Abstract
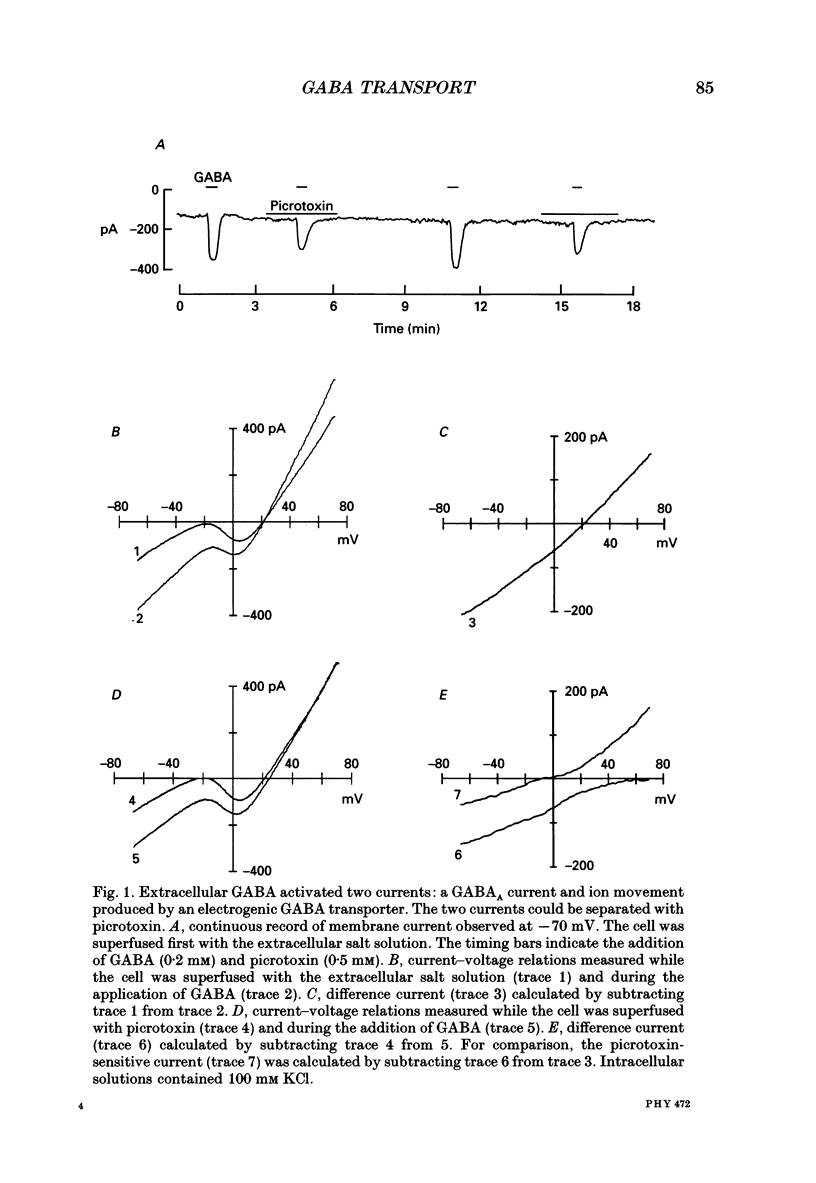
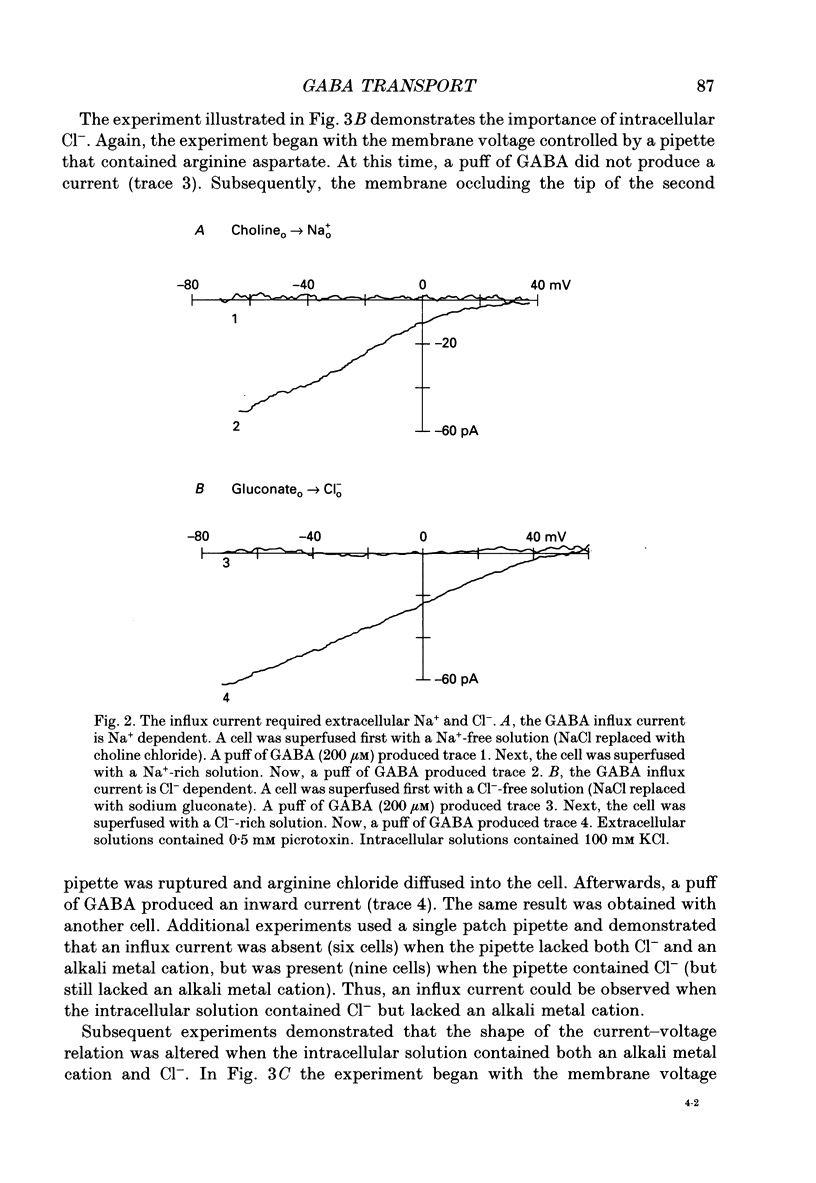
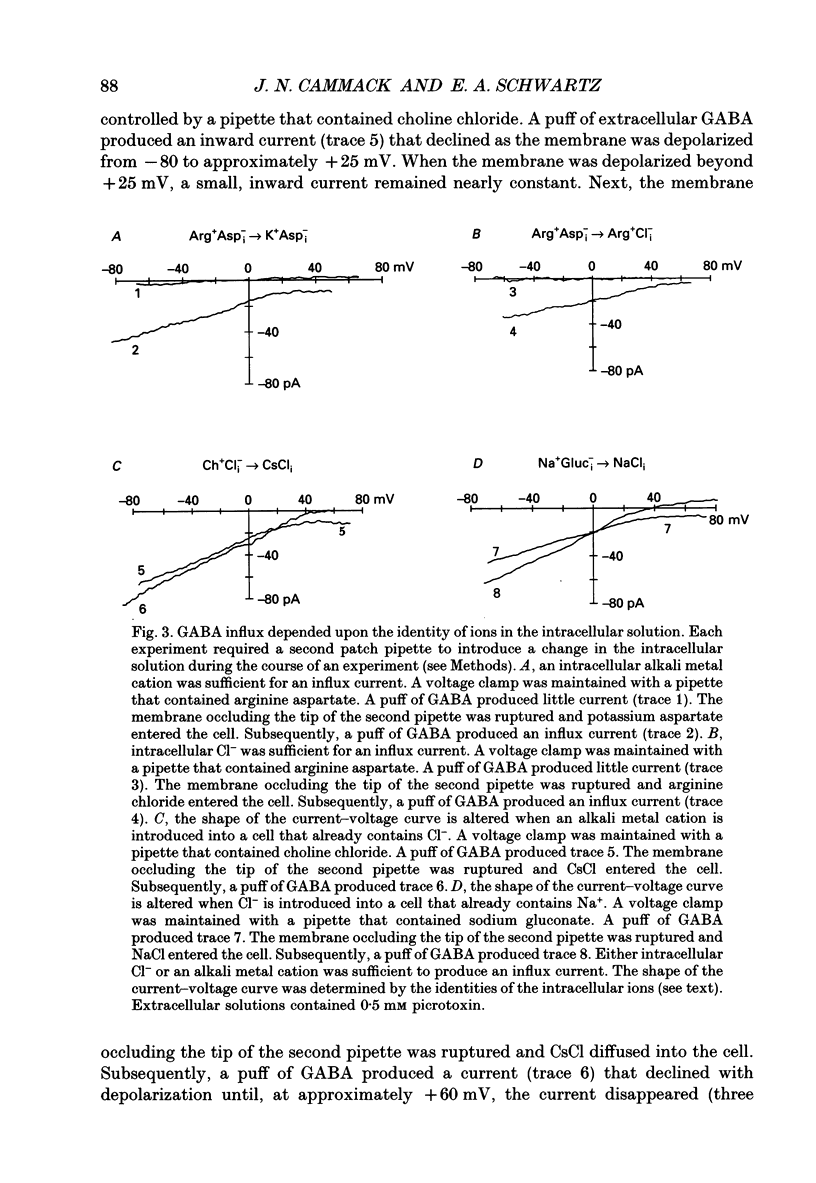
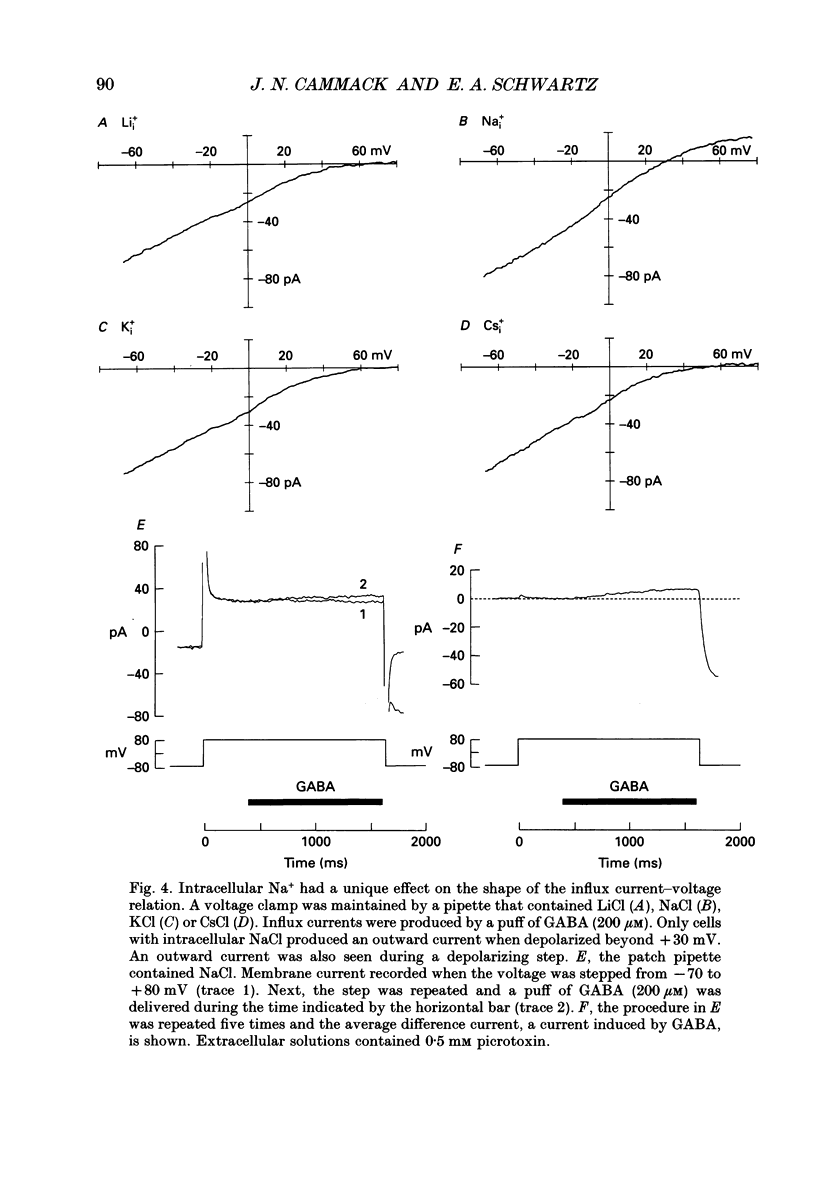
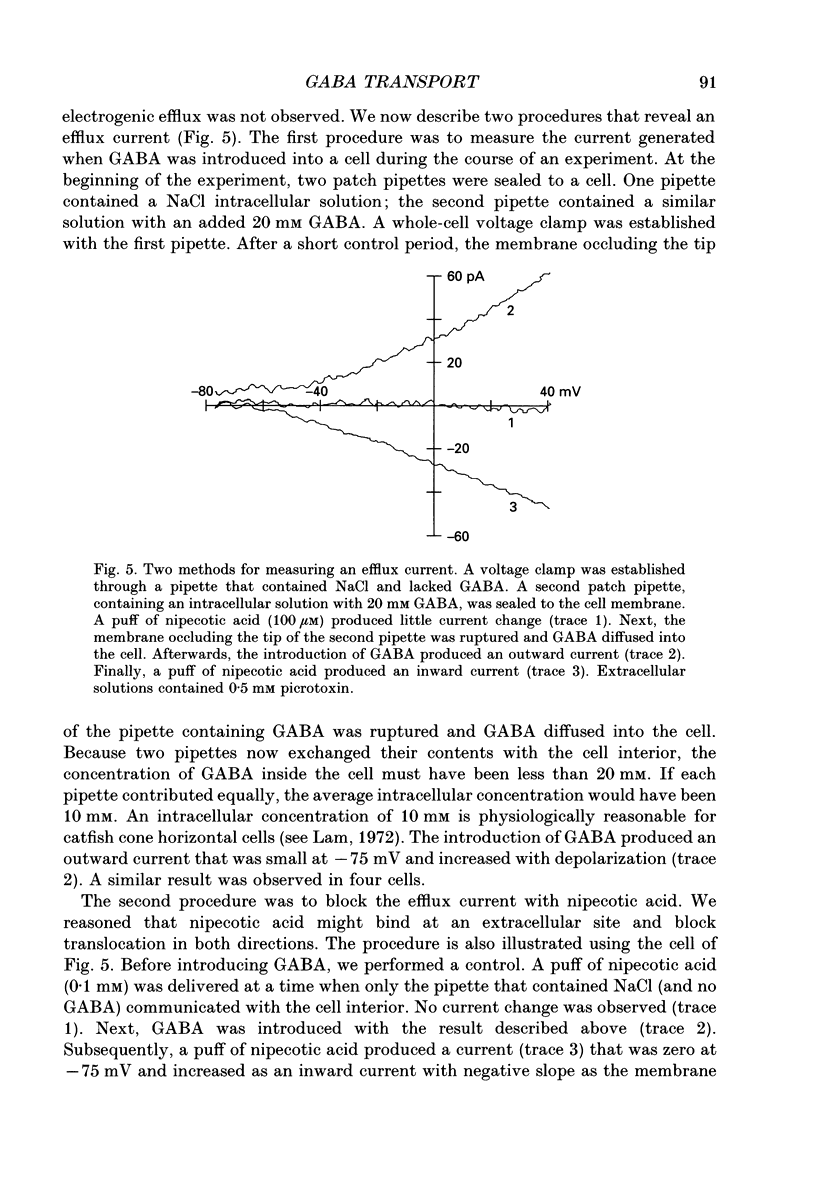
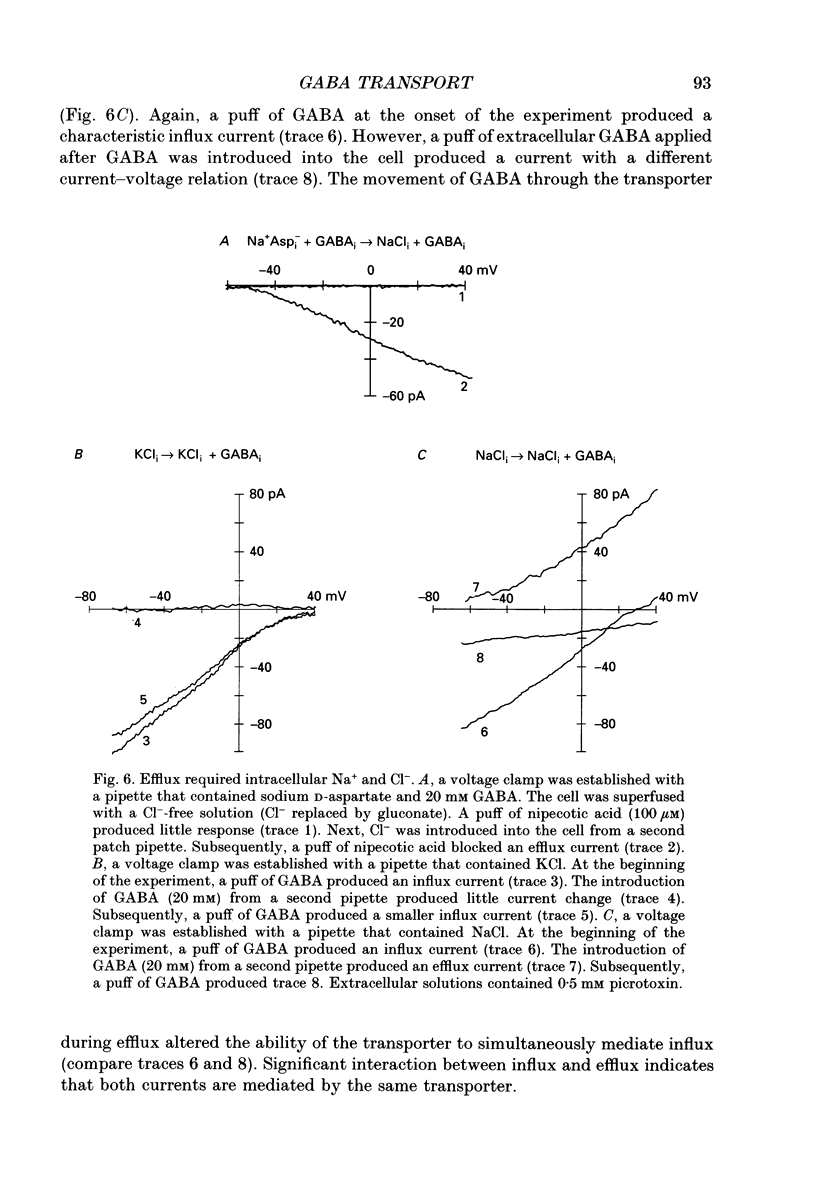
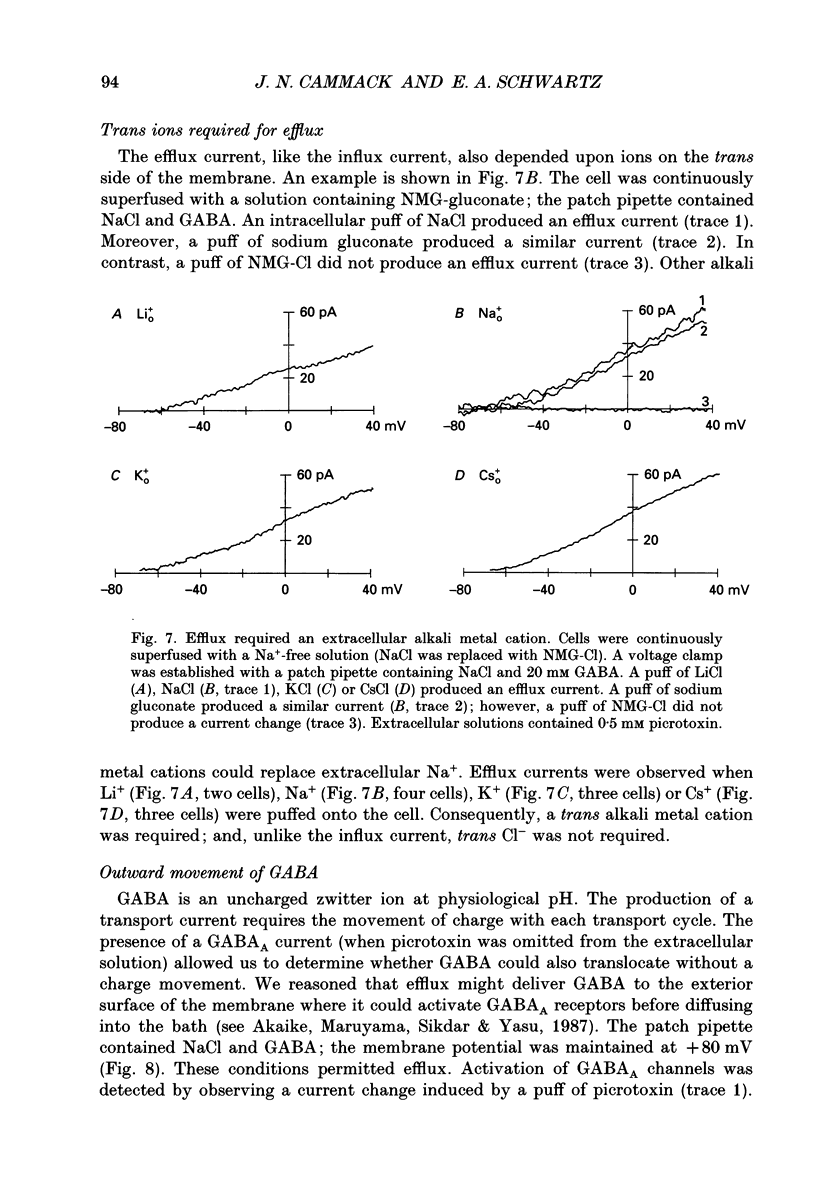
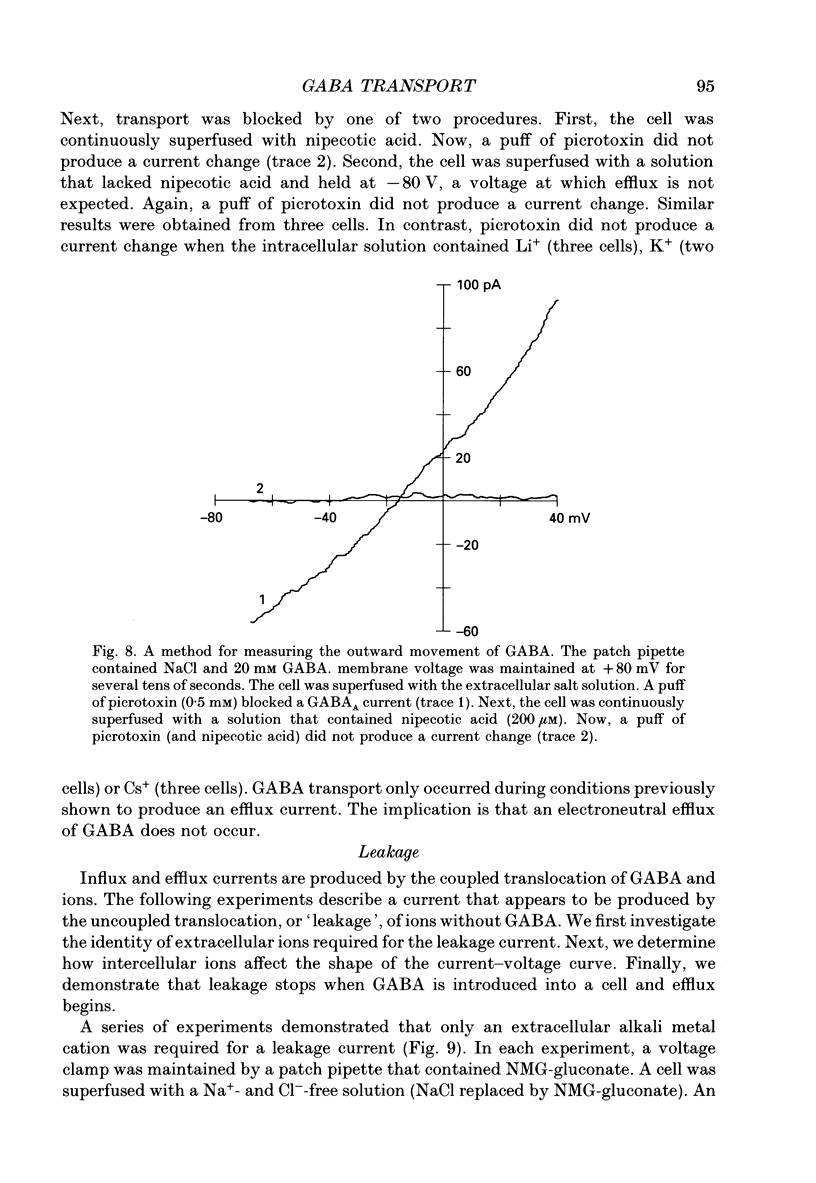
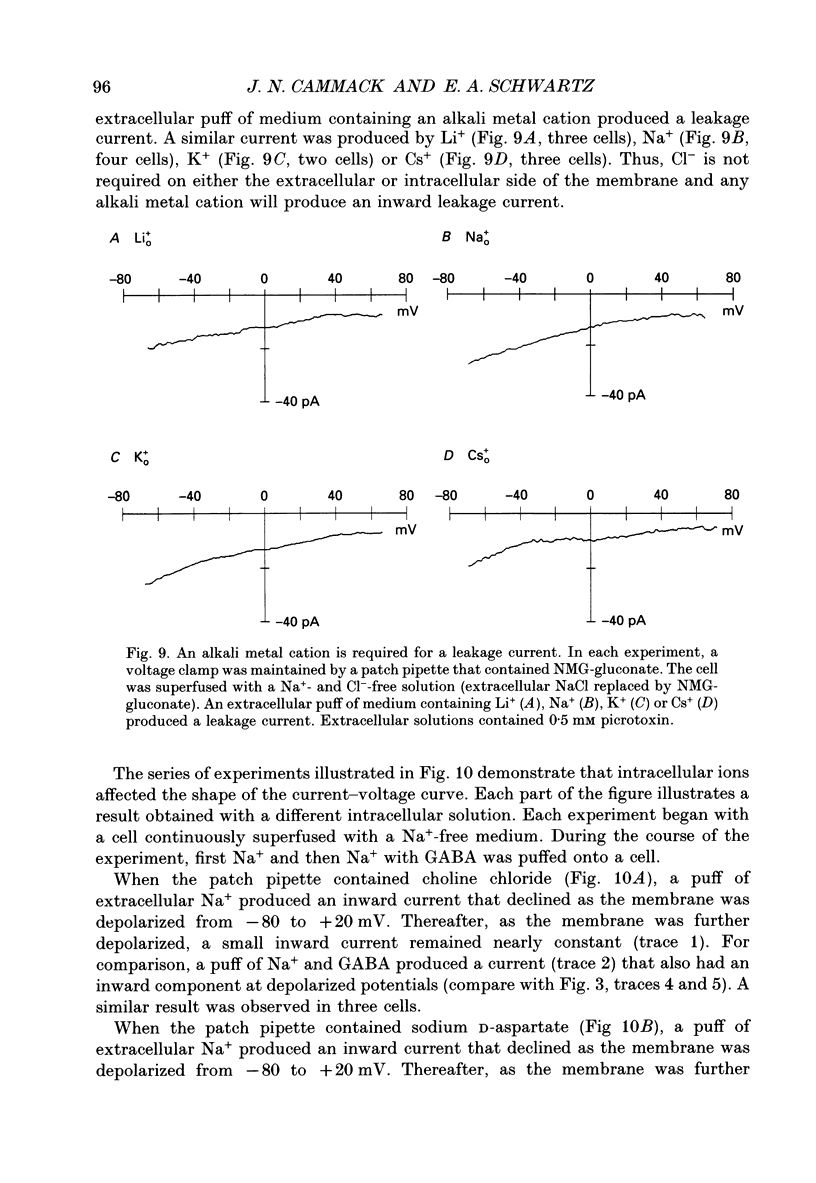
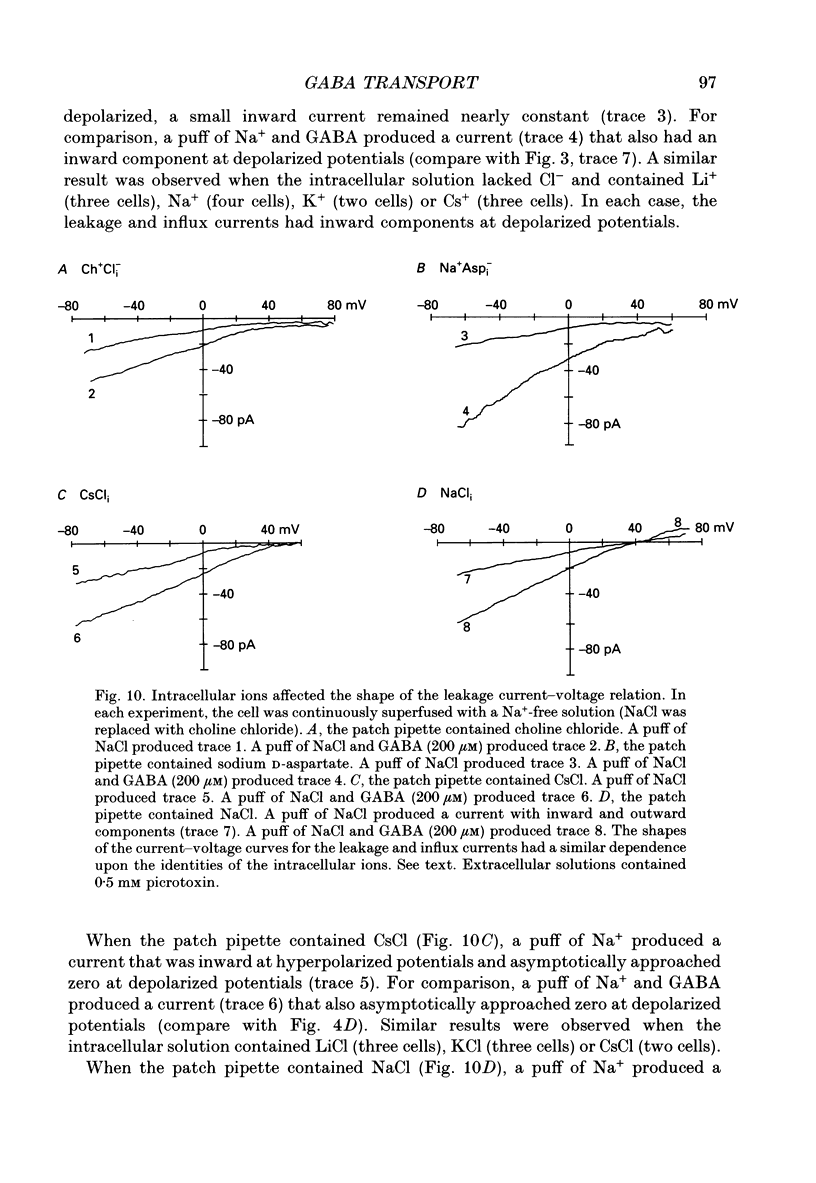
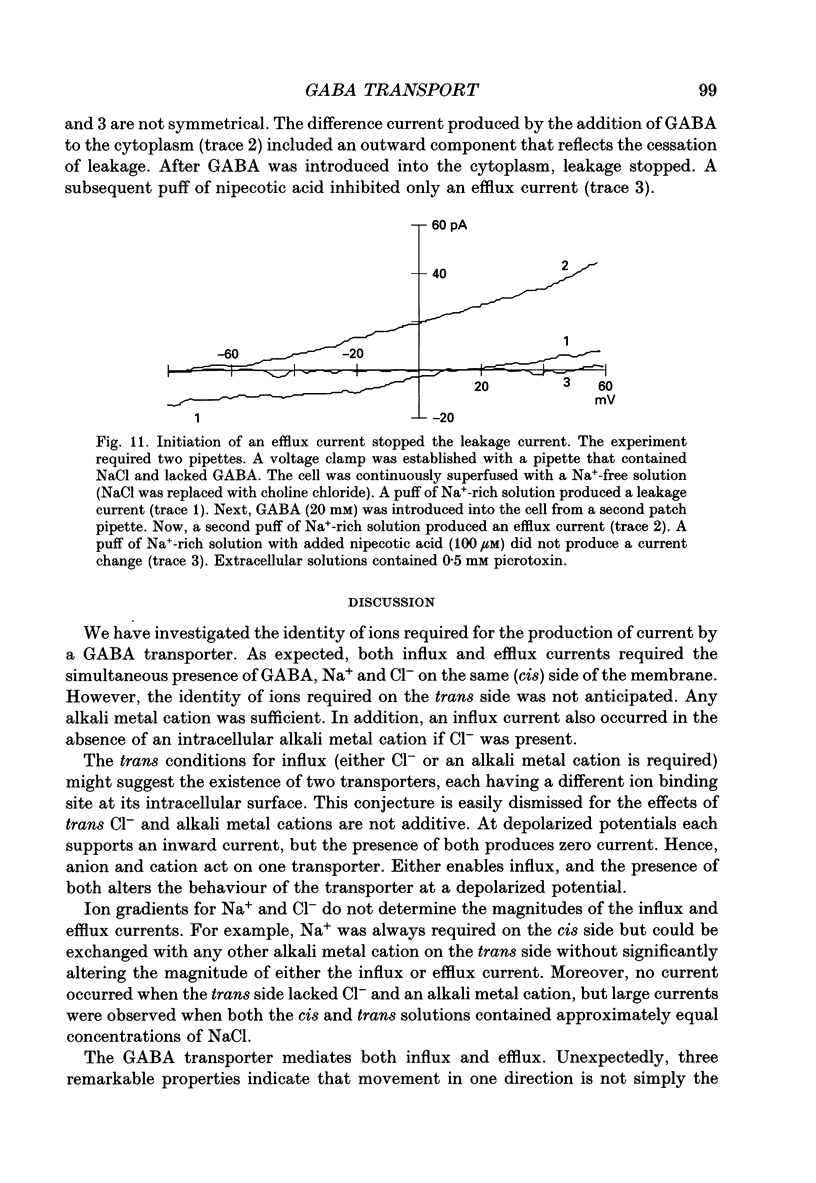
1. Solitary horizontal cells were isolated from catfish retinas. Membrane currents activated by extracellular and intracellular GABA were characterized during a whole-cell voltage clamp. 2. Extracellular GABA activated two currents: a GABAA current, and an 'influx' current mediated by a GABA transporter. The influx current was studied after the GABAA current was blocked with 0.5 mM picrotoxin. The influx current required extracellular Na+ and Cl-. Extracellular Na+ could not be replaced by another alkali metal cation. 