Abstract
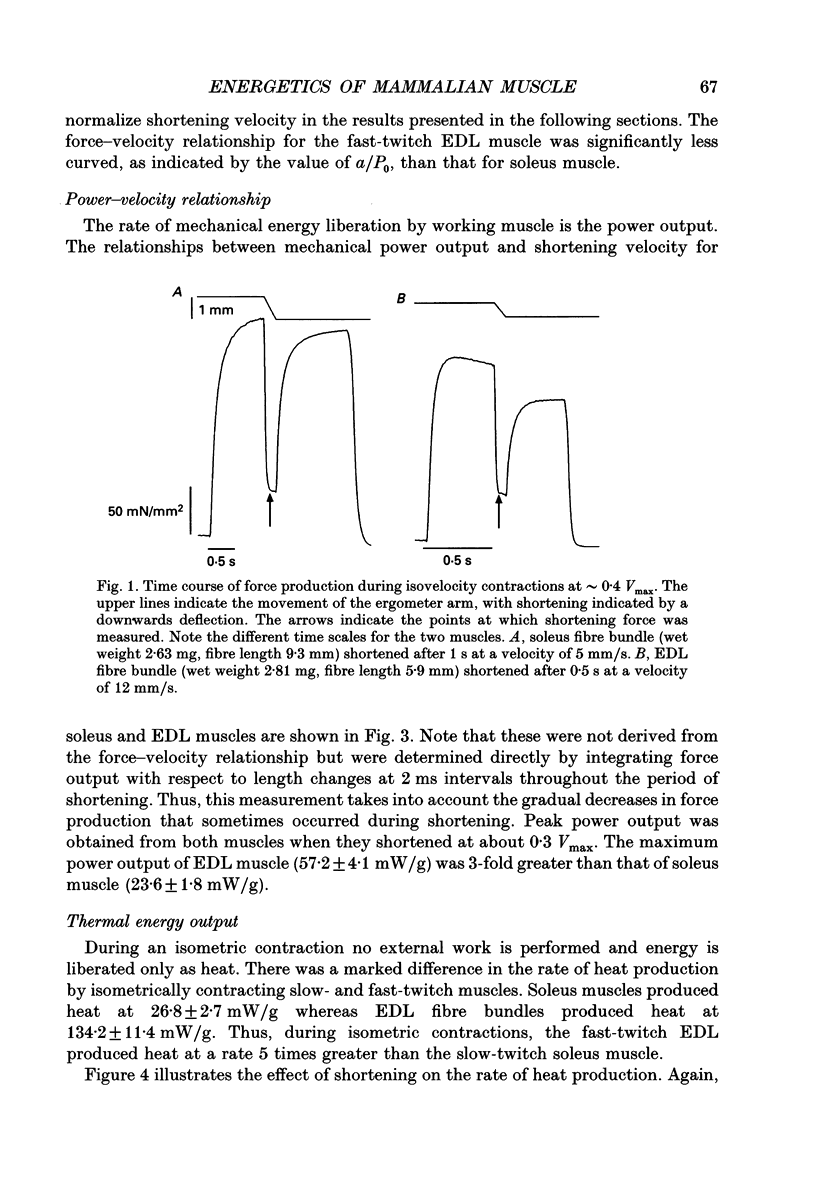
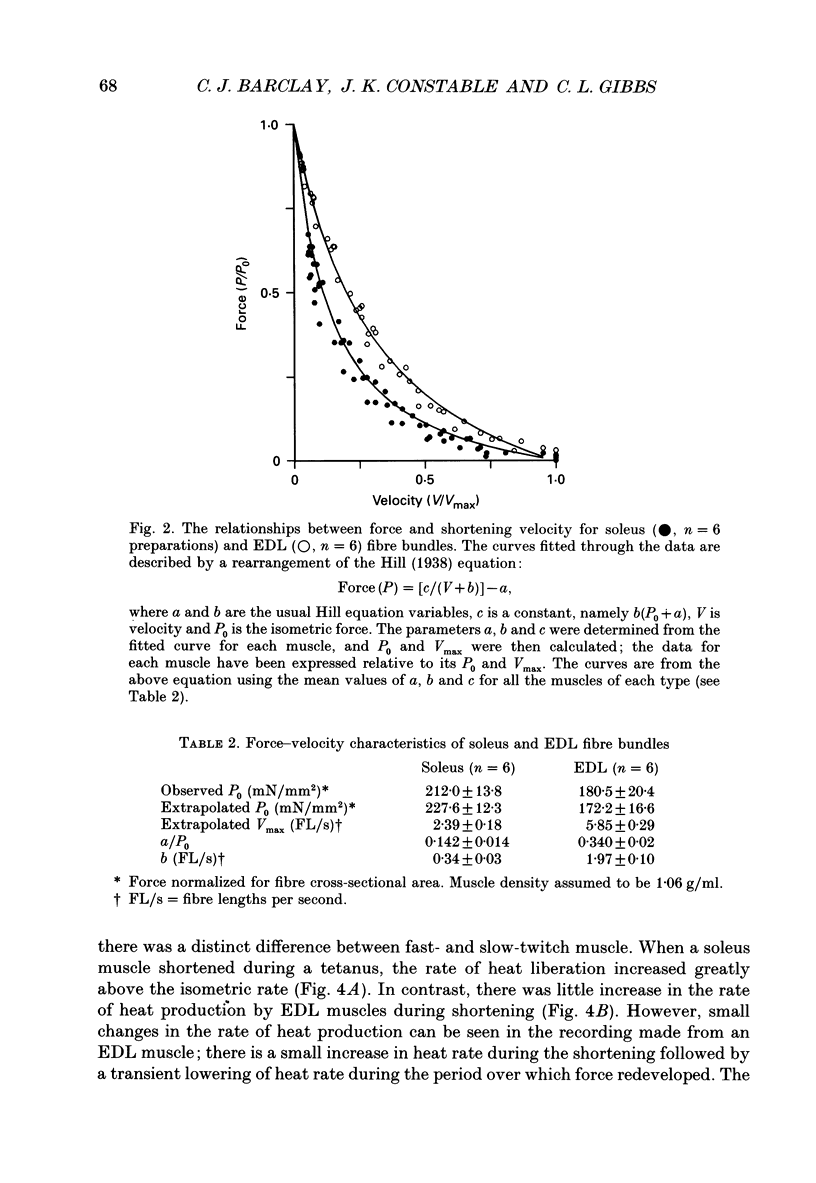
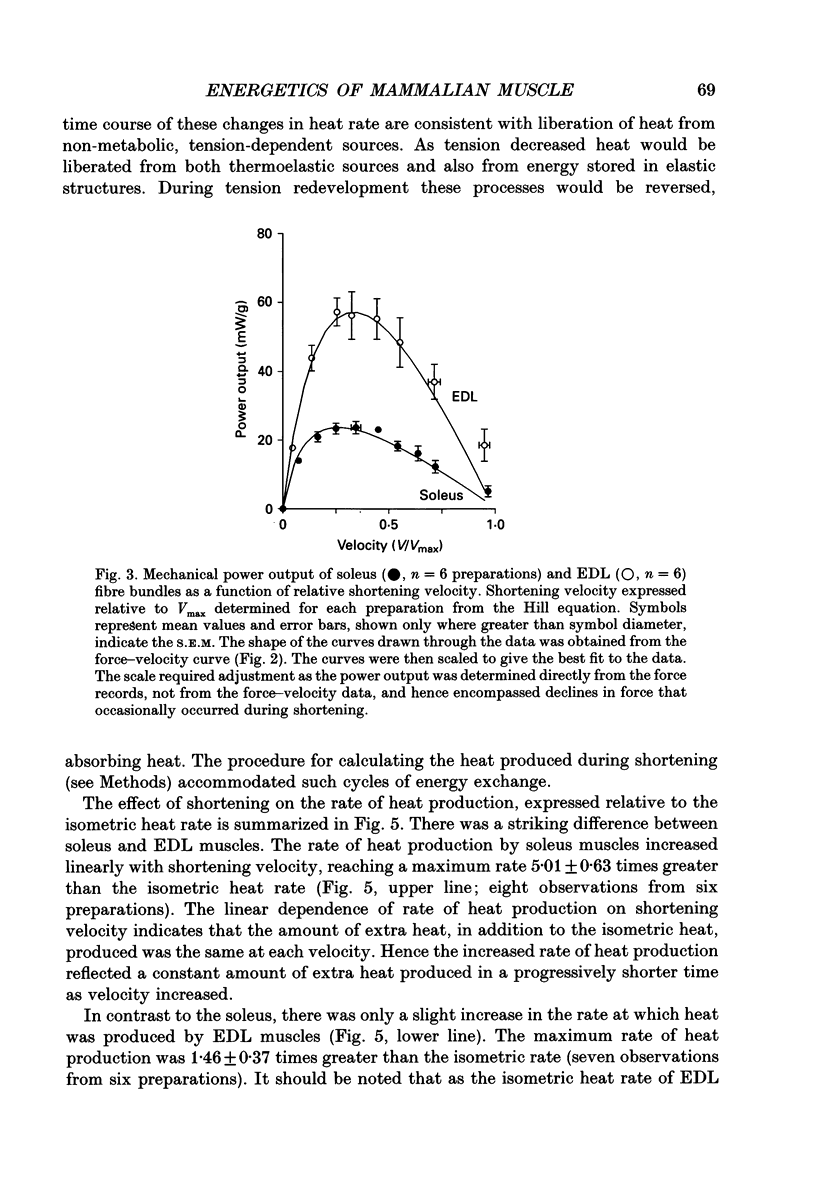
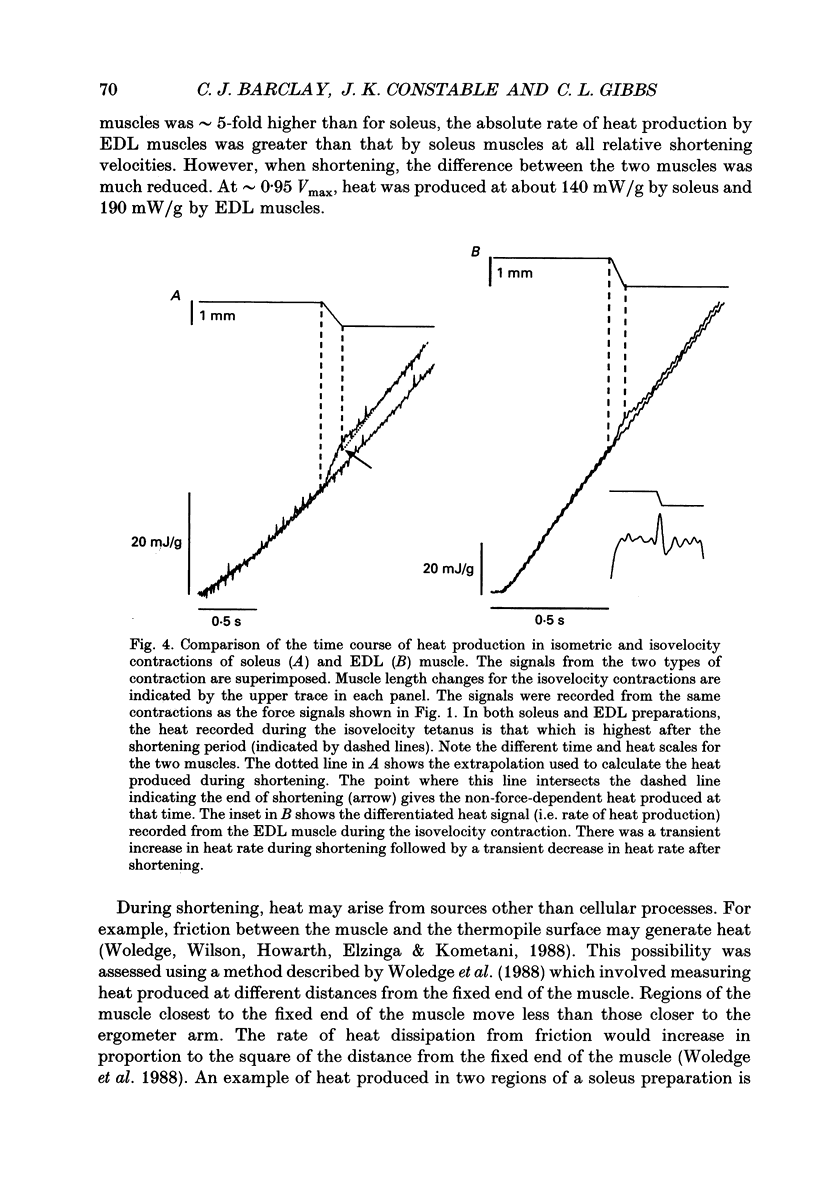
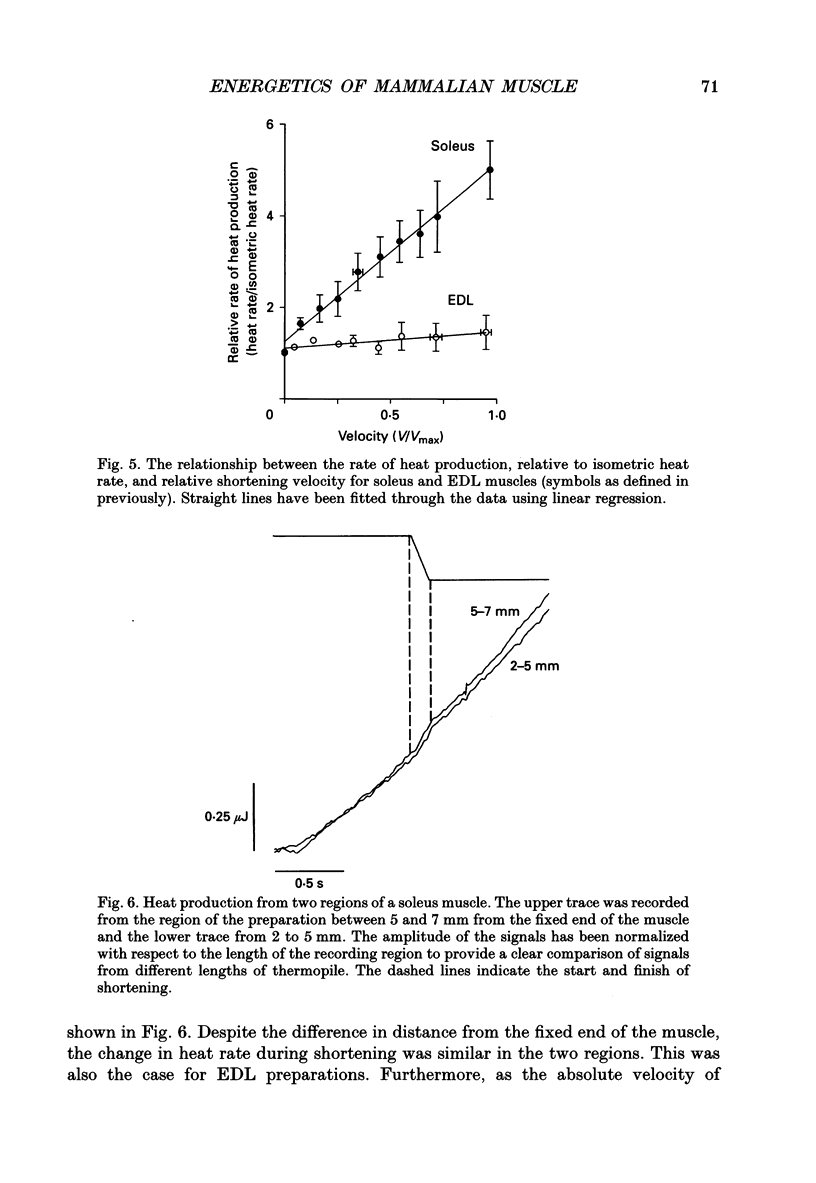
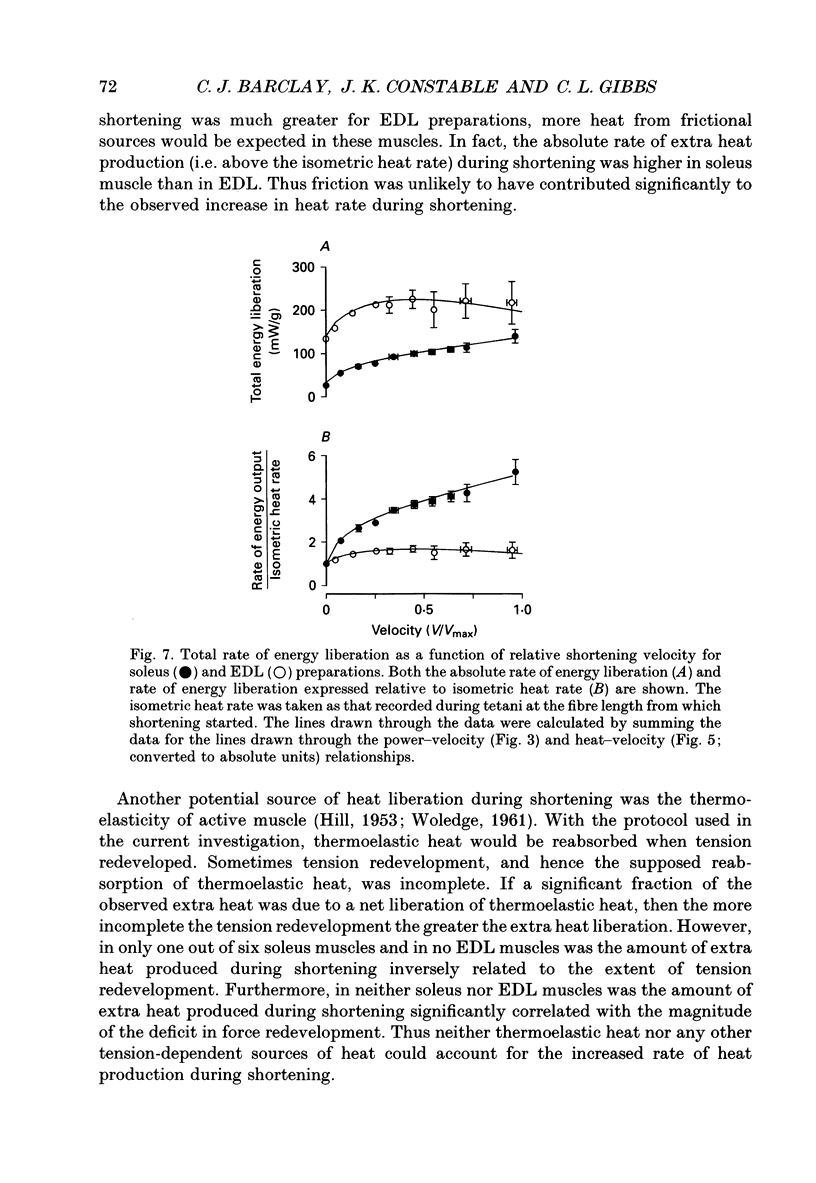
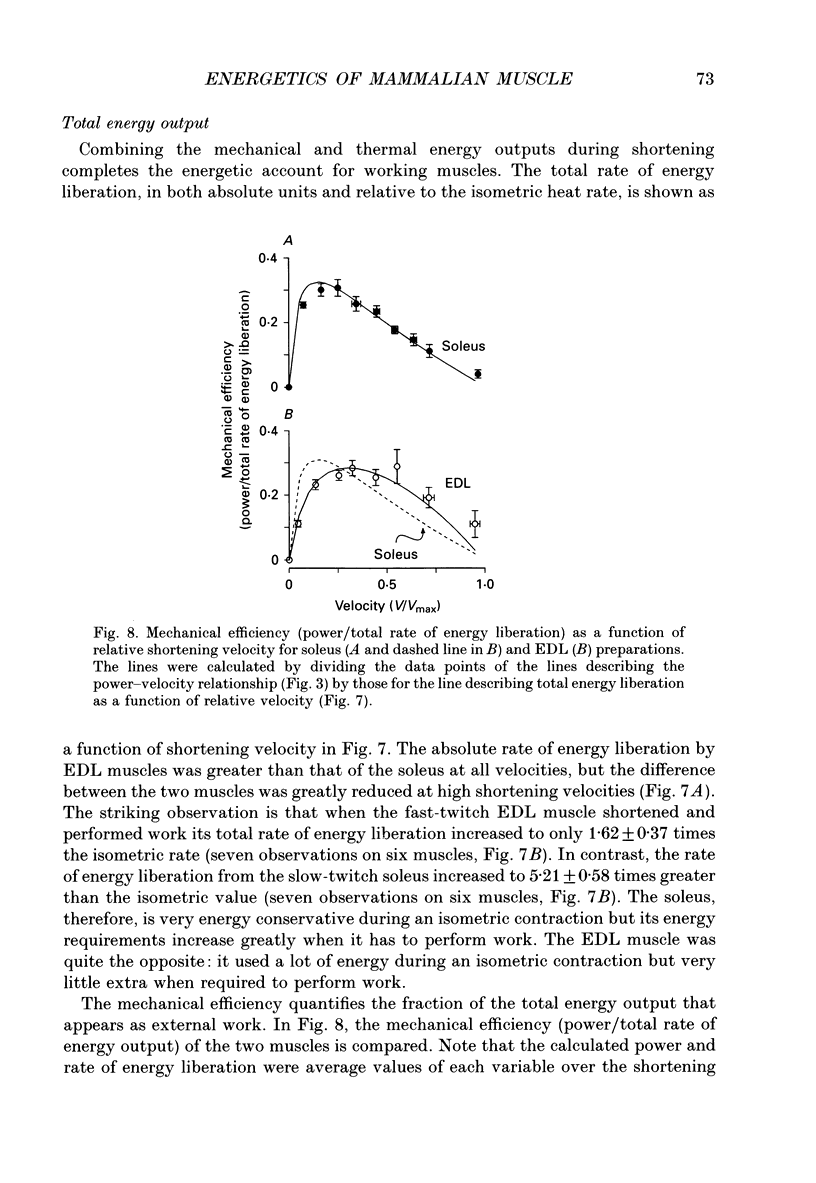
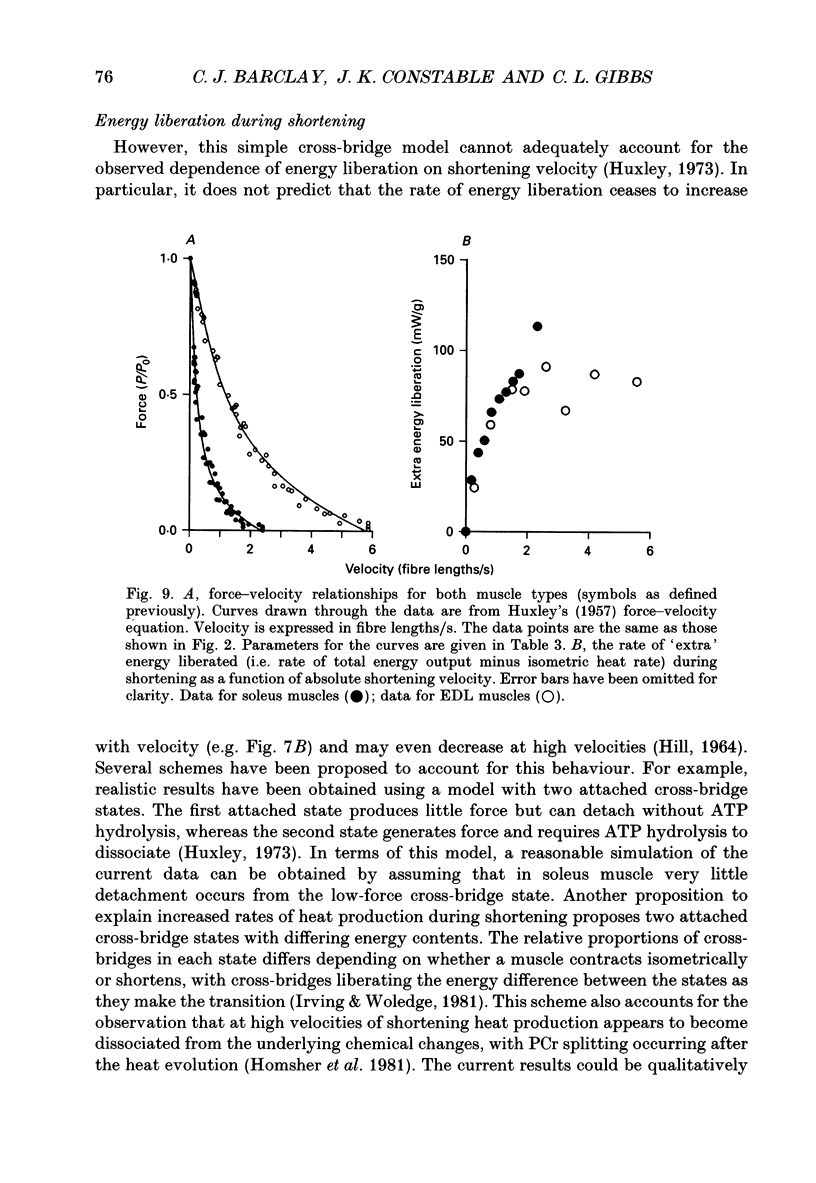
1. The energetic cost of work performance by mouse fast- and slow-twitch muscle was assessed by measuring the rates of thermal and mechanical energy liberation of the muscles at 21 degrees C. Thermal energy (heat) liberation was measured using a fast-responding thermopile. 2. Bundles of muscles fibres from the slow-twitch soleus and fast-twitch extensor digitorum longus (EDL) muscles were used. Work output was controlled