Abstract
Constructing organic fluorophosphines, vital drug skeletons, through the direct fluorination of readily available alkyl phosphonates has been impeded due to the intrinsic low electrophilicity of PV and the high bond energy of P═O bond. Here, alkyl phosphonates are electrophilically activated with triflic anhydride and N-heteroaromatic bases, enabling nucleophilic fluorination at room temperature to form fluorophosphines via reactive phosphine intermediates. This approach facilitates the late-stage (radio)fluorination of broad dialkyl and monoalkyl phosphonates. Monoalkyl phosphonates derived from targeted drugs, including cyclophosphamide, vortioxetine, and dihydrocholesterol, are effectively fluorinated, achieving notable yields of 47−71%. Radiofluorination of medically significant 18F-tracers and synthons are completed in radiochemical conversions (radio-TLC) of 51−88% and molar activities up to 251 ± 12 GBq/μmol (initial activity 11.2 GBq) within 10 min at room temperature. Utilizing a phosphonamidic fluoride building block (BFPA), [18F]BFPA-Flurpiridaz and [18F]BFPA-E[c(RGDyK)]2 demonstrate high-contrast target imaging, excellent pharmacokinetics, and negligible defluorination.
Subject terms: Synthetic chemistry methodology, Drug discovery and development
Constructing organic fluorophosphines via direct fluorination of alkyl phosphonates is challenging. Herein, the authors show that alkyl phosphonates are electrophilically activated with triflic anhydride and N-heteroaromatic bases, enabling nucleophilic fluorination at room temperature to form fluorophosphines via reactive phosphine intermediates.
Introduction
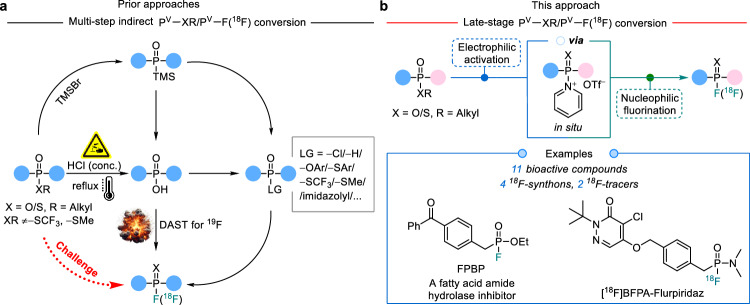
Organic fluorophosphines bearing a PV−F bond are widely employed as agrochemicals1–3, synthetic intermediates4, catalysts5–7, clickable moieties for phosphorus fluoride exchange (PFEx)8, and building blocks for the development of 18F-radiopharmaceuticals9–16. However, the preparation of these fluorophosphines are always constrained by the requirement for pre-modification of leaving groups (LGs), such as, −Cl9,17–20, −H21–24, electron-deficient −O/SAr25, −SR (R = CF3, Me)26,27, or −OH28–33. Symmetrical fluorophosphines are primarily constructed with these conventional precursors that are usually unstable via multi-step synthesis from the ubiquitous alkyl phosphonates (Fig. 1a). The current direct 18F-labeling through nucleophilic substitution of aryl phosphonate precursors, aimed at overcoming the low molar activity (Am) associated with isotope exchange-based 18F-labeling method34 produces ionic 18F-fluorophosphonates that hinder cell uptake and exhibit high bone affinity, limiting their bioavailability. The late-stage (radio)fluorination of broader alkyl phosphonates with a more efficient and streamlined strategy is long expected for the substantial variability of candidate structures and rapid radiosynthesis with 18F35.
Fig. 1. Approaches for fluorination of alkyl phosphonates.
a Prior approaches rely on multi-step conversion from symmetrical alkyl phosphonates and isolation of intermediates. b This late-stage approach from alkyl phosphonates eliminates the requirement for hash conditions, intricate multi-step transformations and separation. OTf− trifluoromethanesulfonate, TMSBr bromotrimethylsilane, DAST diethylaminosulfur trifluoride, Ar aryl, Me methyl. The blue and pink balls represent different substituents.
Although alkyl phosphonates are susceptible to enzymolysis in vivo catalyzed by enzymes like phospholipases, synthetic methods for selective conversion of PV−OR bond under mild conditions remains limited. The presence of p–π conjugation in alkyl phosphonates (bond dissociation energy of P⚍O = 585 kJ/mol) and alkyl phosphonamides significantly diminishes the electrophilicity of the PV atom. Highly electrophilic species, such as CuIII 36 and triflic anhydride (Tf2O)37,38, have been found to effectively activate dialkyl phosphonates through enhancing the electrophilicity of PV. In CuIII-catalyzed oxygen-arylation of dialkyl phosphonates, however, no P−X (X = coordinating atom of P) substitution occurs, in inconsistence with our expectation to form P−F bonds by replacing X. Despite the above, given that the high electronegativity of F and the small size of F− favors the formation of π-bond with the d-orbital of P (pπ-dπ bonding, back-donation)39, N-heteroaromatic bases may serve as active LG through an Arbuzov-like pathway, which are commonly used in Tf2O-mediated electrophilic activation and stabilize reactive intermediates40, allowing for broader screening options due to their various electronic substituents. Therefore, it is hypothesized that the electrophilic PV species generated in situ by Tf2O and N-heteroaromatic bases may mediate the construction of P−F bond under mild conditions (Fig. 1b).
Herein, we present a (radio)fluorination method that enables the late-stage synthesis of fluorophosphines via electrophilic activation of readily accessible alkyl phosphonates. The activation of alkyl phosphonates by diverse bases is investigated, and the effects of different fluoride sources and solvents on the fluorination yield are examined. A comprehensive series of alkyl phosphonates are rationally designed and synthesized, and the fluorination reactions of diverse phosphonates are investigated by modulating the structures of alkyl substituents. The formation and cleavage of chemical bonds during the reaction are elucidated through control experiments. Phosphorus-31 NMR spectroscopy (31P NMR) and mass spectrometry (MS) are employed to monitor potential reaction intermediates in situ. In addition, density functional theory (DFT) calculations are conducted to elucidate the plausible reaction mechanism. The feasibility of late-stage fluorination of alkyl phosphonate substrates with diverse structural variations on benzyls, functional linkers and drugs is explored. The conditions and efficiency of 18F-fluorination are explored based on the non-radioactive reaction conditions. Rapid preparation and positron emission tomography (PET) evaluation of medically significant 18F-tracers and synthons via late-stage 18F-fluorination of the corresponding alkyl phosphonate precursors are conducted for the development of PET tracers.
Results
General compounds information
In this study, the alkyl phosphonate precursors used for fluorination are designated as S1 through S57, with a combination of the letter “S” and Arabic numerals. The fluorinated phosphonate products are numbered sequentially in Arabic numerals from 1 to 46. The resulting products were fully characterized using Proton NMR spectroscopy (1H NMR), Carbon-13 NMR spectroscopy (13C NMR), Fluorine-19 NMR spectroscopy (19F NMR), 31P NMR and high-resolution mass spectrometry (HRMS).
