Abstract
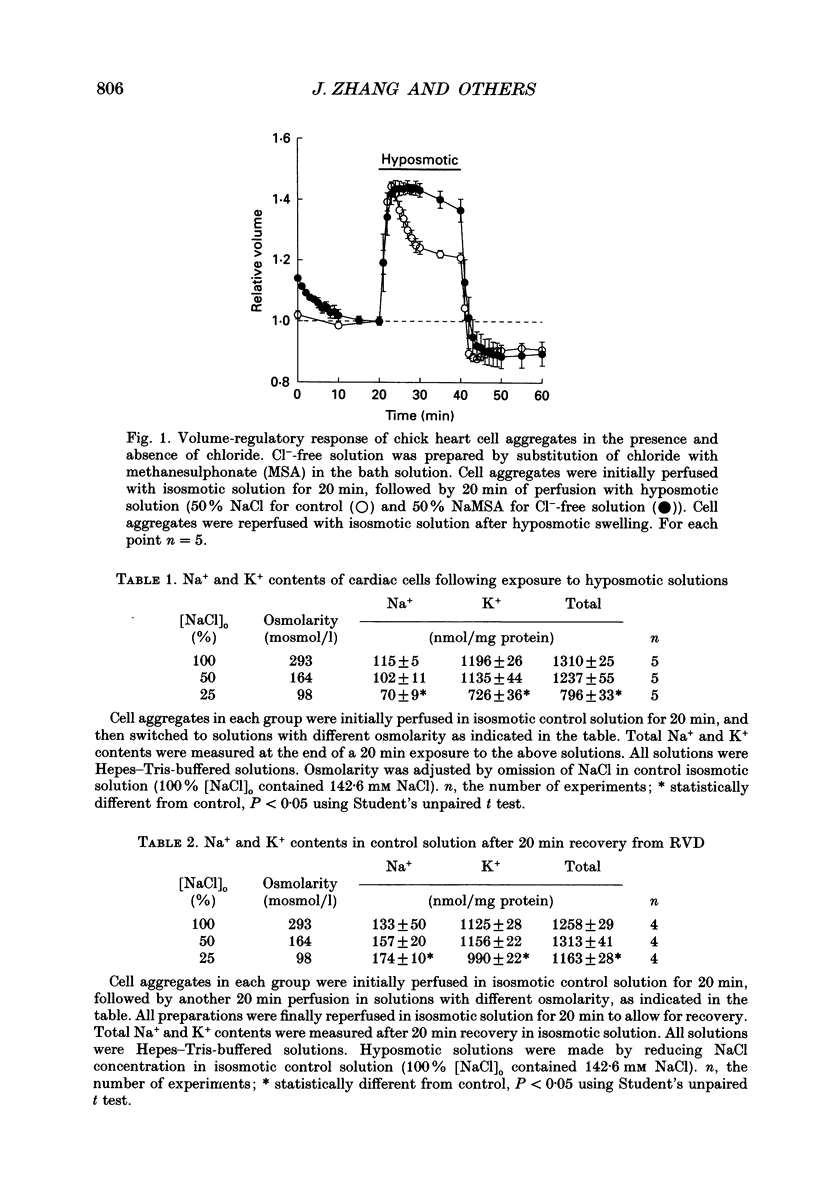
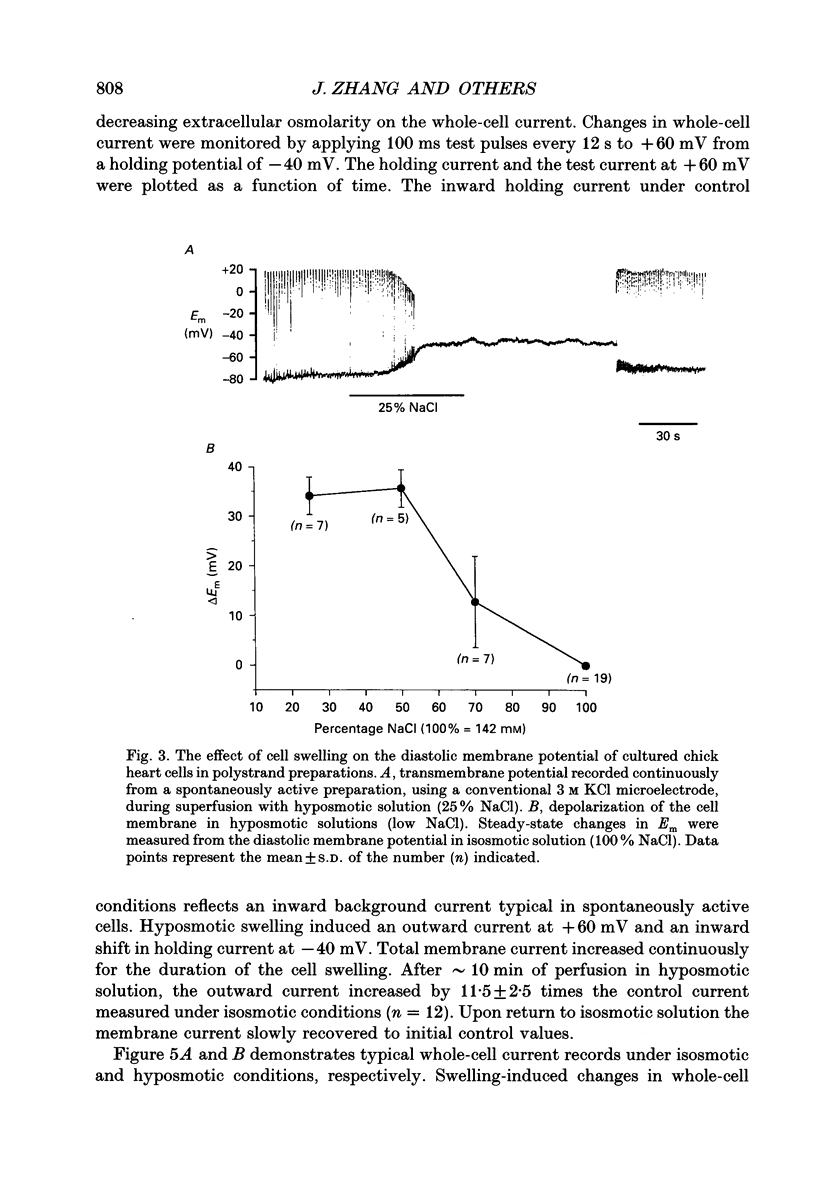
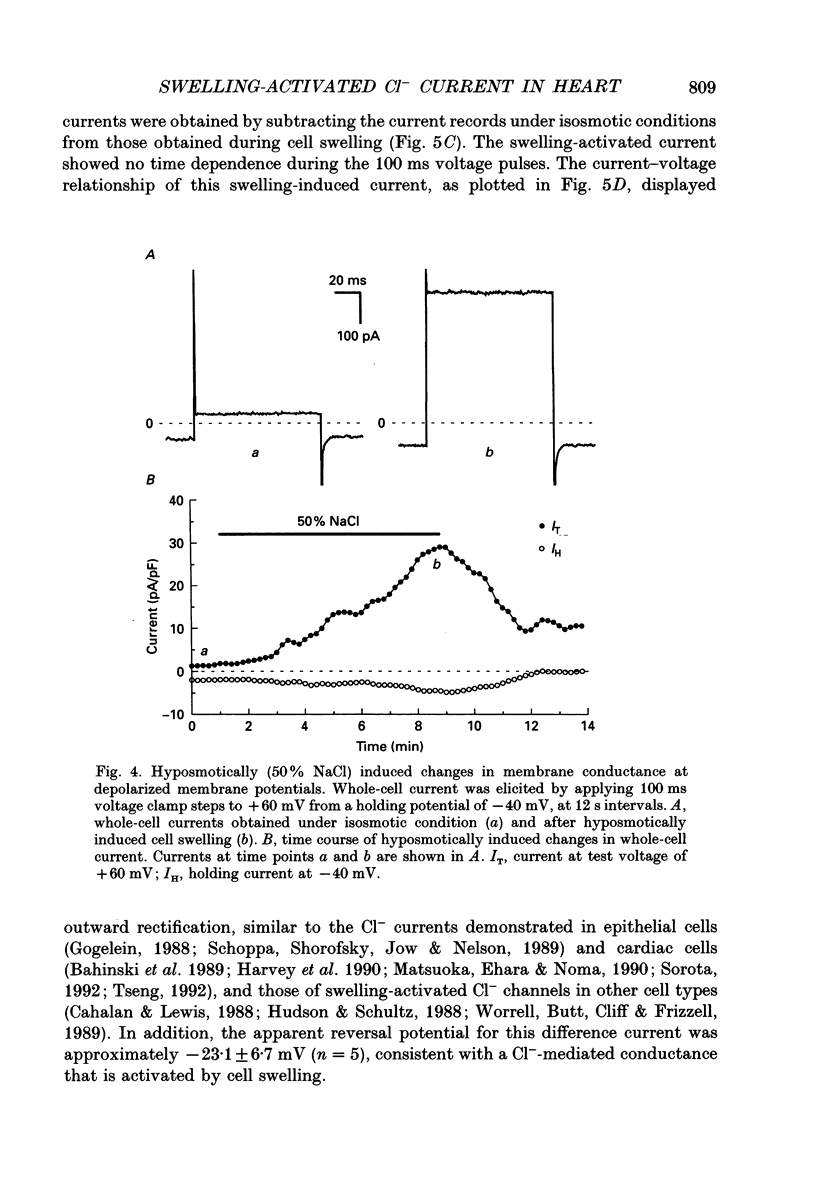
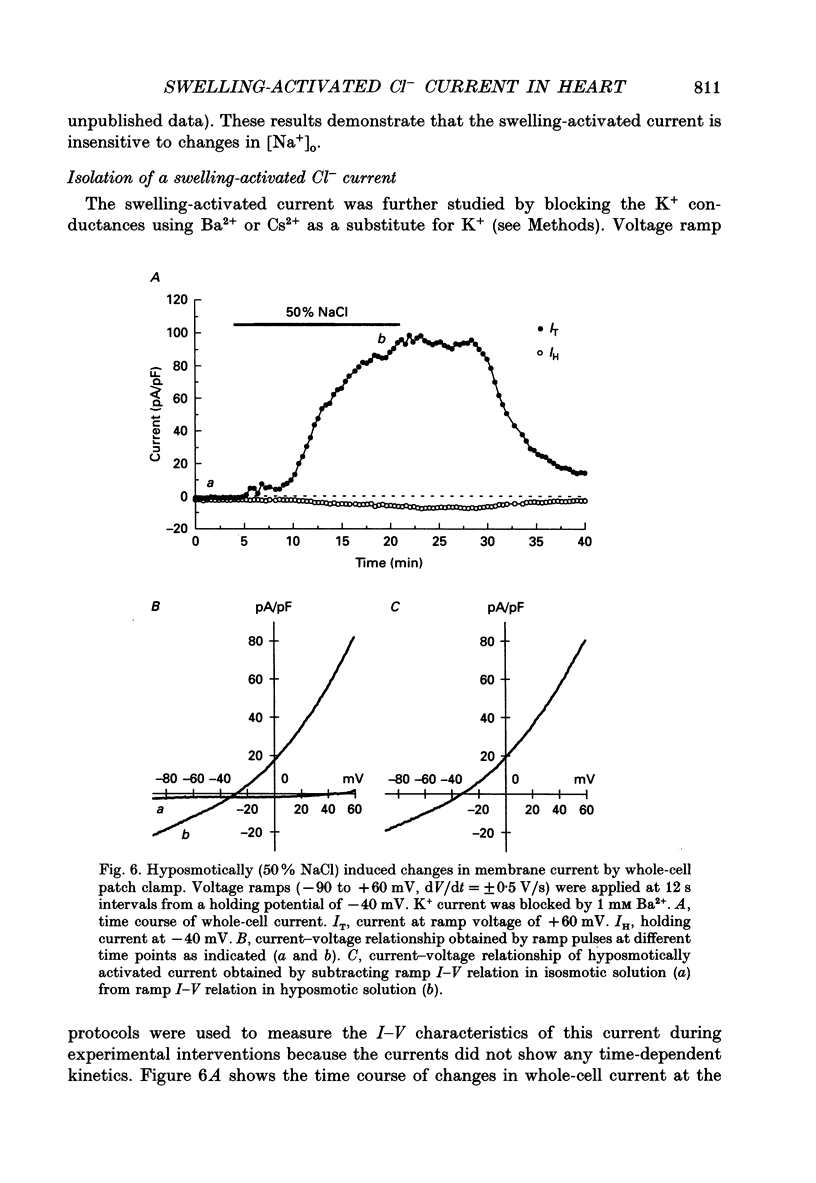
1. Cultured chick heart cells challenged by hyposmotic stress underwent regulatory volume decrease (RVD) that was attenuated by prior depletion of intracellular chloride. 2. During hyposmotic swelling, cell aggregates experienced an initial increase in spontaneous contractile activity followed by eventual quiescence. Conventional microelectrode studies revealed an underlying increase in spontaneous electrical activity, followed by a sustained depolarization beyond threshold. 3. Whole-cell patch clamp studies, with K+ currents blocked, indicated that exposure of cells to hyposmotic solution (NaCl reduction) resulted in a rapid osmotic swelling followed by a substantial increase in whole-cell conductance which persisted for the duration of hyposmotic exposure and was almost completely reversed on return to isosmotic bath solution. 4. For a variety of Cl- concentrations, the reversal potentials (Erev) of the measured swelling-activated current closely followed the calculated Cl- equilibrium potential (ECl) with a linear regression slope of 0.82. When estimated by the Nernst equation, the relationship between Erev and the [Cl-]i/[Cl-]o ratio fitted well with a slope of 51 mV per decade change in the concentration ratio, consistent with a Cl(-)-selective conductance. 5. The permeability ratios of this swelling-activated conductance to chloride, methanesulphonate (MSA) and aspartate (Asp) were calculated as PCl:PMSA:PASP = 1:0.36:0.02, with the ion selectivity sequence of Cl- > MSA- >> Asp-, which suggests the swelling-activated conductance is slightly permeable to other anions. 