by performing isovelocity shortenings during the plateau of an isometric tetanus. A range of shortening velocities, spanning the possible range, was used for each muscle. 3. During tetanic contractions, the rate of heat production from EDL muscle was 134.2 +/- 11.4 mW/g. The rate of heat production by soleus muscle was only one-fifth as great (26.8 +/- 2.7 mW/g). 4. The maximum shortening velocity (Vmax) of EDL muscles was 2.5-fold greater than that for soleus muscles and it's force-velocity relationship was less curved. Peak power output from EDL muscles was 3-fold greater than that from soleus muscle. 5. During shortening, the rate of heat output from soleus muscles increased considerably above the isometric heat rate. In contrast to soleus muscle, the rate of heat production by EDL muscle increased by only a small fraction of the isometric heat rate. The magnitude of the increases in rate was proportional to shortening velocity. 6. The total rate of energy liberation (heat rate + power) by EDL muscle, shortening at 0.95 Vmax was 1.62 +/- 0.37 times greater than the isometric heat rate. In contrast, the rate of energy liberation from soleus muscle shortening at 0.95 Vmax was 5.21 +/- 0.58 times greater than its isometric heat rate. The peak mechanical efficiency (power/total energy rate) of the both muscles was approximately 30%.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mulieri L. A., Scubon-Mulieri B. Non-hyperbolic force-velocity relationship in single muscle fibres. Acta Physiol Scand. 