Abstract
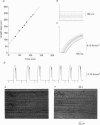
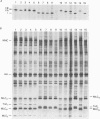
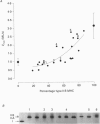
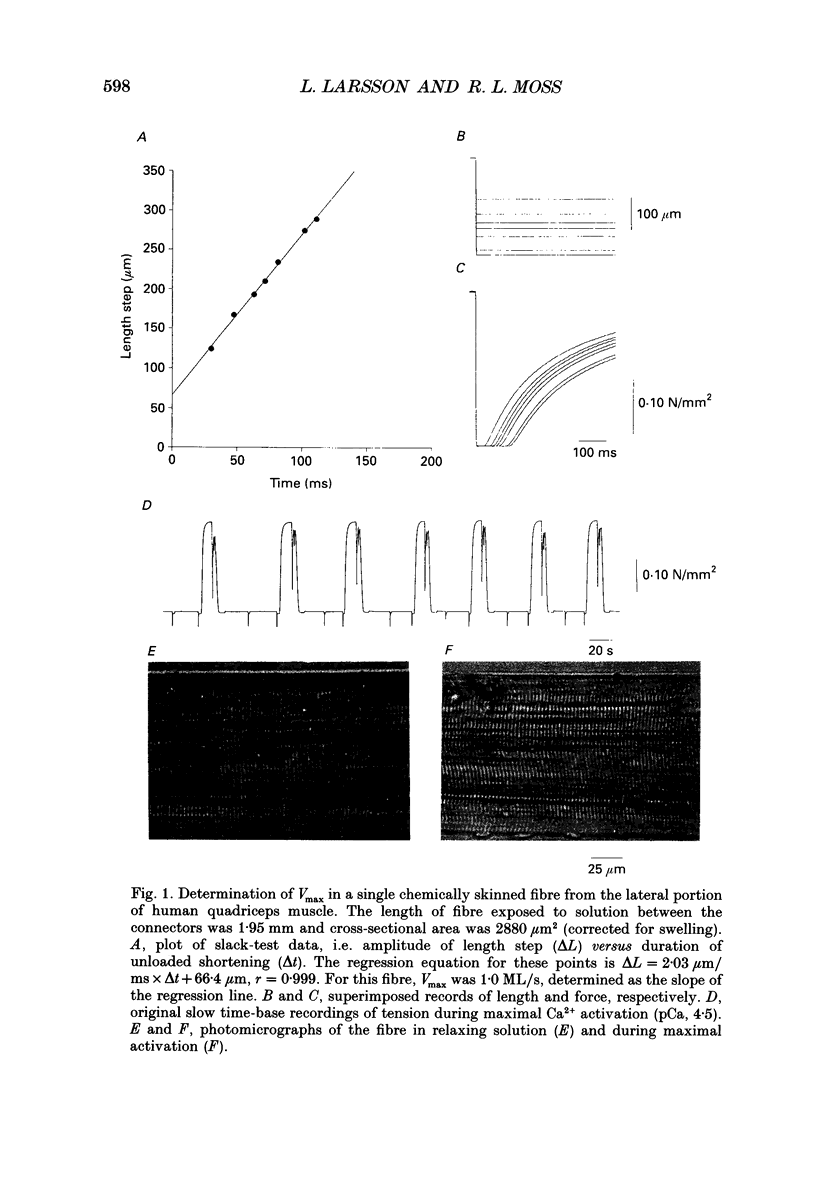
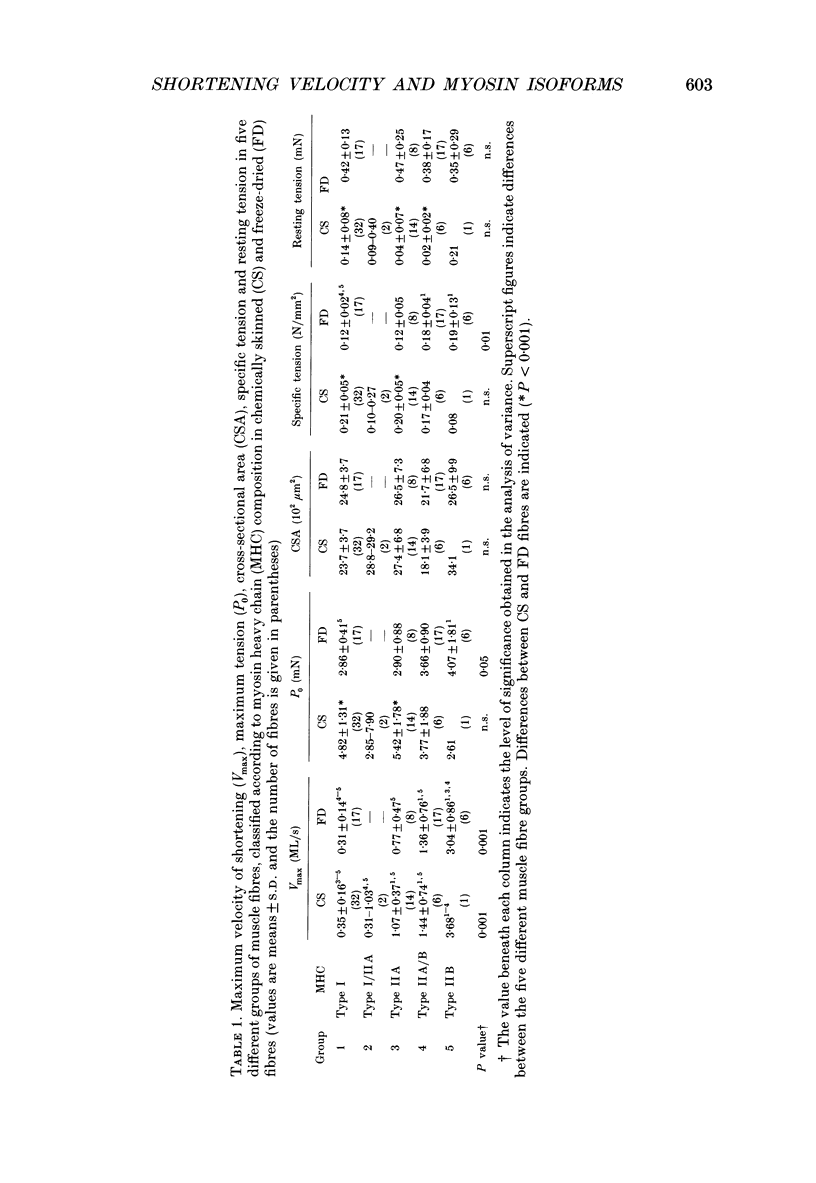
1. Maximum velocity of shortening (Vmax) and compositions of myosin heavy chain (MHC) and myosin light chain (MLC) isoforms were determined in single fibres from the soleus or the lateral region of the quadriceps (vastus lateralis) muscles in man. Muscle samples were obtained by percutaneous biopsy, and membranes were permeabilized by glycerol treatment (chemical skinning) or by freeze-drying. 2. Types I, IIA and IIB MHCs were resolved from single fibre segments by 6% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and five different fibre types were identified: fibres containing type I MHC, types I and IIA MHCs, type IIA MHC, types IIA and IIB MHCs, and type IIB MHC. Only a few fibres co-expressed types I and IIA MHCs but 28% of all quadriceps fibres expressed both IIA and IIB MHCs in variable proportions. Fibres co-expressing types I and IIB MHCs were not found. 3. Alkali (MLC1 and MLC3) and dithio nitrobenzoic acid (DTNB) (MLC2) myosin light chains were observed in all type II fibres in variable proportions. MLC (MLC1s and MLC2s) isoforms from type I fibres had lower migration rates than the corresponding isoforms from type II fibres (MLC1f and MLC2f). More than half of type I fibres in both soleus (65%) and quadriceps (68%) muscles also expressed 'fast' MLC3 and 36% of the type II fibres from quadriceps muscle expressed the slow isoform of MLC2. 