3. The influx current also depended upon the identity of ions in the intracellular solution. Either an intracellular alkali metal cation or Cl- was required to produce an influx current. 4. The influx current was inward at -75 mV and decreased as the membrane was depolarized towards +20 mV. When the membrane was depolarized beyond +25 mV, the polarity of the current depended upon the ion composition of the intracellular solution and could be inward, zero or outward. 5. The introduction of GABA into a cell during the course of an experiment produced an outward current. This 'efflux' current was small at -75 mV and increased with depolarization. The efflux current required intracellular Na+ and Cl-. Intracellular Na+ could not be replaced by another alkali metal cation. 6. The efflux current also depended upon the identity of ions in the extracellular solution. An extracellular alkali metal cation was required to produce an efflux current. Removing extracellular Cl- did not affect the efflux current. 7. The outward movement of GABA produced a local accumulation in extracellular GABA concentration that could be detected by the activation of the GABAA current. GABA efflux only occurred during conditions that produced an efflux current. Electroneutral efflux did not occur. 8. In the absence of GABA, extracellular alkali metal cations produced a 'leakage' current. The leakage current was inward at -75 mV and decreased as the membrane was depolarized towards +20 mV. When the membrane was depolarized beyond +25 mV, the polarity of the leakage current depended, like the GABA influx current, upon the ion composition of the intracellular solution and could be inward, zero or outward. The addition of GABA to the intracellular solution produced an efflux current and suppressed the leakage current. 