1976 Oct;98(2):143–156. doi: 10.1111/j.1748-1716.1976.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Gibbs C. L., Gibson W. R. Energy production of rat soleus muscle. Am J Physiol. 1972 Oct;223(4):864–871. doi: 10.1152/ajplegacy.1972.223.4.864. [DOI] [PubMed] [Google Scholar]
- HILL A. V. THE EFFECT OF LOAD ON THE HEAT OF SHORTENING OF MUSCLE. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:297–318. doi: 10.1098/rspb.1964.0004. [DOI] [PubMed] [Google Scholar]
- HILL A. V. The instantaneous elasticity of active muscle. Proc R Soc Lond B Biol Sci. 1953 Apr 17;141(903):161–178. doi: 10.1098/rspb.1953.0033. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Heglund N. C., Cavagna G. A. Mechanical work, oxygen consumption, and efficiency in isolated frog and rat muscle. Am J Physiol. 1987 Jul;253(1 Pt 1):C22–C29. doi: 10.1152/ajpcell.1987.253.1.C22. [DOI] [PubMed] [Google Scholar]
- Homsher E., Irving M., Wallner A. High-energy phosphate metabolism and energy liberation associated with rapid shortening in frog skeletal muscle. J Physiol. 1981 Dec;321:423–436. doi: 10.1113/jphysiol.1981.sp013994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E., Mommaerts W. F., Ricchiuti N. V. Energetics of shortening muscles in twitches and tetanic contractions. II. Force-determined shortening heat. J Gen Physiol. 1973 Dec;62(6):677–692. doi: 10.1085/jgp.62.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E. Muscle enthalpy production and its relationship to actomyosin ATPase. Annu Rev Physiol. 1987;49:673–690. doi: 10.1146/annurev.ph.49.030187.003325. [DOI] [PubMed] [Google Scholar]