4. Differences were observed in some mechanical characteristics of freeze-dried versus chemically skinned fibres. Maximum tension (P0) and specific tension were lower in freeze-dried types I and IIA fibres than in chemically skinned, while no differences were observed in the IIA/B fibres. The numbers of types I/IIA and IIB fibres were too low to allow statistical comparisons. In chemically skinned fibres, mean specific tension (0.20 +/- 0.01 N/mm2) did not vary with fibre type. In freeze-dried fibres, on the other hand, specific tensions varied according to MHC type: higher (P < 0.01) specific tensions were observed in types IIB (0.19 +/- 0.01 N/mm2) and type IIA/B fibres (0.18 +/- 0.04 N/mm2) than in type I fibres (0.12 +/- 0.02 N/mm2). The specific tension of type IIA fibres (0.12 +/- 0.05 N/mm2) did not differ significantly from the other fibre types. Cross-sectional areas and mean Vmax did not differ between freeze-dried and chemically skinned fibres, either when all fibres were pooled or within respective fibre types. Vmax data from all fibres of a given type, irrespective of membrane permeabilization technique, have therefore been pooled.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



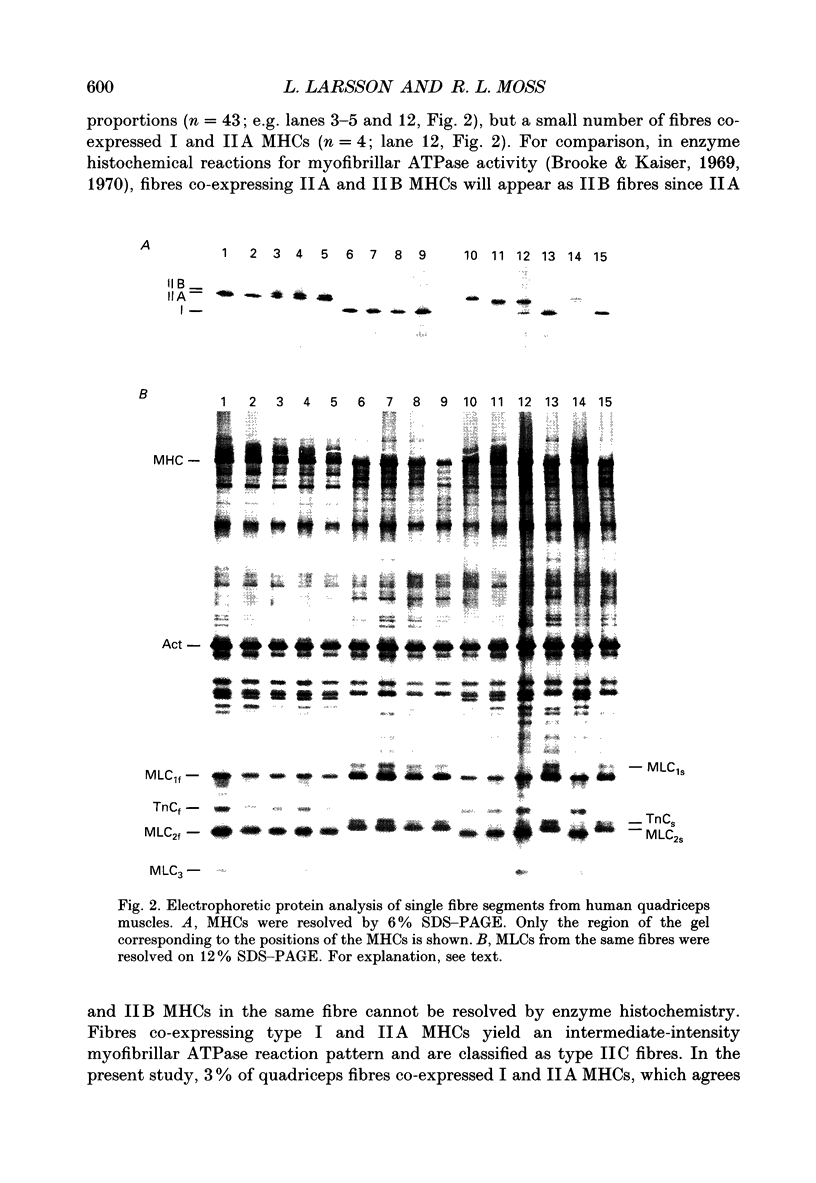


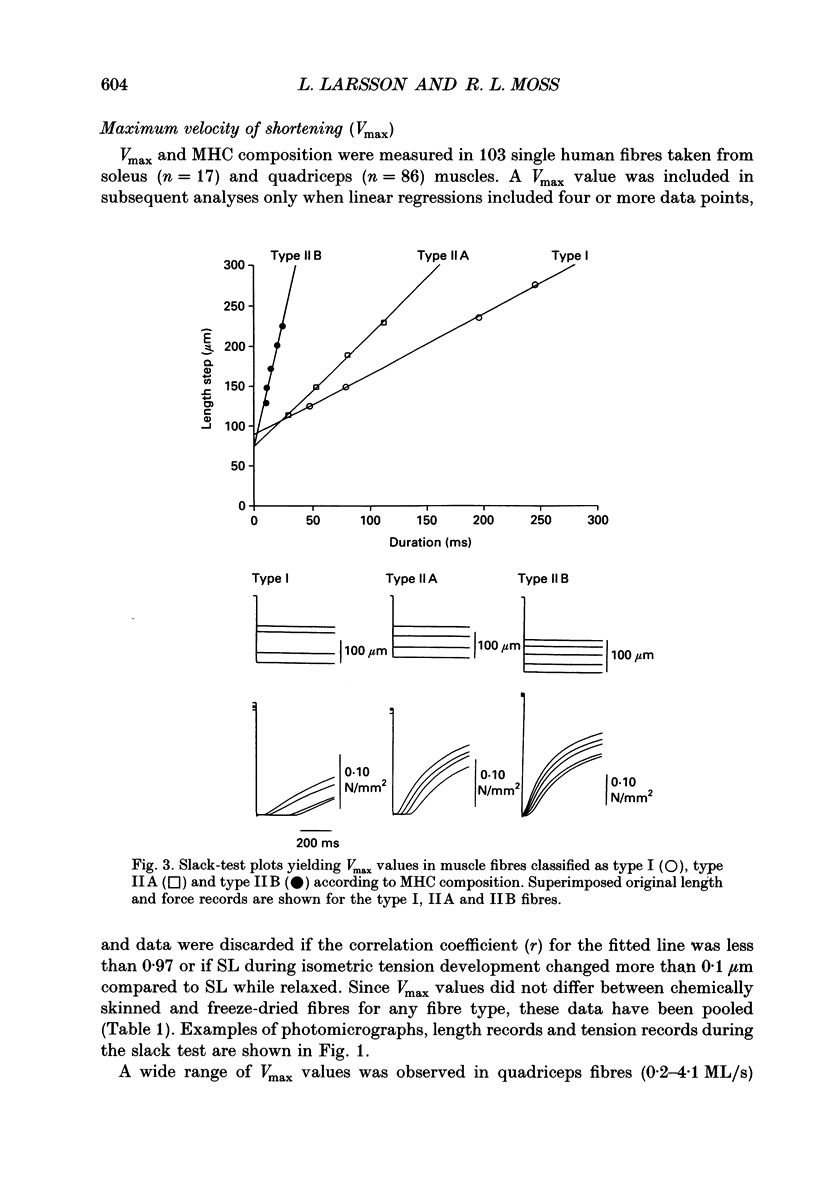
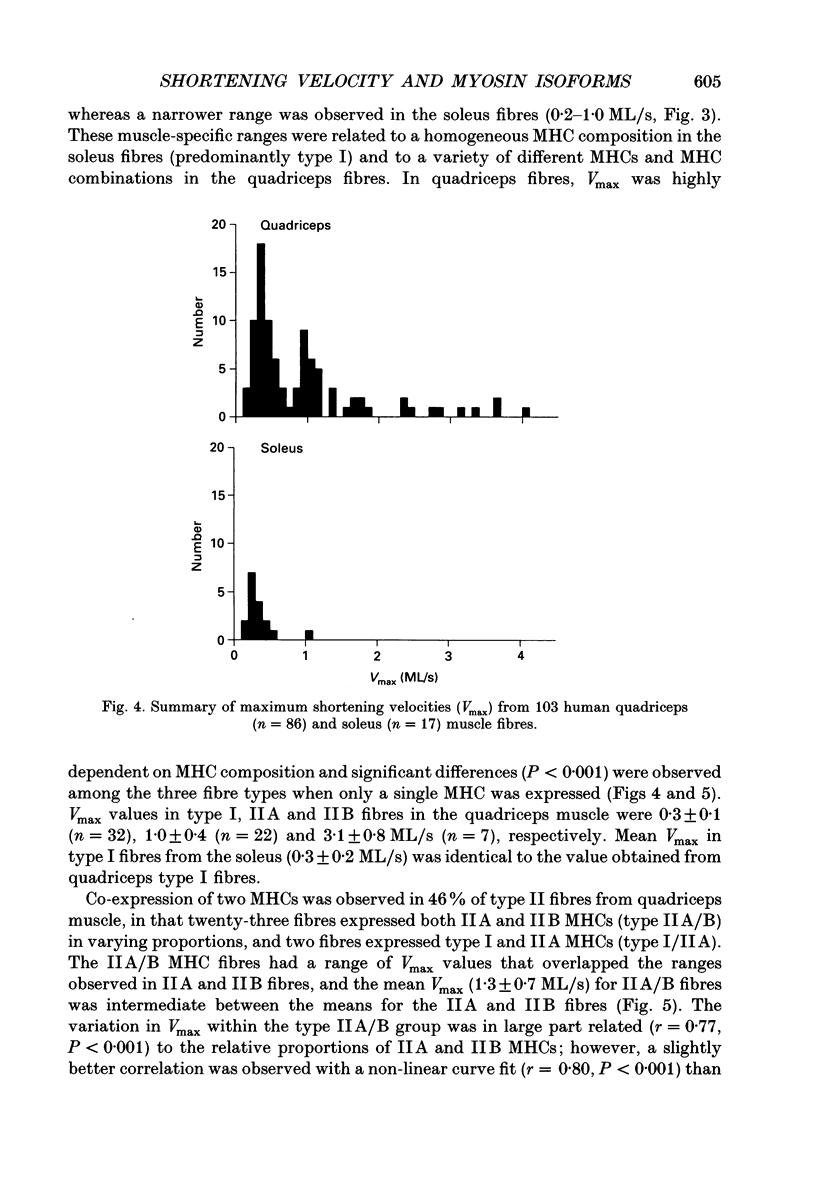
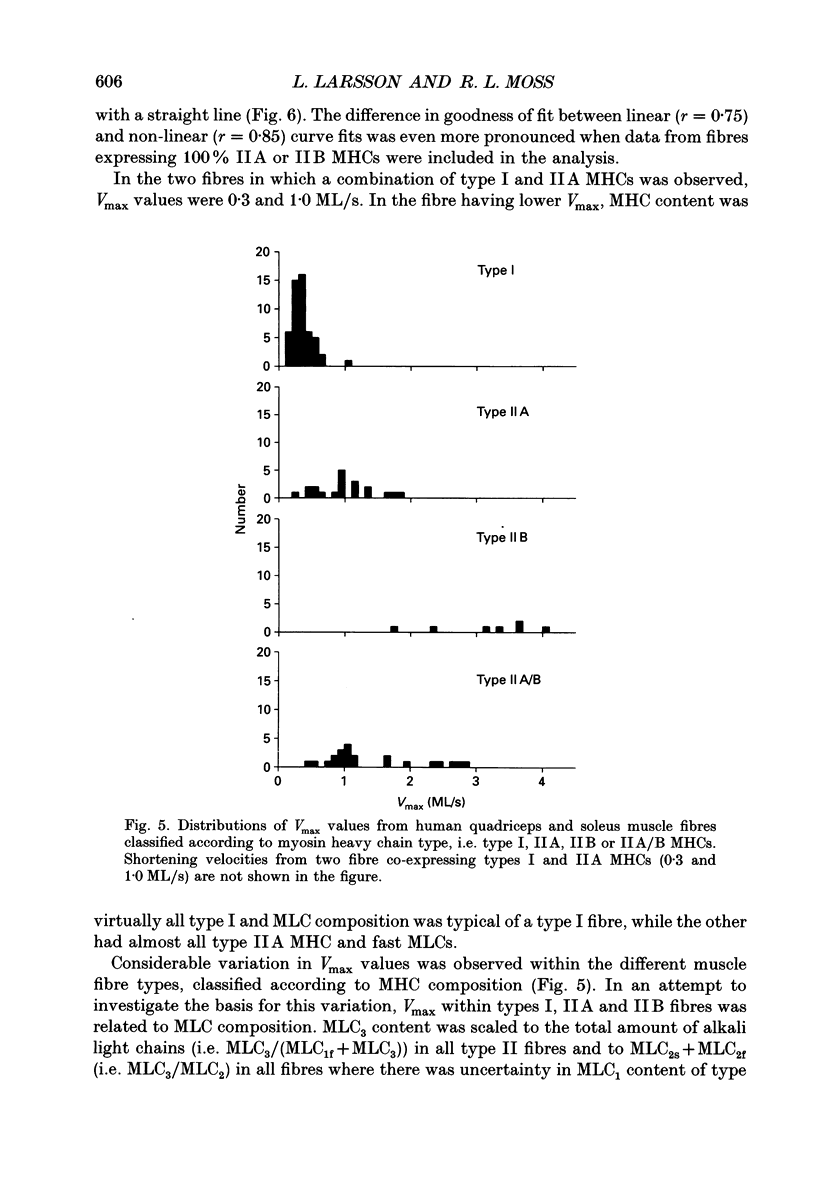
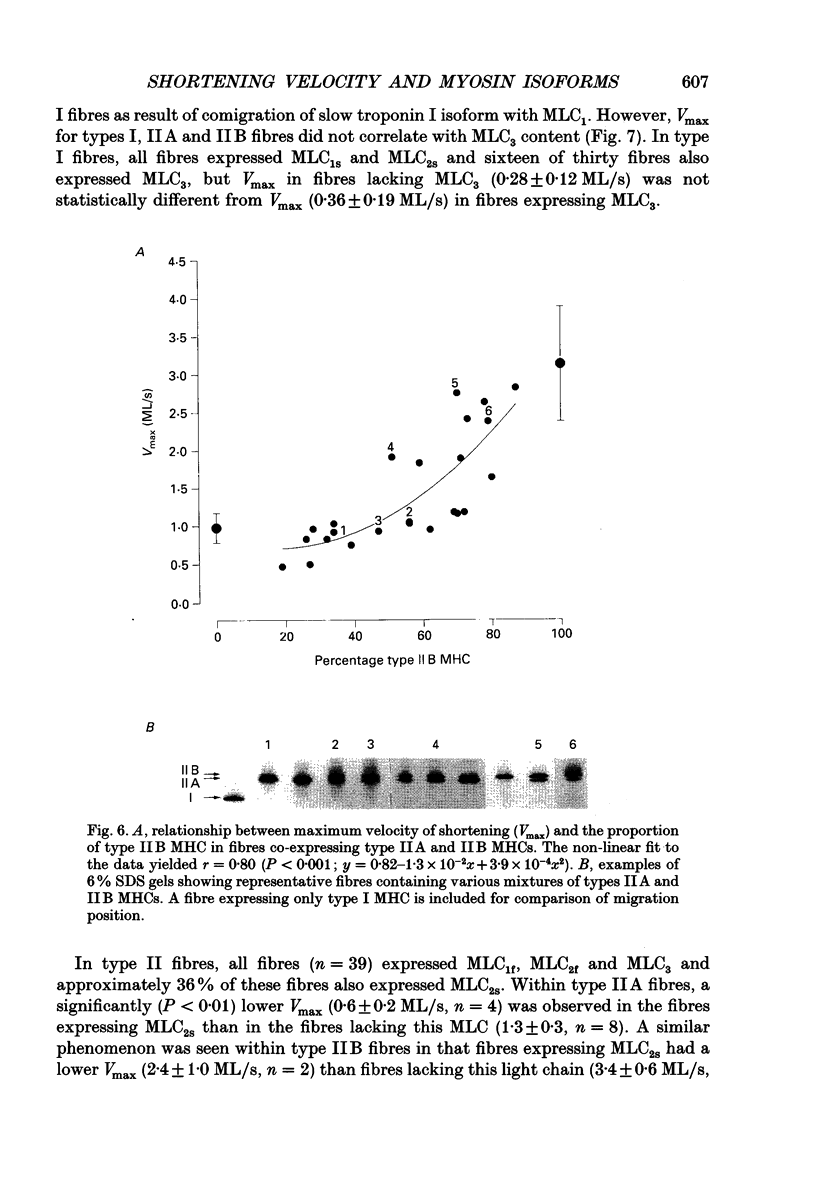
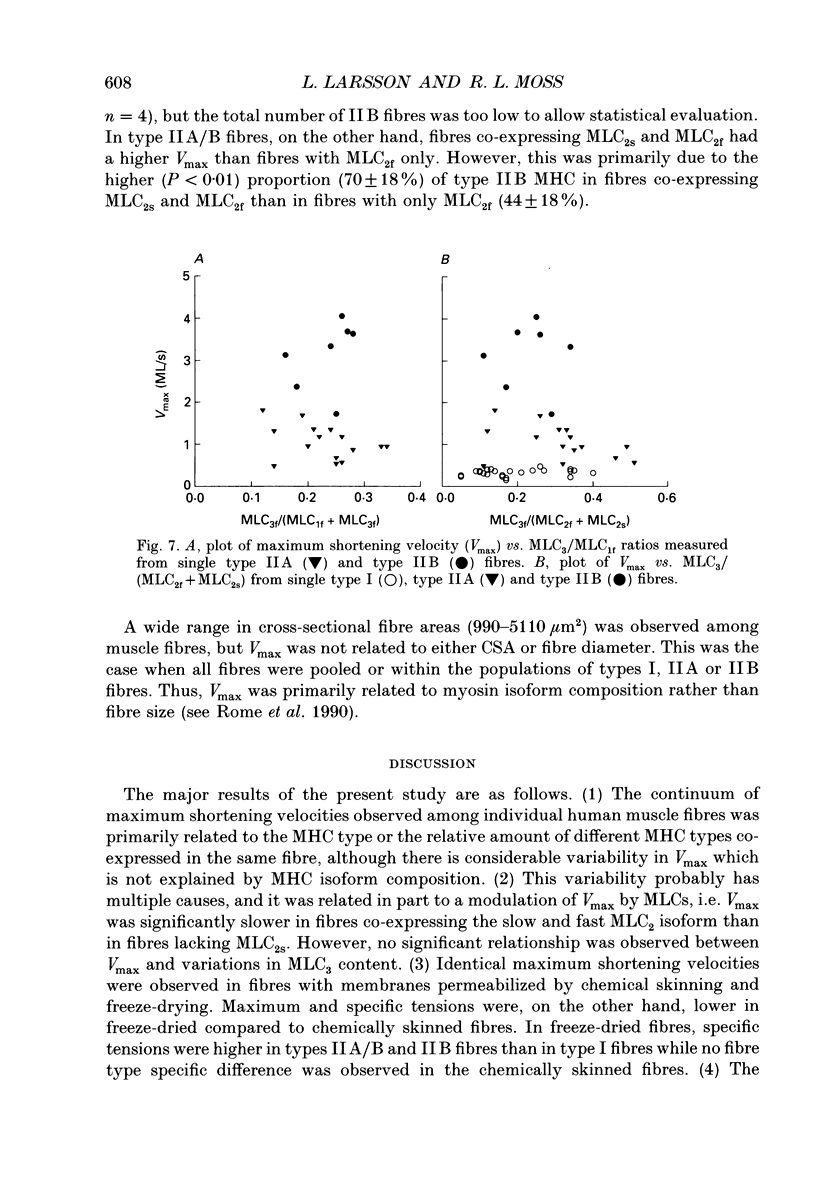