Screening of bases
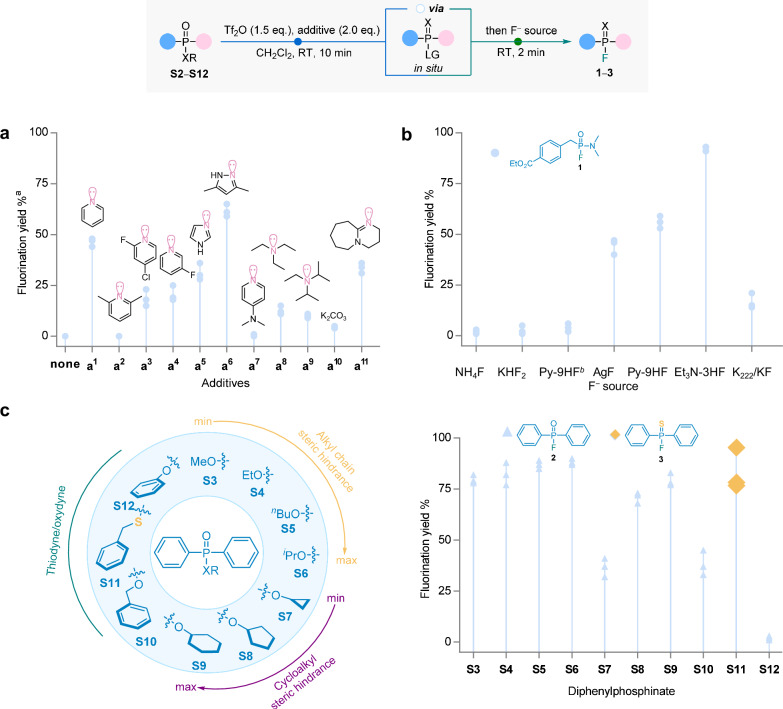
After optimizing the ratio of electrophilic activator and additives (Table S1), screening of N-heteroaromatic bases (2.0 eq.) as additives was conducted using a simple asymmetrical benzylphosphonamide monoalkyl ester (S2, 1.0 eq.) as the model substrate, with tetrabutylammonium fluoride (TBAF, 1.2 eq.) serving as the fluoride source in the presence of 1.5 eq. of electrophilic activator Tf2O (Fig. 2a). It was observed that the absence of basic additives hindered the direct action of F− on the intermediates generated from the S2 and Tf2O, resulting in the inability to obtain the desired fluorophosphine. Upon introducing different N-heteroaromatic bases into the reaction system, it was observed that pyridine (a1) exhibited a higher fluorination yield of 47% compared to the other substituted pyridine additives. This could be attributed to the optimal electron cloud density of pyridine (Py), allowing it to effectively serve as both a nucleophile and a LG. In addition, imidazole (a5) and 3,5-dimethylpyrazole (a6) also exhibited comparable reactivity in the reaction, with a6 achieving the highest fluorination yield of 56%. Interestingly, 31P NMR spectrum revealed that a6 exhibited incomplete conversion of the preceding intermediat, whereas a1 did. In addition to N-heteroaromatic bases, several common bases were also evaluated. Triethylamine (Et₃N, a8) and N,N-diisopropylethylamine (DIPEA, a9) were less effective due to steric hindrance, while potassium carbonate (K₂CO₃, a10) performed poorly because of its low solubility in CH₂Cl₂. In contrast, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, a11) yielded 33%. Therefore, Py is considered to be the optimal N-heteroaromatic base.
Fig. 2. Optimization of conditions for late-stage fluorination of alkyl phosphonates via electrophilic activation.
All reactions were conducted using 0.2 mmol model compounds, with a gradual addition of Tf2O (1.5 eq., 0.3 mmol) and the additive (2.0 eq., 0.4 mmol), followed by the subsequent addition of the F− source (1.5 eq., 0.3 mmol), in CH2Cl2 (1.0 mL, 0.2 M) under room temperature (RT). a Optimization of bases, ethyl 4-(((dimethylamino)(ethoxy)phosphoryl)methyl)benzoate (S2) as a model compound. aYields determined by 31P NMR. 31P NMR was conducted by adding CD₂Cl₂ after the reaction, with the preliminary conversion proportion calculated from the ratio of product to non-product peak areas. b Screening of fluorine sources with S2 as the model compound and Py as an additive. bNo additive is added. c, Study of direct fluorination of different diphenylphosphonates (S3−S12).
Screening of fluoride sources
Based on the 31P NMR monitoring result, the high alkalinity of TBAF (with a pKa > 16 in THF)41,42 is postulated to lead to the decomposition of intermediates and thus reduce the yield of fluorination. Consequently, various mild neutral fluoride sources were utilized in this study (Fig. 2b). NH4F and KHF2 were found to be almost ineffective in CH2Cl2 due to their limited solubility. AgF as a fluorination reagent had a fluorination yield similar to TBAF of 46%, while Py-9HF as a fluorination reagent provided a slightly higher yield of 54%. Given the presence of Py in the Py-9HF complex, efforts were made to employ Py-9HF as a fluoride source without the addition of extra Py. The unsuccessful outcome of this endeavor was attributed to the notable stability of the complex under these conditions, making it challenging to liberate free Py with the necessary nucleophilic ability to engage in the reaction. Notably, Et3N·3HF demonstrated remarkable efficacy, achieving a satisfactory fluorination yield of 92%.
Fluorination of diverse alkyl phosphonates
The reactivity of alkyl ester substituents with varying degrees of steric hindrance towards fluorination was investigated using diphenylphosphonates (S3−S12) as precursors (Fig. 2c). The results showed that substrates with varying degrees of steric hindrance had negligible impact on fluorination yields. Moreover, both cyclic and linear alkyl esters displayed effective participation in the fluorination reaction, achieving yields ranging from 32% to 90%. Interestingly, fluorination was nearly absent when phenyl diphenylphosphonate (S12) was used as a precursor, highlighting the high selectivity of this fluorination strategy for alkyl phosphonates. This selectivity was further confirmed by the 18F-fluorination of a mixed phosphonate precursor (S15) substituted with −OEt and −OPh groups (see Fig. S4 for details). It is worth noting that when S-benzyl diphenylphosphinothioate (S11) was used as the precursor, the fluorination exhibited an exceptional yield of 83%, resulting in the formation of diphenylphosphinothioic fluoride 3 as the product. Consequently, this observation prompted our interest in the reaction and motivated us to explore the corresponding mechanism of fluoridation.
Mechanism
In contrast to carbonyl and sulfoxide structures, phosphorus oxides typically demonstrate reduced reactivity, thus limiting the development of their interaction with Tf2O for electrophilic activation43. The general process of Tf2O-mediated electrophilic activation involves the electrophilic attack of anhydrides on the electron-rich center of X═O (X = C, S, Se, P, Sb, I)44. This results in the cleavage of the double bond and the formation of trifluoromethyl ester intermediate (X+), which then react and transform with nucleophilic reagents.
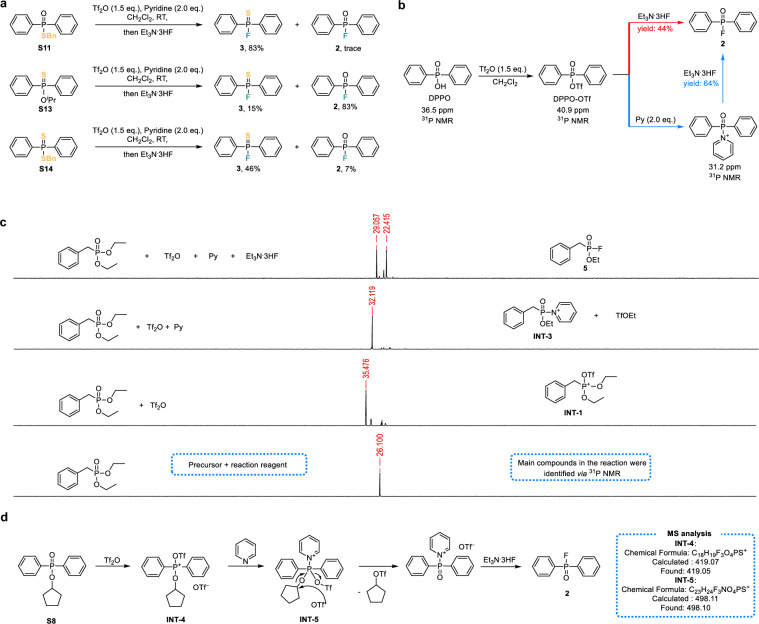
The foremost among our inquiries was determining whether the reaction proceeded through the direct cleavage of the P−O bond in alkyl phosphonates, resulting in the departure of −OR and facilitating the nucleophilic substitution fluorination reaction—a pivotal step in this process. To investigate the bond cleavage and formation in the reaction, control experiments using thio-precursors were conducted (Fig. 3a). In the case where S13 served as the precursor, the primary product obtained was diphenylphosphinic fluoride 2, accompanied by a minor quantity of diphenylphosphine thiofluoride 3. These results clearly demonstrated that the P−O or P−S single bonds in phosphonates with P−OiPr or P−SBn groups remained intact during the fluorination of alkyl phosphonates. Instead, cleavage of the C−O or C−S bonds occurred, leading to the separation of isopropyl or benzyl segments and the formation of a new P═X bond, resembling the classical Arbuzov reaction pathway45. Furthermore, the aforementioned conclusion was further corroborated by the fluorination results of precursor benzyl diphenylphosphinodithioate S14. A method for constructing structurally diverse P═X (X = S, Se) compounds was provided, inspiring a strategy that bypasses the traditional selenization/sulfidation steps46.