6. Application of a Cl- channel blocker, diphenylamine-2-carboxylate (DPC, 200 microM), substantially suppressed the swelling-activated current without shifting the Erev of this current. The effect of DPC was independent of membrane potential. 7. This evidence demonstrates that hyposmotic swelling of cultured chick heart cells activates a channel-mediated Cl- conductance which may be associated with the integrated response of volume-regulatory mechanisms.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahinski A., Nairn A. C., Greengard P., Gadsby D. C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989 Aug 31;340(6236):718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D., Lewis R. S. Role of potassium and chloride channels in volume regulation by T lymphocytes. Soc Gen Physiol Ser. 1988;43:281–301. [PubMed] [Google Scholar]
- Campbell D. L., Rasmusson R. L., Strauss H. C. Ionic current mechanisms generating vertebrate primary cardiac pacemaker activity at the single cell level: an integrative view. Annu Rev Physiol. 1992;54:279–302. doi: 10.1146/annurev.ph.54.030192.001431. [DOI] [PubMed] [Google Scholar]
- Di Stefano A., Wittner M., Schlatter E., Lang H. J., Englert H., Greger R. Diphenylamine-2-carboxylate, a blocker of the Cl(-)-conductive pathway in Cl(-)-transporting epithelia. Pflugers Arch. 1985;405 (Suppl 1):S95–100. doi: 10.1007/BF00581787. [DOI] [PubMed] [Google Scholar]
- Drewnowska K., Baumgarten C. M. Regulation of cellular volume in rabbit ventricular myocytes: bumetanide, chlorothiazide, and ouabain. Am J Physiol. 1991 Jan;260(1 Pt 1):C122–C131. doi: 10.1152/ajpcell.1991.260.1.C122. [DOI] [PubMed] [Google Scholar]
- Drewnowska K., Clemo H. F., Baumgarten C. M. Prevention of myocardial intracellular edema induced by St. Thomas' Hospital cardioplegic solution. J Mol Cell Cardiol. 1991 Nov;23(11):1215–1221. doi: 10.1016/0022-2828(91)90079-2. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Vander Heide R. S. Irreversible injury of isolated adult rat myocytes. Osmotic fragility during metabolic inhibition. Am J Pathol. 1988 Aug;132(2):212–222. [PMC free article] [PubMed] [Google Scholar]