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottinelli R., Schiaffino S., Reggiani C. Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol. 1991 Jun;437:655–672. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Some comments on the histochemical characterization of muscle adenosine triphosphatase. J Histochem Cytochem. 1969 Jun;17(6):431–432. doi: 10.1177/17.6.431. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Three "myosin adenosine triphosphatase" systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970 Sep;18(9):670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Brown W. E., Salmons S., Whalen R. G. The sequential replacement of myosin subunit isoforms during muscle type transformation induced by long term electrical stimulation. J Biol Chem. 1983 Dec 10;258(23):14686–14692. [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro U., Catani C. A sensitive SDS-PAGE method separating myosin heavy chain isoforms of rat skeletal muscles reveals the heterogeneous nature of the embryonic myosin. Biochem Biophys Res Commun. 1983 Nov 15;116(3):793–802. doi: 10.1016/s0006-291x(83)80212-5. [DOI] [PubMed] [Google Scholar]
- Close R. The relation between intrinsic speed of shortening and duration of the active state of muscle. J Physiol. 1965 Oct;180(3):542–559. doi: 10.1113/jphysiol.1965.sp007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danieli Betto D., Zerbato E., Betto R. Type 1, 2A, and 2B myosin heavy chain electrophoretic analysis of rat muscle fibers. Biochem Biophys Res Commun. 1986 Jul 31;138(2):981–987. doi: 10.1016/s0006-291x(86)80592-7. [DOI] [PubMed] [Google Scholar]