9. We conclude that the transporter mediates electrogenic influx, efflux and leakage. Each mode of operation depends upon ions on both sides of the membrane. Influx and efflux are not symmetrical.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Maruyama T., Sikdar S. K., Yasui S. Sodium-dependent suppression of gamma-aminobutyric-acid-gated chloride currents in internally perfused frog sensory neurones. J Physiol. 1987 Nov;392:543–562. doi: 10.1113/jphysiol.1987.sp016796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub G. S., Lam D. M. The release of gamma-aminobutyric acid from horizontal cells of the goldfish (Carassius auratus) retina. J Physiol. 1984 Oct;355:191–214. doi: 10.1113/jphysiol.1984.sp015414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., King A. C. Influence of membrane potential on the sodium-dependent uptake of gamma-aminobutyric acid by presynaptic nerve terminals: experimental observations and theoretical considerations. J Membr Biol. 1976 Dec 28;30(2):153–173. doi: 10.1007/BF01869665. [DOI] [PubMed] [Google Scholar]
- Borden L. A., Smith K. E., Hartig P. R., Branchek T. A., Weinshank R. L. Molecular heterogeneity of the gamma-aminobutyric acid (GABA) transport system. Cloning of two novel high affinity GABA transporters from rat brain. J Biol Chem. 1992 Oct 15;267(29):21098–21104. [PubMed] [Google Scholar]
- Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988 Mar;11(3):112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- Clark J. A., Deutch A. Y., Gallipoli P. Z., Amara S. G. Functional expression and CNS distribution of a beta-alanine-sensitive neuronal GABA transporter. Neuron. 1992 Aug;9(2):337–348. doi: 10.1016/0896-6273(92)90172-a. [DOI] [PubMed] [Google Scholar]
- DeVries S. H., Schwartz E. A. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol. 1989 Jul;414:351–375. doi: 10.1113/jphysiol.1989.sp017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella J., Nelson N., Nelson H., Czyzyk L., Keynan S., Miedel M. C., Davidson N., Lester H. A., Kanner B. I. Cloning and expression of a rat brain GABA transporter. Science. 1990 Sep 14;249(4974):1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Neal M. J. The uptake of [3H]GABA by slices of rat cerebral cortex. J Neurochem. 1968 Oct;15(10):1141–1149. doi: 10.1111/j.1471-4159.1968.tb06831.x. [DOI] [PubMed] [Google Scholar]
- Kaila K., Rydqvist B., Pasternack M., Voipio J. Inward current caused by sodium-dependent uptake of GABA in the crayfish stretch receptor neurone. J Physiol. 1992;453:627–645. doi: 10.1113/jphysiol.1992.sp019248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynan S., Kanner B. I. gamma-Aminobutyric acid transport in reconstituted preparations from rat brain: coupled sodium and chloride fluxes. Biochemistry. 1988 Jan 12;27(1):12–17. doi: 10.1021/bi00401a003. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P., Johnston G. A. Inhibition of GABA uptake in rat brain slices by nipecotic acid, various isoxazoles and related compounds. J Neurochem. 1975 Dec;25(6):797–802. doi: 10.1111/j.1471-4159.1975.tb04410.x. [DOI] [PubMed] [Google Scholar]
- Lam D. M. The biosynthesis and content of gamma-aminobutyric acid in the goldifsh retina. J Cell Biol. 1972 Aug;54(2):225–231. doi: 10.1083/jcb.54.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q. R., Mandiyan S., Nelson H., Nelson N. A family of genes encoding neurotransmitter transporters. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6639–6643. doi: 10.1073/pnas.89.14.6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow R. P., Ripps H. Effects of gamma-aminobutyric acid on skate retinal horizontal cells: evidence for an electrogenic uptake mechanism. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8945–8949. doi: 10.1073/pnas.87.22.8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. L. Kinetics of the sodium-dependent transport of gamma-aminobutyric acid by synaptosomes. J Neurochem. 1973 Aug;21(2):345–356. doi: 10.1111/j.1471-4159.1973.tb04255.x. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Pastuszko A., Wilson D. F., Erecinska M. Energetics of gamma-aminobutyrate transport in rat brain synaptosomes. J Biol Chem. 1982 Jul 10;257(13):7514–7519. [PubMed] [Google Scholar]
- Schwartz E. A. Calcium-independent release of GABA from isolated horizontal cells of the toad retina. J Physiol. 1982 Feb;323:211–227. doi: 10.1113/jphysiol.1982.sp014069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science. 1987 Oct 16;238(4825):350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- Yazulla S., Kleinschmidt J. Carrier-mediated release of GABA from retinal horizontal cells. Brain Res. 1983 Mar 14;263(1):63–75. doi: 10.1016/0006-8993(83)91201-5. [DOI] [PubMed] [Google Scholar]