Fig. 3. The mechanism study of late-stage fluorination of alkyl phosphonates via electrophilic activation.
a Control experiment on the direct fluorination of thiophosphonic alkyl ester. b Control experiment of Py with diphenylphosphinic acid (DPPO) as the precursor. c 31P NMR monitoring of stepwise reaction intermediates in situ. d Stepwise in situ mass spectrometric monitoring of fluorination using precursor compound S8.
Subsequently, commercially available diethyl benzylphosphonate (S16) was employed as a precursor dissolved in CD2Cl2, successively activated with Tf2O and Py, and then reacted with Et3N·3HF, while in situ monitoring of 31P NMR was performed (Fig. 3c). Each addition of a reaction reagent resulted in a pronounced and complete shift in the 31P NMR spectrum. When diethyl benzylphosphonate was incubated with Tf2O in CD2Cl2 solution for 5 min, the main product exhibited a new 31P NMR spectrum shift of ~35 ppm, indicating a noticeable low field movement trend. This shift was attributed to the decrease in electron cloud density at the P+ center of the λ4σ4-phosphinic cation intermediate INT-1, resulting from the electrophilic activation by Tf2O. Given that the intermediate INT-1 cannot react with Et3N·3HF to yield the desired product without Py, the OTf-substituted λ5σ5-type intermediate INT-S2 was excluded (Fig. S49).
The −OTf, serving as an excellent LG, has been demonstrated to facilitate nucleophilic substitution by F− on OTf-substituted λ5σ5-type substrate, as evidenced by the reaction of DPPO (Fig. 3b). Subsequent introduction of Py caused further shifting in the 31P NMR spectrum, resulting in a single peak of ~32 ppm. Concurrently, only OTf− and TfOEt fluorine-containing structures were identifiable in situ 19F NMR (Fig. S45), the compound was inferred to be a non-fluorine-λ5σ4 electrophilic phosphine-pyridinium intermediate denoted as INT-3. Formation of INT-3 proceeds through two possible pathways for the departure of the ethyl fragment via Arbuzov-like processes. One route involves direct nucleophilic attack by Py on the methylene of the ester in INT-1, yielding the 1-ethylpyridinium byproduct with an activation barrier of 16.7 kcal/mol (Fig. S49). Another pathway involves initial interaction between Py and INT-1 to form INT-2, followed by OTf− attacking the methylene of the ester in INT-2, which leads to the departure of TfOEt and the formation of INT-3, requiring a lower activation barrier of 12.9 kcal/mol (Fig. S47). The latter pathway is energetically favored. Furthermore, in situ 1H NMR analysis revealed that the TfOEt byproduct was ~9 times more abundant than 1-ethylpyridinium (Fig. S44)47, supporting this mechanistic proposal. Ultimately, after Et3N·3HF treatment, INT-3 intermediate was nearly completely converted into fluorophosphine 5.
Our efforts to detect these active intermediates by MS or isolate them have been unsuccessful. Nevertheless, to further support the proposed mechanistic hypothesis, cyclopentyl diphenylphosphinate (S8) was employed as a precursor due to the potential contributions of the diphenyl conjugated system on stabilizing certain reaction intermediates (Fig. 3d). Encouragingly, intermediates INT-4 and INT-5 corresponding to INT-1 and INT-2 were both detected through in situ MS monitoring (Fig. S46). Notably, for the first time, the possible involvement of a λ5σ5 transient intermediate in Tf2O-mediated P═O electrophilic activation has been proposed. This insight may help in developing strategies for inducing highly stereoselective P−F compounds.
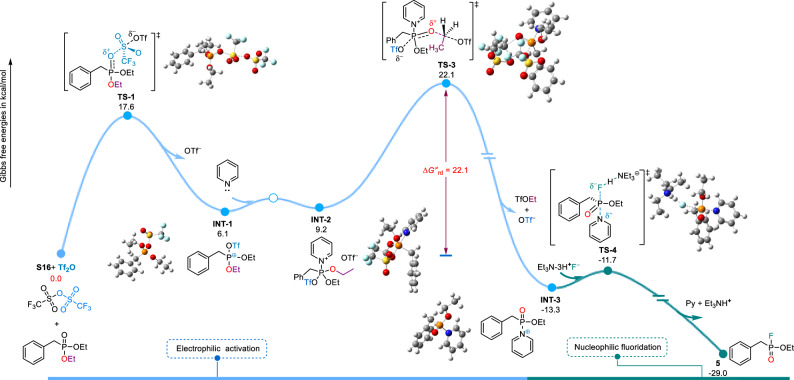
Based on experimental results, a plausible fluorination reaction mechanism is presented in Fig. 4. The change in Gibbs free energy (ΔG) for the reaction of diethyl benzylphosphonate with Et3N·3HF to give fluorophosphine 5 was calculated by the DFT method (using the ‘Gaussian 09’ software; see Supplementary Information section 2.7.2), and whether the conversion of PV−OR to PV−F could be thermodynamically advantageous was evaluated. The proposed fluorination process of diethyl benzylphosphinate proceeds as follows. Initially, the precursor undergoes activation by Tf2O, resulting in the formation of the λ4σ4 P+ intermediate INT-1 via transition state 1 (TS-1). Subsequently, the INT-1 interacts with the newly introduced Py to form intermediate INT-2 (transition state TS-2 has not been optimized, refer to Fig. S48). Following this, functioning as a nucleophilic reagent, OTf− initiates an attack on the methylene of the ester, resulting in the cleavage of the C−O bond. Transitioning through TS-3, the λ5σ4 phosphine-pyridinium INT-3 is generated, accompanied by the release of the byproduct TfOEt and the departure of another OTf−. It is noteworthy that the rate-determining step of the reaction only requires crossing a barrier of 22.1 kcal/mol. Finally, through nucleophilic substitution with F−, intermediate INT-3 transforms into the corresponding fluorophosphine. Throughout the reaction process, which is characterized by a low energy barrier, it proceeds smoothly at room temperature (< 24 kcal/mol) and can be completed quickly and efficiently within 12 min. The entire process is spontaneous, with a total ΔG of −29 kcal/mol, indicating a strong thermodynamic driving force.
Fig. 4. The calculated mechanism of late-stage fluorination of alkyl phosphonates via electrophilic activation.
The energy distribution of alkyl phosphonate (S16) activated by Tf2O for fluorination and the optimal structure of key stability points were investigated using DFT calculations. The calculated Gibbs energy (ΔG and ΔG≠rd, 298.15 K, 1.0 atm) is expressed in kcal/mol.