- Eddinger T. J., Cassens R. G., Moss R. L. Mechanical and histochemical characterization of skeletal muscles from senescent rats. Am J Physiol. 1986 Sep;251(3 Pt 1):C421–C430. doi: 10.1152/ajpcell.1986.251.3.C421. [DOI] [PubMed] [Google Scholar]
- Eddinger T. J., Moss R. L. Mechanical properties of skinned single fibers of identified types from rat diaphragm. Am J Physiol. 1987 Aug;253(2 Pt 1):C210–C218. doi: 10.1152/ajpcell.1987.253.2.C210. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Reggiani C., Schiaffino S., te Kronnie G. Maximum velocity of shortening related to myosin isoform composition in frog skeletal muscle fibres. J Physiol. 1988 Jan;395:679–694. doi: 10.1113/jphysiol.1988.sp016941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fitts R. H., Costill D. L., Gardetto P. R. Effect of swim exercise training on human muscle fiber function. J Appl Physiol (1985) 1989 Jan;66(1):465–475. doi: 10.1152/jappl.1989.66.1.465. [DOI] [PubMed] [Google Scholar]
- Galler S., Rathmayer W. Shortening velocity and force/pCa relationship in skinned crab muscle fibres of different types. Pflugers Arch. 1992 Feb;420(2):187–193. doi: 10.1007/BF00374989. [DOI] [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem. 1983 Mar;129(2):277–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- Greaser M. L., Moss R. L., Reiser P. J. Variations in contractile properties of rabbit single muscle fibres in relation to troponin T isoforms and myosin light chains. J Physiol. 