- Greger R. Chloride channel blockers. Methods Enzymol. 1990;191:793–810. doi: 10.1016/0076-6879(90)91048-b. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A., Sarkadi B., Gelfand E. W. Responses of lymphocytes to anisotonic media: volume-regulating behavior. Am J Physiol. 1984 Mar;246(3 Pt 1):C204–C215. doi: 10.1152/ajpcell.1984.246.3.C204. [DOI] [PubMed] [Google Scholar]
- Gögelein H. Chloride channels in epithelia. Biochim Biophys Acta. 1988 Oct 11;947(3):521–547. doi: 10.1016/0304-4157(88)90006-8. [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Masuda H., Shoda M., Irisawa H. Stretch-activated anion currents of rabbit cardiac myocytes. J Physiol. 1992 Oct;456:285–302. doi: 10.1113/jphysiol.1992.sp019337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Clark C. D., Hume J. R. Chloride current in mammalian cardiac myocytes. Novel mechanism for autonomic regulation of action potential duration and resting membrane potential. J Gen Physiol. 1990 Jun;95(6):1077–1102. doi: 10.1085/jgp.95.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989 Apr;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Houser S. R., Freeman A. R. A simple method for volumetric measurements in isolated cardiac muscle. Am J Physiol. 1979 Mar;236(3):H519–H524. doi: 10.1152/ajpheart.1979.236.3.H519. [DOI] [PubMed] [Google Scholar]
- Hudson R. L., Schultz S. G. Sodium-coupled glycine uptake by Ehrlich ascites tumor cells results in an increase in cell volume and plasma membrane channel activities. Proc Natl Acad Sci U S A. 1988 Jan;85(1):279–283. doi: 10.1073/pnas.85.