Substrate scope of alkyl phosphonates
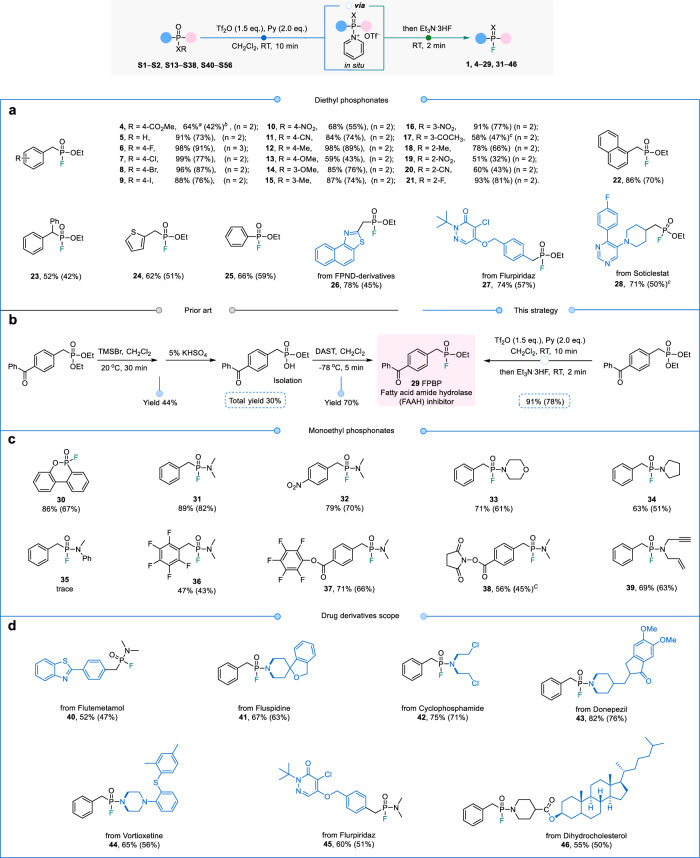
The scope of fluorination for alkyl phosphonates was investigated, as illustrated in Fig. 5. Initially, commercially available diethyl benzylphosphonates bearing halogen substituents on the benzyl, such as −F, −Cl, −Br, and −I (6−9) exhibited excellent yields ranging from 76% to 91%. Substrates with strong electron-withdrawing groups such as −NO2 or −CN (10, 11, 16, 19, 20) and electron-donating groups such as −Me or −OMe (12−15, 18) were well-tolerated. The conversions of ortho-substituted substrates with −NO2 and −CN decreased to 51% (19) and 60% (20), respectively, likely due to increased steric hindrance. Notably, the substrate with a −COCH3 substituent required specific conditions—Tf2O (3.0 eq.) and pyridine (4.0 eq.)—to successfully yield the desired fluorophosphonate (17). This necessity is likely due to the reversible interaction between Tf2O and the −COCH3. However, when the α position of the benzyl derivatives was substituted with a −Ph (23), a significantly lower conversion of 52% was obtained, possibly due to increased steric hindrance.
Fig. 5. Substrate scope for late-stage fluorination of alkyl phosphonates via electrophilic activation.
a Fluorination of diethyl phosphonates. aConversions determined by 31P NMR. bIsolated yield. cTf2O (3.0 eq.), Py (4.0 eq.). b An exemplification of the method’s application in the synthesis of FPBP, a fatty acid amide hydrolase inhibitor. c Fluorination of monoethyl phosphonates. d Late-stage fluorination of alkyl phosphonates derived from drug molecules.
In addition to benzyl derivatives, we also investigated naphthyl (22), thiophene (24) and phenyl (25) substrates, all of which provided good fluorination yields. Furthermore, highly functionalized substrates derived from FPND derivative (26), Flurpiridaz (27), and Socticlestat (28), which feature aromatic heterocycles and lactams, were successfully fluorinated with conversions ranging from 71% to 78%. Importantly, our strategy enabled the one-pot synthesis of 4-(benzoylbenzyl)fluorophosphonic acid ethyl ester (FPBP, 29), a fatty acid amide hydrolase inhibitor, from the same starting material. This method achieved a high isolated yield of 78% within just 12 min, in contrast to the previous three-step process that required intermediate isolation and utilized the potentially hazardous fluorination reagent DAST, which yielded only 30% (Fig. 4b)48. To the best of our knowledge, this work demonstrates the late-stage selective fluorination of alkyl phosphonates to convert PV−OEt to PV−F for the construction of fluorophosphines.
The structural diversity of monoethyl phosphonate and its derived drug molecules or functional linker substrates was explored. These entities exhibited various structures and incorporate multiple functional groups that could potentially impact the P−F bond formation process. An ethylphosphonate lactone 30 can be well fluorinated with a good conversion of 86%. Subsequently, the conversion of different secondary amine substituents, such as dimethylamine, morpholine amine and tetrahydropyrrole substituted benzylphosphonamide monoethyl substrate can also be carried out smoothly (31−34), with conversions ranging from 63% to 89%. However, when N-methylaniline group was introduced, the strategy failed to generate the desired product 35, since the phenyl (−Ph) group acts as an electron-withdrawing group in N-methylaniline, which can reduce the nucleophilicity of O, possibly accounting for the hindrance encountered by O in attacking Tf2O. Furthermore, several monoethyl phosphinamide-derived substrates with bifunctional linkers (36−39) can be directly synthesized into the corresponding fluorophosphine derivatives in late-stage process with yields of 43–66%, offering an advantageous approach for synthesizing challenging structures that are typically difficult to access using existing methods. Notably, certain drug molecules derived from monoethyl phosphonate (40−46), such as nitrogen mustard, flutemetamol, fluspidine, vortioxetine, flurpiridaz and cholestanol, undergo rapid and highly selective late-stage one pot fluorination under mild conditions, resulting in yields of up to 76%.
Condition optimazation for late-stage 18F-fluorination of alkyl phosphonates
The most commonly used [18F]Fluoride ([18F]F−) was employed as a default fluoride source for 18F-fluorination. The transition from F− to [18F]F− necessitates a reevaluation of the fluoride source and reaction time. A high Am [18F]F− aqueous solution is obtained through proton bombardment of [18O]H2O, followed by elution of [18F]F− using tetrabutylammonium bicarbonate/Cs2CO3 to obtain [18F]TBAF/[18F]CsF or potassium carbonate complexed with the amine-poly-ether crown ligand Kryptofix 222/18-crown-6, resulting in the formation of [18F]KF/K222 or [18F]KF/18-crown-6. Among these systems, [18F]KF/K222 exhibited the most significant impact on the fluorination of [18F]1 synthesis (Table S2). We optimized the precursor loading and reaction time to achieve an optimal radiochemical conversion (RCCTLC) of 62 ± 3% (Table S3). Furthermore, we have observed the P-benzyl fluorophosphonamide to possess excellent stability under both in vitro and in vivo conditions, suggesting its potential utilization in PET tracer development upon successful 18F-labeling. With the optimal 18F-fluoride source identified, our focus shifted towards assessing the applicability of this approach across a range of tracer and synthon substrates.
Design and synthesis of alkyl phosphonate precursors for 18F-tracers
The design principle for the alkyl phosphonate precursor of a radiotracer is rooted in subtle structural adjustments to a primary compound, ensuring the preservation of its core activity or function. Alkyl fluorophosphonamide is typically incorporated into the lead compound using an ‘embedded’ strategy as an ¹⁸F-building block. Considering that nitrogen-containing active pharmaceuticals make up 85% of the market49, this adaptability is essential. The amino group of the ethyl P-benzyl phosphonamidate building block can align with the amino group in the lead compound (e.g., cyclophosphamide). This enables the rapid construction of the precursor through the reaction of the amino lead compound with readily available ethyl benzylphosphonochloridate. Furthermore, the phosphonamide is a bioisostere of amide, containing a secondary amide moiety in the original molecule (e.g., vortioxetine). The precursor can be formed by reacting an amino-carrying lead compound with ethyl benzylphosphonochloridate. Similarly, the benzyl in ethyl P-benzyl phosphonamidate can also originate from the phenyl or benzyl in the lead compound (e.g., Flurpiridaz derivative that targets mitochondrial complex I (MC I)). More importantly, alkyl fluorophosphonamide favorable water solubility contributes to its excellent pharmacokinetic properties. By using the Arbuzov reaction, the corresponding halide compound was converted to diethyl benzylphosphonate derivative, and then subjected to amidation to obtain the precursor.