1988 Dec;406:85–98. doi: 10.1113/jphysiol.1988.sp017370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann P. A., Metzger J. M., Greaser M. L., Moss R. L. Effects of partial extraction of light chain 2 on the Ca2+ sensitivities of isometric tension, stiffness, and velocity of shortening in skinned skeletal muscle fibers. J Gen Physiol. 1990 Mar;95(3):477–498. doi: 10.1085/jgp.95.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Moss R. L., Waller G. S. Mechanical properties and myosin light chain composition of skinned muscle fibres from adult and new-born rabbits. J Physiol. 1981 Feb;311:201–218. doi: 10.1113/jphysiol.1981.sp013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda K., Hashizume K. Factors causing difference in force output among motor units in the rat medial gastrocnemius muscle. J Physiol. 1992 Mar;448:677–695. doi: 10.1113/jphysiol.1992.sp019064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFramboise W. A., Daood M. J., Guthrie R. D., Moretti P., Schiaffino S., Ontell M. Electrophoretic separation and immunological identification of type 2X myosin heavy chain in rat skeletal muscle. Biochim Biophys Acta. 1990 Jul 20;1035(1):109–112. doi: 10.1016/0304-4165(90)90181-u. [DOI] [PubMed] [Google Scholar]
- Larsson L., Edström L., Lindegren B., Gorza L., Schiaffino S. MHC composition and enzyme-histochemical and physiological properties of a novel fast-twitch motor unit type. Am J Physiol. 1991 Jul;261(1 Pt 1):C93–101. doi: 10.1152/ajpcell.1991.261.1.C93. [DOI] [PubMed] [Google Scholar]
