Neufeld T P, Kwan E, Rubin G M. PHYL acts to down regulate TTK88, a transcriptional repressor of neural cell fates by a SINA-dependent mechanism. Cell. 1997;90:459–467. doi: 10.1016/s0092-8674(00)80506-1. [DOI] [PubMed] [Google Scholar]
- 44.Tilley W D, Marcelli M, Wilsom J D, McPhaul M M. Characterization and expression of a cDNA encoding the human androgen receptor. Proc Natl Acad Sci USA. 1989;86:327–331. doi: 10.1073/pnas.86.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tjian R, Maniatis T. Transcriptional activation: a complex puzzle with few easy pieces. Cell. 1994;77:5–8. doi: 10.1016/0092-8674(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 46.Torchia J, Rose D W, Inostroza J, Kamei Y, Westin S, Glass C K, Rosenfeld M J. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 47.Tsai M-J, O’Malley B W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 48.Tukeshita A, Cardona G R, Koibuchi N, Sue C S, Chin W W. TRAM-1, a novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1. J Biol Chem. 1997;272:27629–27634. doi: 10.1074/jbc.272.44.27629. [DOI] [PubMed] [Google Scholar]
- 49.Van der Spek P T, Smith E M, Beverloo H B, Sugasawa K, Masutani C, Hanaoka F, Hoeijimakers J H, Hagermeijier A. Chromosomal localization of three repair genes: the xeroderma pigmentosum group C gene and two human homologs of yeast RA23. Genomics. 1994;23:651–658. doi: 10.1006/geno.1994.1554. [DOI] [PubMed] [Google Scholar]
- 50.Vegeto E, Allan G F, Schrader W T, Tsai M-J, McDonnell D P, O’Malley B W. The mechanism of RU486 antagonism is dependent on the conformation of the carboxy-terminal tail of human progesterone receptor. Cell. 1992;69:703–713. doi: 10.1016/0092-8674(92)90234-4. [DOI] [PubMed] [Google Scholar]
- 51.Voegel J J, Heine M J, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 52.Xu J, Nawaz Z, Tsai S Y, Tsai M-J, O’Malley B W. The extreme C-terminus of progesterone receptor contains a transcriptional repressor domain that functions through a putative corepressor. Proc Natl Acad Sci USA. 1996;93:12195–12199. doi: 10.1073/pnas.93.22.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu J, Qiu Y, DeMayo F J, Tsai S Y, Tsai M-J, O’Malley B W. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1924. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 54.Yeh S, Chang C. Cloning and characterization of a specific coactivator, ARA70, for androgen receptor in human prostate cells. Proc Natl Acad Sci USA. 1996;93:5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J M, Guenther M G, Carthew R W, Lazar M A. Proteasomal regulation of nuclear receptor corepressor-mediated repression. Genes Dev. 1998;12:1775–1780. doi: 10.1101/gad.12.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]