Late-stage 18F-fluorination of 18F-synthons
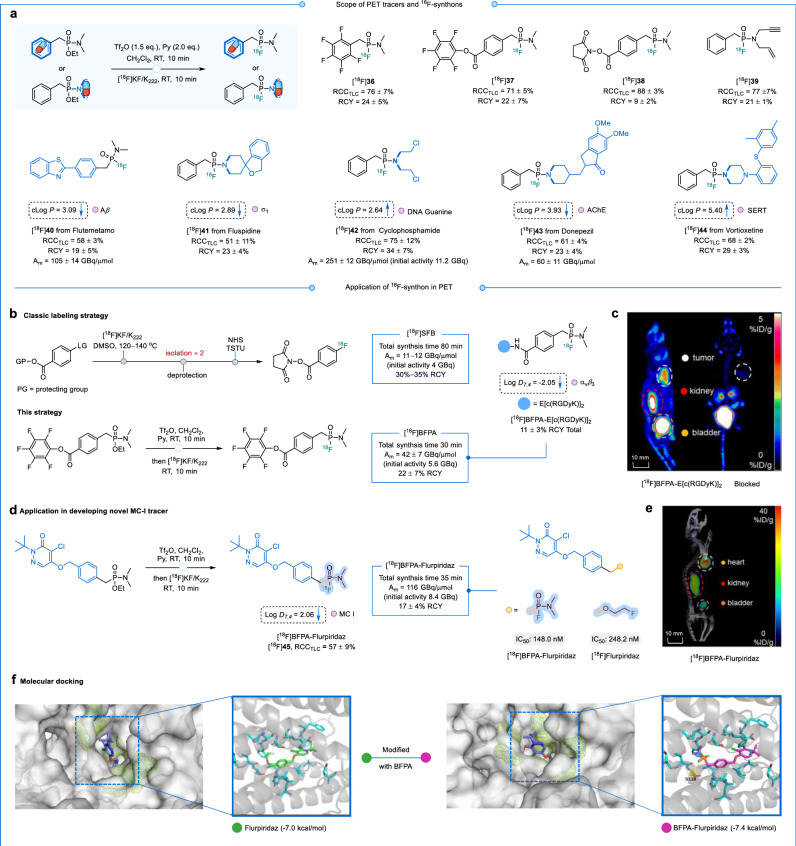
The classical method for preparing N-succinimidyl-4-[18F]fluorobenzoate ([18F]SFB) requires three-step of synthesis and two isolations, with a total radiosynthesis time of 80 min and an Am of 11−12 GBq/μmol50. In contrast, our phosphonamidic fluoride synthon ([18F]BFPA, [18F]37) offers a more efficient approach, as it can be synthesized from ~5.6 GBq [18F]F− at room temperature with a total radiosynthesis time of 30 min, yielding an Am of 42 ± 7 GBq/μmol (Fig. 6b). The activated ester [18F]38, amenable for amine-specific coupling, delivered 9 ± 2% RCYs. Moreover, the thiol-specific coupling with pentafluorobenzene [18F]36 provided 24 ± 5% RCYs. The bifunctional synthon [18F]39, suitable for alkyne-azide cycloaddition, provided 21 ± 1% RCYs.
Fig. 6. PET tracers and 18F-synthons synthesized by late-stage radiofluorination of alkyl phosphonates.
a Late-stage radiofluorination of PET tracers and 18F-synthons. RCCTLC determined by radio-TLC (n = 3); RCY = isolated 18F-product activity amount/starting amount of radioactivity (decay corrected). cLog P values were predicted using ALOGPS 2.1 (http://www.vcclab.org/lab/alogps). Blue arrows pointing upward indicate an increase in cLog P/Log D7.4 compared to the parent compound, while downward arrows indicate a decrease. The small pink ball after cLog P/Log D7.4 represents the target of the parent compound. b Preparation αvβ3 integrin receptor developer [18F]BFPA-E[c(RGDyK)]2. c MicroPET images of [18F]BFPA-E[c(RGDyK)]2 in U87MG xenograft mice at 30 min after tail vein injection. 200 μg of E[c(RGDyK)]2 was used to block the tumor uptake of [18F]BFPA-E[c(RGDyK)]2. The white circle is the tumor area. d Preparation of PET tracer [18F]BFPA-Flurpiridaz targeting MC I. e MicroPET/CT images of healthy mice [18F]BFPA-Flurpiridaz 60 min after caudal vein injection. f Molecular Docking. Flurpiridaz and BFPA-Flurpiridaz to MC I (PDB: 7ZM8). Cyan: residues composing the substrate-binding cavity; yellow: residues forming hydrogen bonds; yellow dashed lines: locations of hydrogen bond.
When the precursor structure contains electron-rich functional groups—such as amino, carboxyl, hydroxyl, or amide groups—that are incompatible with electrophilic activation conditions, a two-step strategy can be employed to achieve ¹⁸F-labeling through [¹⁸F]BFPA. [18F]BFPA was readily employed to facilitate the 18F-labeling of the integrin αvβ3-targeting peptide E[c(RGDyK)]2, yielding [18F]BFPA-E[RGDyk]2 with RCYs of 11 ± 3% in >99% radiochemical purity (RCP). As depicted in Fig. 6c, at 30 min after injection, U87MG xenografted mice (glioma) exhibited significant tumor-specific uptake of [18F]BFPA-E[RGDyk]2 (RCP > 99%). When blocked by E[c(RGDyK)]2, tumor uptake was substantially reduced from 3.3 ± 0.2 %ID/g (injected dose per gram) to 0.8 ± 0.2 %ID/g. [18F]BFPA-E[RGDyk]2 showed superior in vivo stability in blood, maintaining 94% stability at 60 min post-intravenous administration (Fig. S38d), compared to 74.2% for the NHS-modified 18F-FPRGD251. Additionally, the [18F]BFPA-modified tracers exhibited faster background clearance within 30 min than the [18F]DBPOF-modified tracers.
Late-stage 18F-fluorination of 18F-tracers
Building upon the P-benzyl fluorophosphonamide scaffold, we designed and developed 18F-tracers [18F]40−[18F]45 (Fig. 6a). Drug-derived alkyl phosphonates were also subjected to late-stage ¹⁸F-fluorination under mild conditions. The RCYs for the flutemetamol derivative [18F]40, designed to target amyloid-β (Aβ), and the fluspidine derivative [18F]41, targeting σ1 receptors, were 19 ± 5% and 23 ± 4%, respectively. Furthermore, molecular docking studies revealed that the modified drug molecules can effectively bind in the same structural domain as their parent compounds52–54, In some cases, the introduction of P═O led to additional hydrogen bonding (as shown in Fig. S40c, d, e, f), resulting in both a lower binding energy—typically reduced by 0.4−1.0 kcal/mol—and a decreasing cLog P value55.
Iterative optimization, guided by the outcomes of non-radioactive reactions, has culminated in the development of an automated method for radiopharmaceutical synthesis. For (pre)clinical applications, the automation of radiosynthesis is crucial (Fig. S32). Employing an AllinOne automated synthesis module enabled the radiosynthesis of the cyclophosphamide derivative [18F]42, which was tailored to target DNA guanine. The automated process allowed for the use of higher starting activities (an initial activity of 11.2 GBq), leading to an approximately tenfold increase in Am (251 ± 12 GBq/μmol, n = 3). Additionally, the achieved RCY was 34 ± 7%, with the entire synthesis completed in a significantly shorter time frame. While 18F/19F-isotope exchange facilitated direct 18F-labeling of highly functionalized peptides in one step, a significant limitation was the inseparability of the precursors from the 18F-product, resulting in an Am of only 2.22–4.81 GBq/μmol12. The labeling method described in this study demonstrates the potential to enhance the Am by nearly 100-fold. Additionally, tracer [18F]43 derived from donepezil (targeting AChE) and tracer [18F]44 derived from vortioxetine (targeting serotonin transporter) achieved RCYs of 23 ± 4% and 29 ± 3%, respectively.
The incorporation of phosphine oxide (P═O) as an atypical hydrogen bond acceptor has proven instrumental in achieving the requisite potency and metabolic stability56. In the context of myocardial perfusion PET imaging, minimizing uptake in adjacent organs such as lung and liver is essential to provide high imaging contrast with clinical diagnostics. To address this issue, [18F]BFPA-Flurpiridaz (Log D7.4 = 2.06) with a P-benzyl fluorophosphonamide motif integrated into the structure of Flurpiridaz (Log P = 2.73)57 was developed to reduced liver uptake. Subsequent IC50 assays affirmed that BFPA-Flurpiridaz (148.0 nM) exhibits a higher affinity compared to Flurpiridaz (248.2 nM) (Section 2.5.2 in the Supplementary Information). In vivo PET imaging in healthy mice showed high specific myocardial uptake of the tracer at 60 min post-intravenous injection, providing clear delineation between the heart and surrounding organs (Fig. 6e). The binding free energy of BFPA-Flurpiridaz with the MC I protein (PDB: 7ZM8) was found to be −7.4 kcal/mol, compared to −7.0 kcal/mol for Flurpiridaz (Fig. 6f). This suggests that the binding affinity of the tracer is slightly higher than that of the original drug after the incorporation of BFPA. This increased affinity is likely due to the introduction of the P═O moiety, which enables the formation of an additional hydrogen bond with residue S118.