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R., Lieberman M., Liu S. Electrogenic sodium-calcium exchange in cultured embryonic chick heart cells. J Physiol. 1987 Jun;387:567–588. doi: 10.1113/jphysiol.1987.sp016589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings R. B., Reimer K. A., Steenbergen C. Myocardial ischemia revisited. The osmolar load, membrane damage, and reperfusion. J Mol Cell Cardiol. 1986 Aug;18(8):769–780. doi: 10.1016/s0022-2828(86)80952-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu S., Jacob R., Piwnica-Worms D., Lieberman M. (Na + K + 2Cl) cotransport in cultured embryonic chick heart cells. Am J Physiol. 1987 Nov;253(5 Pt 1):C721–C730. doi: 10.1152/ajpcell.1987.253.5.C721. [DOI] [PubMed] [Google Scholar]
- Liu S., Piwnica-Worms D., Lieberman M. Intracellular pH regulation in cultured embryonic chick heart cells. Na(+)-dependent Cl-/HCO3- exchange. J Gen Physiol. 1990 Dec;96(6):1247–1269. doi: 10.1085/jgp.96.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S., Ehara T., Noma A. Chloride-sensitive nature of the adrenaline-induced current in guinea-pig cardiac myocytes. J Physiol. 1990 Jun;425:579–598. doi: 10.1113/jphysiol.1990.sp018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Aiton J. F., Horres C. R., Lieberman M. Calcium elevation in cultured heart cells: its role in cell injury. Am J Physiol. 1983 Nov;245(5 Pt 1):C316–C321. doi: 10.1152/ajpcell.1983.245.5.C316. [DOI] [PubMed] [Google Scholar]
- Page E., Storm S. R. Cat heart muscle in vitro. IX. Cell ion and water contents in anisosmolal solutions. J Gen Physiol. 1966 Mar;49(4):641–653. doi: 10.1085/jgp.49.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms D., Jacob R., Horres C. R., Lieberman M. Transmembrane chloride flux in tissue-cultured chick heart cells. J Gen Physiol. 1983 May;81(5):731–748. doi: 10.1085/jgp.81.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson R. L., Davis D. G., Lieberman M. Amino acid loss during volume regulatory decrease in cultured chick heart cells. Am J Physiol. 1993 Jan;264(1 Pt 1):C136–C145. doi: 10.1152/ajpcell.1993.264.1.C136. [DOI] [PubMed] [Google Scholar]