- Larsson L., Salviati G. A technique for studies of the contractile apparatus in single human muscle fibre segments obtained by percutaneous biopsy. Acta Physiol Scand. 1992 Dec;146(4):485–495. doi: 10.1111/j.1748-1716.1992.tb09450.x. [DOI] [PubMed] [Google Scholar]
- Larsson L., Sjödin B., Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22--65 years. Acta Physiol Scand. 1978 May;103(1):31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- Lännergren J. Contractile properties and myosin isoenzymes of various kinds of Xenopus twitch muscle fibres. J Muscle Res Cell Motil. 1987 Jun;8(3):260–273. doi: 10.1007/BF01574594. [DOI] [PubMed] [Google Scholar]
- Lännergren J., Hoh J. F. Myosin isoenzymes in single muscle fibres of Xenopus laevis: analysis of five different functional types. Proc R Soc Lond B Biol Sci. 1984 Sep 22;222(1228):401–408. doi: 10.1098/rspb.1984.0072. [DOI] [PubMed] [Google Scholar]
- Maruyama K. Connectin, an elastic filamentous protein of striated muscle. Int Rev Cytol. 1986;104:81–114. doi: 10.1016/s0074-7696(08)61924-5. [DOI] [PubMed] [Google Scholar]
- Miller D. J. Are cardiac muscle cells 'skinned' by EGTA or EDTA? Nature. 1979 Jan 11;277(5692):142–143. doi: 10.1038/277142a0. [DOI] [PubMed] [Google Scholar]
- Moss R. L., Giulian G. G., Greaser M. L. Physiological effects accompanying the removal of myosin LC2 from skinned skeletal muscle fibers. J Biol Chem. 1982 Aug 10;257(15):8588–8591. [PubMed] [Google Scholar]