Discussion
In conclusion, a method for the late-stage fluorination of alkyl phosphonates by F− was presented. Highly electrophilic PV species have been generated from alkyl phosphonates through a sequential activation strategy of Tf2O and Py, which were subsequently reacted with F− to obtain the corresponding P−F bond-containing compounds. Through the combination of controlled experiments and theoretical calculations, the underlying reconfiguration of chemical bonds was revealed, and the fluorination mechanism was thoroughly elucidated, increasing the comprehensibility of our reaction while also providing possibilities for other synthetic designs based on this strategy. This strategy enables direct conversion of alkyl phosphonates from P−XR (R = alkyl) to P−F bonds in the late-stage. A series of ethyl benzylphosphonofluoridates and some fluorophosphines derivatives of drugs were successfully synthesized. Particularly, significant improvements were made regarding the synthesis of FPBP, which previously required a multi-step approach. The method was also employed in 18F-fluorination to synthesize a series of 18F-tracers and synthons derived from benzyl fluorophosphonamide, resulting in the successful development of a [18F]BFPA with a high Am and exhibiting in vivo stability as an 18F-synthon. Furthermore, our research endeavors extended to the conceptualization and development of [18F]BFPA-Flurpiridaz, a myocardial perfusion imaging agent that exhibits considerable clinical promise, leveraging the favorable pharmacokinetic effects embedded in its structural design. In many instances, the incorporation of the building block into the targeted molecule facilitated a late-stge nucleophilic substitution of the 18F-labeling through this strategy, offering a compelling tool for the ongoing development of PET tracers. Specifically, integrating the P═O skeleton is foreseen as an innovative and effective strategy for reducing the Log P of drugs and augmenting affinity.
Methods
General procedure for alkyl phosphates fluorination
Alkyl phosphonates (0.2 mmol) was dissolved in 1 mL CH2Cl2 at RT, and then Tf2O (0.3 mmol, 50.5 μL, 1.5 eq.) was added to the reaction mixture for 5 min, followed by Py (0.4 mmol, 32 μL, 2.0 eq.) for an additional 5 min. Following this, the fluorinating reagent Et3N·3HF (0.5 eq.) was introduced into the system. The progress of the reaction was monitored using TLC or 31P NMR, and after 2 min, the reaction was completed. The reaction mixture, with a volume of approximately 1 mL, underwent direct purification through silica gel column chromatography to afford the desired products.
General procedure for alkyl phosphates radiofluorination
All 18F-labeling reactions were performed following the specified protocol: 3.0 μmol of precursor were dissolved in 300 μL CH2Cl2. Then, 2.0 eq. of Tf2O were added for 5 min, followed by the addition of 1.5 eq. of Py for another 5 min. The resulting intermediate solution was subsequently divided into three equal portions and introduced into separate glass vial reactors, each containing pre-prepared 37−55.5 MBq dried [18F]KF/K222. The reactors were continuously oscillated at RT for 10 min. Upon completion of the reaction, 9900 μL of water was added to quench the reaction. The RCCs of the reaction were determined using radio-TLC with methanol as the developing agent (n = 3). The resulting reaction mixture was then subjected to simple purification using a Sep-Pak® Plus Short C18 cartridge (Waters, Part No. WAT020515), followed by separation and purification on a SEP Basic-C18 semi-preparative column (120 A 5 μm 10 × 250 mm). The traces for each radiolabelled product are detailed in Supplementary Section 2.3.3. The desired 18F-products were confirmed via co-injection of the purified 18F-labelled species and reference compound.
Stability evaluation
Purified [18F]BFPA-E[c(RGDyK)]2 and [18F]BFPA-Flurpiridaz were dried under N2 at room temperature and reconstituted with ultrapure H2O to achieve an activity of 1−3 MBq/100 μL. Then, 10 μL of [18F]BFPA-E[c(RGDyK)]2 or [18F]BFPA-Flurpiridaz was added to 90 μL of mouse serum, and the mixture was incubated at 37 °C for 60 min, respectively. After adding 100 μL of CH3CN and vigorously vortexing to generate flocculent precipitate, the supernatant was collected after centrifugation at 7043 × g for 8 min. The supernatant was filtered through a 0.22 μm micropore filter, and the RCP of the filtrate was analyzed by radio-HPLC. Subsequently, the purified [18F]BFPA-E[c(RGDyK)]2 and [18F]BFPA-Flurpiridaz (0.1−0.3 MBq, 10 μL) were dissolved in 90 μL of saline and then incubated at 37 °C for 60 min, respectively. The RCP was determined by radio-HPLC. To evaluate the in vivo metabolic stability of [18F]BFPA-E[c(RGDyK)]2, administer 30−37 MBq intravenously via the tail vein. After 60 min, collect blood and urine samples. Process the samples by adding 100 μL of CH3CN, mixing thoroughly, and centrifuging. Subsequently, filter the supernatant and analyze it using radio-HPLC to assess metabolic stability.
Cell culture
Cells H9C2 (A cardiomyocyte line derived from rat embryonic heart tissue), purchased from Cell Cook, are maintained, incubated, and washed using a cardiomyocyte-specific modified DMEM medium. In a 24-well plate, seed 105 H9C2 cells per well and add 1 mL of complete growth medium to each well. The glioma cell line U87MG was obtained from the China Center for Type Culture Collection of the Chinese Academy of Sciences. The cells were cultured in Leibovitz L15 medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum and penicillin/streptomycin (each at a concentration of 100 U/mL). All cells were regularly tested for mycoplasma contamination and cultured or incubated (as in cell-based assays) in a 5% CO2 incubator at 37 °C.
IC50 determination via cell competitive binding assay
To determine the IC50, cells were incubated in a cell culture incubator overnight until they adhered and nearly covered the wells. Following this, the cells were washed twice with pre-chilled PBS to remove any residual medium. A solution of BFPA-Flurpiridaz or Flurpiridaz (concentrations ranging from 10−12 M to 10−4 M) was added to each well at a volume of 100 μL, and the cells were incubated for 10 min to evaluate their inhibitory effects on [18F]Flurpiridaz uptake in H9C2 cells, with four replicate wells established for each concentration to ensure reliability. Afterward, 400 μL of a radioactively labeled sample with a specific activity of 0.37 MBq was added to each well, and the cells were incubated for an additional 60 min. Once incubation was complete, the medium was completely aspirated, and each well was washed twice with 0.5 mL of pre-chilled PBS containing 0.2% BSA to remove any unbound label. Then, 1 mL of 1 M NaOH solution was added to each well, followed by a 5–10 min incubation period. The cells were gently dislodged and scraped from the bottom of the wells using a pipette, and all liquid and cells were transferred into a marked plastic tube. Three aliquots of the labeled medium were taken from the tube to serve as standard samples. Finally, the radioactivity count of all samples was measured using a γ-counter (WIZARD2 2480), and data processing, graphing, and calculation of cellular probe uptake at various time points were performed using Graphpad Prism 8.2.1. Detailed data processing can be found in Supplementary Information 2.5.2.
Molecular docking
In the molecular docking study, AutoDock Vina 1.2.0 was employed to dock two ligands—the parent drug molecule and its BFPA-modified derivative—onto the target protein. For each ligand, the most favorable binding conformation was selected based on the docking score and the plausibility of the interaction.