- Roos K. P. Length, width, and volume changes in osmotically stressed myocytes. Am J Physiol. 1986 Dec;251(6 Pt 2):H1373–H1378. doi: 10.1152/ajpheart.1986.251.6.H1373. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Parker J. C. Activation of ion transport pathways by changes in cell volume. Biochim Biophys Acta. 1991 Dec 12;1071(4):407–427. doi: 10.1016/0304-4157(91)90005-h. [DOI] [PubMed] [Google Scholar]
- Schoppa N., Shorofsky S. R., Jow F., Nelson D. J. Voltage-gated chloride currents in cultured canine tracheal epithelial cells. J Membr Biol. 1989 Apr;108(1):73–90. doi: 10.1007/BF01870427. [DOI] [PubMed] [Google Scholar]
- Sorota S. Swelling-induced chloride-sensitive current in canine atrial cells revealed by whole-cell patch-clamp method. Circ Res. 1992 Apr;70(4):679–687. doi: 10.1161/01.res.70.4.679. [DOI] [PubMed] [Google Scholar]
- Steadman B. W., Moore K. B., Spitzer K. W., Bridge J. H. A video system for measuring motion in contracting heart cells. IEEE Trans Biomed Eng. 1988 Apr;35(4):264–272. doi: 10.1109/10.1375. [DOI] [PubMed] [Google Scholar]
- Steenbergen C., Hill M. L., Jennings R. B. Volume regulation and plasma membrane injury in aerobic, anaerobic, and ischemic myocardium in vitro. Effects of osmotic cell swelling on plasma membrane integrity. Circ Res. 1985 Dec;57(6):864–875. doi: 10.1161/01.res.57.6.864. [DOI] [PubMed] [Google Scholar]
- Tseng G. N. Cell swelling increases membrane conductance of canine cardiac cells: evidence for a volume-sensitive Cl channel. Am J Physiol. 1992 Apr;262(4 Pt 1):C1056–C1068. doi: 10.1152/ajpcell.1992.262.4.C1056. [DOI] [PubMed] [Google Scholar]