- Moss R. L. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979 Jul;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser P. J., Greaser M. L., Moss R. L. Myosin heavy chain composition of single cells from avian slow skeletal muscle is strongly correlated with velocity of shortening during development. Dev Biol. 1988 Oct;129(2):400–407. doi: 10.1016/0012-1606(88)90387-9. [DOI] [PubMed] [Google Scholar]
- Reiser P. J., Kasper C. E., Moss R. L. Myosin subunits and contractile properties of single fibers from hypokinetic rat muscles. J Appl Physiol (1985) 1987 Dec;63(6):2293–2300. doi: 10.1152/jappl.1987.63.6.2293. [DOI] [PubMed] [Google Scholar]
- Reiser P. J., Moss R. L., Giulian G. G., Greaser M. L. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985 Aug 5;260(16):9077–9080. [PubMed] [Google Scholar]
- Rome L. C., Sosnicki A. A., Goble D. O. Maximum velocity of shortening of three fibre types from horse soleus muscle: implications for scaling with body size. J Physiol. 1990 Dec;431:173–185. doi: 10.1113/jphysiol.1990.sp018325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R. L., Whittlesey D. Ca-, Sr-tension relationships and contraction velocities of human muscle fibers. Muscle Nerve. 1991 Dec;14(12):1219–1226. doi: 10.1002/mus.880141214. [DOI] [PubMed] [Google Scholar]
- Sawchak J. A., Lewis S., Shafiq S. A. Coexpression of myosin isoforms in muscle of patients with neurogenic disease. Muscle Nerve. 1989 Aug;12(8):679–689. doi: 10.1002/mus.880120809. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Ausoni S., Gorza L., Saggin L., Gundersen K., Lomo T. Myosin heavy chain isoforms and velocity of shortening of type 2 skeletal muscle fibres. Acta Physiol Scand. 