Octanol/water partition coefficient (Log D7.4)
The log D7.4 values of [18F]BFPA-Flurpiridaz and [18F]BFPA-E[c(RGDyK)]2 in the octanol/water system were determined. Briefly, 100 μL of the radiotracer solution was diluted with 1 mL PBS (0.15 M, pH 7.4) and 0.9 mL octanol, vortexed for 5 min, and centrifuged at 7043 × g for 5 min. For [18F]BFPA-Flurpiridaz, a 100 μL aliquot of the organic phase was transferred to a fresh mixture of 0.9 mL octanol and 1 mL PBS for further vortexing and centrifugation. For [18F]BFPA-E[c(RGDyK)]2, a 100 μL aliquot of the aqueous phases was transferred. Radioactivity in the organic and aqueous phases (100 μL) was measured using a γ-counter. All experiments were performed in triplicate, with results reported as mean ± SD. cLog P values were predicted using ALOGPS 2.1 (http://www.vcclab.org/lab/alogps).
Animal
All the animal experiments were carried out in accordance with the Instructions of Laboratory Animal Ethics Committee of Xiamen University (240506 XMULAC20240099).
Nude mice (4–8 weeks old) and male BALB/c mice (7–8 weeks old) were purchased from Xiamen University Laboratory Animal Center. The mice were housed in the Laboratory Animal Center of Xiamen University under a 12-h light/dark cycle at 22 ± 2 °C and 40–60% humidity.To establish U87MG tumor-bearing mice, U87MG cells were injected subcutaneously into the right forelimb of 4 to 8-week-old nude mice (5 × 106 in 100 μL PBS). The mice were used for tumor imaging studies when the tumor size reached 100–150 mm3 (2–3 weeks post-inoc ulation).
PET/CT imaging
All MicroPET/CT images were acquired using an Inveon MicroPET/CT scanner (Siemens) and subsequently analyzed with the Inveon Research Workplace 4.2 (Siemens). For the MicroPET imaging study, mice received a bolus tail-vein injection of a solution of 18F-tracer (~3.7 MBq) dissolved in 0.9% saline (100 μL). The mice were anesthetized with inhaled 2% isoflurane continuously for 5 min before imaging and then positioned on the MicroPET/CT scanner bed for a 5-min PET scan.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
National Natural Science Foundation of China 22476167, 81971674 (Z.L.), the National University of Singapore NUHSRO/2020/133/Startup/08, UHSRO/2023/008/NUSMed/TCE/LOA, NUHSRO/2021/034/TRP/09/Nanomedicine (X.C.), National Medical Research Council MOH-001388-00, MOH-001041, CG21APR1005 (X.C.), Singapore Ministry of Education MOE-000387-00 (X.C.), and National Research Foundation NRF-000352-00 (X.C.). We extend their gratitude to the Theoretical and Computational Chemistry Team from Shiyanjia Lab (www.shiyanjia.com) for their assistance. We would also like to thank J.Y. and X.W. from the School of Public Health at Xiamen University for their help and analysis in molecular docking.
Author contributions
Z.L. conceived the study and coordinated all the research. K.Z. originated the (radio)fluorination of alkyl phosphonates via electrophilic activation. W.F., Z.M. offered help in chemical synthesis and radiolabeling. Computational chemistry was carried out by L.Z., M.M., Z.Z. and X.L. offered help in biology and microPET imaging experiments. Results were collaboratively analyzed, and the manuscript was prepared by Z.L., K.Z. and X.C. All authors participated in research discussions.
Peer review
Peer review information
Nature Communications thanks Tian-Yu Sun, Zehui Wu and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The authors declare that the data supporting the findings of this study are available within the Article and its Supplementary Information Files or from the corresponding author upon request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kaiqiang Zhang, Wanru Feng, Zhaobiao Mou.
Supplementary information
The online version contains Supplementary Material available at 10.1038/s41467-024-54208-y.
References
- 1.Jang, Y. J., Kim, K., Tsay, O. G., Atwood, D. A. & Churchill, D. G. Update 1 of: destruction and detection of chemical warfare agents. Chem. Rev.115, PR1–PR76 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Mercey, G. et al. Reactivators of acetylcholinesterase inhibited by organophosphorus nerve agents. Acc. Chem. Res.45, 756–766 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Worek, F., Wille, T., Koller, M. & Thiermann, H. Toxicology of organophosphorus compounds in view of an increasing terrorist threat. Arch. Toxicol.90, 2131–2145 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Franca, T. C. C. et al. Novichoks: the dangerous fourth generation of chemical weapons. Int. J. Mol. Sci.20, 1222 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caputo, C. B., Houmjet, L. J., Dobrovetsky, R. & Stephan, D. W. Lewis acidity of organofluorophosphonium salts: hydrodefluorination by a saturated acceptor. Science341, 1374–1376 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Hounjet, L. J., Caputo, C. B. & Stephan, D. W. Phosphorus as a Lewis Acid: CO2 Sequestration with Amidophosphoranes. Angew. Chem. Int. Ed.51, 4714–4717 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Pérez, M., Caputo, C. B., Dobrovetsky, R. & Stephan, D. W. Metal-free transfer hydrogenation of olefins via dehydrocoupling catalysis. Proc. Natl Acad. Sci. USA111, 10917–10921 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun, S. et al. Phosphorus fluoride exchange: multidimensional catalytic click chemistry from phosphorus connective hubs. Chem9, 2128–2143 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Studenov, A. R., Adam, M. J., Wilson, J. S. & Ruth, T. J. New radiolabelling chemistry: synthesis of phosphorus–[18F]fluorine compounds. J. Label. Compd. Radiopharm.48, 497–500 (2005). [Google Scholar]
- 10.Vabre, B. et al. Radiofluorination of a NHC–PF5 adduct: toward new probes for 18F PET imaging. Chem. Commun.53, 8657–8659 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang, H. & DeGrado, T. R. [18F]Tetrafluoroborate ([18F]TFB) and its analogs for PET imaging of the sodium/iodide symporter. Theranostics8, 3918–3931 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong, H. et al. Rapid one-step 18F-radiolabeling of biomolecules in aqueous media by organophosphine fluoride acceptors. Nat. Commun.10, 989 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mou, Z. et al. Nucleophilic 18F-fluorination of phosphorofluoridates and phosphonofluoridic acids via imidazole-activated precursors. Tetrahedron Lett.68, 152917 (2021). [Google Scholar]
- 14.Tang, X., Lv, S., Mou, Z., Liu, X. & Li, Z. Cu(II)-Mediated direct 18F-dehydrofluorination of phosphine oxides in high molar activity. EJNMMI Radiopharm. chem.9, 4 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang, H. et al. Simplified one-pot 18F-labeling of biomolecules with in situ generated fluorothiophosphate synthons in high molar activity. Theranostics13, 472–482 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, Z. et al. Surfactants accelerate isotope exchange-based 18F‑Fluorination in water. Langmuir39, 9007–9016 (2023). [DOI] [PubMed] [Google Scholar]
- 17.Corriu, R. J. P. et al. Silicon phosphorus analogies. Fluoride activation of nucleophilic displacement at the tetrahedral phosphorus: an example of nucleophilic assistance to nucleophilic substitution. J. Am. Chem. Soc.106, 1060–1065 (1984). [Google Scholar]
- 18.Janssen, A. P. A. et al. Development of a multiplexed activity-based protein profiling assay to evaluate activity of endocannabinoid hydrolase inhibitors. ACS Chem. Biol.13, 2406–2413 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Timperley, C. M., Arbon, R. E., Saunders, S. A. & Water, M. J. Fluorinated phosphorus compounds: Part 6. The synthesis of bis(fluoroalkyl) phosphites and bis(fluoroalkyl) phosphorohalidates. J. Fluor. Chem.113, 65–78 (2002). [Google Scholar]
- 20.Gupta, H. K., Pardasani, D., Mazumder, A., Purohit, A. K. & Dubey, D. K. Tetrabutylammonium tetra (tert-butyl alcohol) coordinated fluoride-an efficient reagent for the synthesis of fluorine derivatives of phosphorus(V) compounds. Tetrahedron Lett.50, 2697–2699 (2009). [Google Scholar]