- Huxley A. F. A note suggesting that the cross-bridge attachment during muscle contraction may take place in two stages. Proc R Soc Lond B Biol Sci. 1973 Feb 27;183(1070):83–86. doi: 10.1098/rspb.1973.0006. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Irving M., Woledge R. C. The dependence on extent of shortening of the extra energy liberated by rapidly shortening frog skeletal muscle. J Physiol. 1981 Dec;321:411–422. doi: 10.1113/jphysiol.1981.sp013993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar K. M., Wilkie D. R. A new method for absolute heat measurement, utilizing the Peltier effect. J Physiol. 1972 Jul;224(1):18P–21P. [PubMed] [Google Scholar]
- Leijendekker W. J., Elzinga G. Metabolic recovery of mouse extensor digitorum longus and soleus muscle. Pflugers Arch. 1990 Apr;416(1-2):22–27. doi: 10.1007/BF00370217. [DOI] [PubMed] [Google Scholar]
- Leijendekker W. J., van Hardeveld C., Elzinga G. Heat production during contraction in skeletal muscle of hypothyroid mice. Am J Physiol. 1987 Aug;253(2 Pt 1):E214–E220. doi: 10.1152/ajpendo.1987.253.2.E214. [DOI] [PubMed] [Google Scholar]
- Mulieri L. A., Luhr G., Trefry J., Alpert N. R. Metal-film thermopiles for use with rabbit right ventricular papillary muscles. Am J Physiol. 1977 Nov;233(5):C146–C156. doi: 10.1152/ajpcell.1977.233.5.C146. [DOI] [PubMed] [Google Scholar]
- WOLEDGE R. C. The thermoelastic effect of change of tension in active muscle. J Physiol. 1961 Jan;155:187–208. doi: 10.1113/jphysiol.1961.sp006622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt I. R., Barclay J. K. Effects of dantrolene on the energetics of fast- and slow-twitch muscles of the mouse. Am J Physiol. 1980 Jan;238(1):C56–C61. doi: 10.1152/ajpcell.1980.238.1.C56. [DOI] [PubMed] [Google Scholar]
- Wendt I. R., Gibbs C. L. Energy production of rat extensor digitorum longus muscle. Am J Physiol. 1973 May;224(5):1081–1086. doi: 10.1152/ajplegacy.1973.224.5.1081. [DOI] [PubMed] [Google Scholar]
- Woledge R. C., Wilson M. G., Howarth J. V., Elzinga G., Kometani K. The energetics of work and heat production by single muscle fibres from the frog. Adv Exp Med Biol. 1988;226:677–688. [PubMed] [Google Scholar]