1988 Dec;134(4):575–576. doi: 10.1111/j.1748-1716.1998.tb08539.x. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Gorza L., Sartore S., Saggin L., Ausoni S., Vianello M., Gundersen K., Lømo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989 Jun;10(3):197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Sugiura T., Matoba H., Murakami N. Myosin light chain patterns in histochemically typed single fibers of the rat skeletal muscle. Comp Biochem Physiol B. 1992 Jul;102(3):617–620. doi: 10.1016/0305-0491(92)90056-w. [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J., Mabuchi K., Gergely J., Sréter F. A. Velocity of shortening and myosin isozymes in two types of rabbit fast-twitch muscle fibers. Am J Physiol. 1986 Sep;251(3 Pt 1):C431–C434. doi: 10.1152/ajpcell.1986.251.3.C431. [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J., Mabuchi K., Sréter F. A., Gergely J. Myosin alkali light chain and heavy chain variations correlate with altered shortening velocity of isolated skeletal muscle fibers. J Biol Chem. 1988 Jun 25;263(18):9034–9039. [PubMed] [Google Scholar]
- Termin A., Staron R. S., Pette D. Myosin heavy chain isoforms in histochemically defined fiber types of rat muscle. Histochemistry. 1989;92(6):453–457. doi: 10.1007/BF00524756. [DOI] [PubMed] [Google Scholar]
- Webster C., Silberstein L., Hays A. P., Blau H. M. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell. 1988 Feb 26;52(4):503–513. doi: 10.1016/0092-8674(88)90463-1. [DOI] [PubMed] [Google Scholar]