- 21.Liu, N., Mao, L.-L., Yang, B. & Yang, S.-D. Copper-promoted oxidative-fluorination of arylphosphine under mild conditions. Chem. Commun.50, 10879–10882 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Purohit, A. K. et al. A single-step one pot synthesis of dialkyl fluorophosphates from dialkylphosphites. Tetrahedron Lett.56, 4593–4595 (2015). [Google Scholar]
- 23.Li, Q.-W. et al. TFAA/DMSO-Promoted Fluorination of P(O)–H and P(O)–OH compounds: compatible access to Fluorophosphonates and Phosphonofluoridates. Adv. Synth. Catal.364, 938–946 (2022). [Google Scholar]
- 24.Chen, Q. et al. Electrophilic Fluorination of secondary Phosphine Oxides and its application to P−O bond construction. J. Org. Chem.81, 10043–10048 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Bigley, A. N., Harvey, S. P., Narindoshvili, T. & Raushel, F. M. Substrate analogues for the Enzyme-Catalyzed detoxification of the Organophosphate nerve agents—Sarin, Soman, and Cyclosarin. Biochemistry60, 2875–2887 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chworoś, A. & Woźniak, L. A. A Facile conversion of Thio- and Selenophosphoric acids and their derivatives into Fluoridates by means of reaction with Silver Fluoride. Tetrahedron Lett.40, 9337–9340 (1999). [Google Scholar]
- 27.Lopusiński, A. Chemistry of S-trifluoromethyl organophosphorothioates and their steructural analogs a convenient synthesis of organophosphorus fluoridates. Phosphorus, Sulfur Silicon Relat. Elem.45, 137–143 (1989). [Google Scholar]
- 28.Guo, L., Auarez, A. I., Braden, M. R., Gerdes, J. M. & Thompson, C. M. Inhibition of acetylcholinesterase by chromophore-linked fluorophosphonates. Bioorg. Med. Chem. Lett.20, 1194–1197 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tully, S. E. & Cravatt, B. F. Activity-based probes that target functional subclasses of phospholipases in proteomes. J. Am. Chem. Soc.132, 3264–3265 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao, S. et al. Deoxyfluorination of carboxylic, sulfonic, phosphinic acids and phosphine oxides by perfluoroalkyl ether carboxylic acids featuring CF2O units. Chin. J. Chem.39, 1225–1232 (2021). [Google Scholar]
- 31.Miller, L. P., Vogel, J. A., Harel, S., Krussman, J. M. & Melvin, P. R. Rapid generation of P(V)−F bonds through the use of sulfone iminium fluoride reagents. Org. Lett.25, 1834–1838 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Łopusiński, A. & Michalski, J. Novel application of Sulfonyl Chloride Fluoride in the synthesis of Organophosphorus Fluorine Compounds: direct conversion of and groups into groups. Angrew. Chem. Int. Ed.21, 294 (1982). [Google Scholar]
- 33.Zhang, G.-F., Han, L.-J., Guan, C.-F. & Ding, C.-R. SO2F2-mediated Fluorination of P(O)–H and P(O)–OH compounds under mild conditions. J. Org. Chem.88, 13142–13148 (2023). [DOI] [PubMed] [Google Scholar]
- 34.Wang, C. et al. Direct 18F-labeling of biomolecules via spontaneous site-specific nucleophilic substitution by F− on phosphonate prostheses. Org. Lett.23, 4261–4266 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Preshlock, S., Tredwell, M. & Gouverneur, V. 18F-labeling of arenes and heteroarenes for applications in positron emission Tomography. Chem. Rev.116, 719–766 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Fañanás-Mastral, M. & Feringa, B. L. Copper-catalyzed synthesis of mixed Alkyl Aryl phosphonates. J. Am. Chem. Soc.136, 9894–9897 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Huang, H., Denne, J., Yang, C.-H., Wang, H. & Kang, J. Y. Direct Aryloxylation/Alkyloxylation of Dialkyl phosphonates for the synthesis of mixed phosphonates. Angew. Chem. Int. Ed.57, 6624–6628 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Adler, P. et al. Chemoselective activation of diethyl phosphonates: modular synthesis of biologically relevant phosphonylated scaffolds. Angew. Chem. Int. Ed.57, 13330–13334 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarak, J. H. Fluoride Ion as a base in organic synthesis. Chem. Rev.80, 429–452 (1980). [Google Scholar]
- 40.Tang, Z., Mo, K., Ma, X., Huang, J. & Zhao, D. para-selective Radical Trifluoromethylation of Benzamide Derivatives via Iminium Intermediates. Angew. Chem. Int. Ed.61, 1–7 (2022). [DOI] [PubMed] [Google Scholar]
- 41.Hagiwara, H., Watanabe, N., Ijuin, H. K., Yamada, M. & Matsumoto, M. Synthesis of bicyclic Dioxetanes bearing a 4-(Benzimidazol-2-yl)−3-hydroxyphenyl group and their base-induced Chemiluminescent decomposition in an aprotic medium and in an aqueous medium. Heterocyclics87, 65–78 (2013). [Google Scholar]
- 42.Leclercq, L., Suisse, I., Nowogrocki, G. & gbossou-Niedercorn, F. Halide-free highly-pure imidazolium triflate ionic liquids: preparation and use in palladium-catalysed allylic alkylation. Green. Chem.9, 1097–1103 (2007). [Google Scholar]
- 43.Huang, H. & Kang, J. Y. Triflic anhydride (Tf2O)-activated transformations of amides, sulfoxides and phosphorus oxides via nucleophilic trapping. Synthesis54, 1157–1202 (2022). [Google Scholar]
- 44.Baraznenok, I. L., Nenajdenko, V. G. & Balenkova, E. S. Chemical transformations induced by Triflic Anhydride. Tetrahedron56, 3077–3119 (2000). [Google Scholar]
- 45.Michaelis, A. & Kaehne, R. The reaction of alkyl iodides with phosphites. Chem. Ber.31, 1048–1058 (1898). [Google Scholar]
- 46.Ozturk, T., Ertas, E. & Mert, O. Use of Lawesson’s Reagent in Organic Syntheses. Chem. Rev.107, 5210–5278 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Barthen, P., Frank, W. & Ignatiev, N. Development of low viscous ionic liquids: the dependence of the viscosity on the mass of the ions. Ionics21, 149–159 (2015). [Google Scholar]
- 48.Patricelli, M. P. & Cravatt, B. F. Characterization and manipulation of the Acyl chain selectivity of fatty acid amide hydrolase. Biochemistry40, 6107–6115 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Heravi, M. M. & Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: an overview. RSC Adv.10, 44247–44311 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaidyanathan, G. & Zalutsky, M. R. Synthesis of N-succinimidyl 4-[18F]fluorobenzoate, an agent for labeling proteins and peptides with 18F. Nat. Protoc.1, 1655–1661 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Wu, Z. et al. 18F-labeled mini-PEG spacered RGD dimer (18F-FPRGD2): synthesis and microPET imaging of αvβ3 integrin expression. Eur. J. Nucl. Med. Mol. Imaging34, 1823–1831 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eberhardt, J., Santos-Martins, D., Tillack, A. F. & Forli, S. AutoDock Vina 1.2.0: new docking methods, expanded force field, and python bindings. J. Chem. Inf. Model.61, 3891–3898 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem.31, 455–461 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stierand, K. & Rarey, M. Drawing the PDB-protein-ligand complexes in two dimensions. ACS Med. Chem. Lett.1, 540–545 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tetko, I. V. & Tanchuk, V. Y. Application of associative neural networks for prediction of lipophilicity in ALOGPS 2.1 program. J. Chem. Inf. Comput. Sci.42, 1136–1145 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Finkbeiner, P., Hehn, J. P. & Gnamn, C. Phosphine oxides from a medicinal chemist’s perspective: physicochemical and in vitro parameters relevant for drug discovery. J. Med. Chem.63, 7081–7107 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Nekolla, S. G. et al. Evaluation of the novel myocardial perfusion positron-emission tomography tracer 18F-BMS-747158-02: comparison to 13N-ammonia and validation with microspheres in a pig model. Circulation119, 2333–2342 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the Article and its Supplementary Information Files or from the corresponding author upon request. Source data are provided with this paper.