Abstract
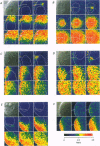
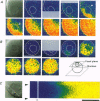
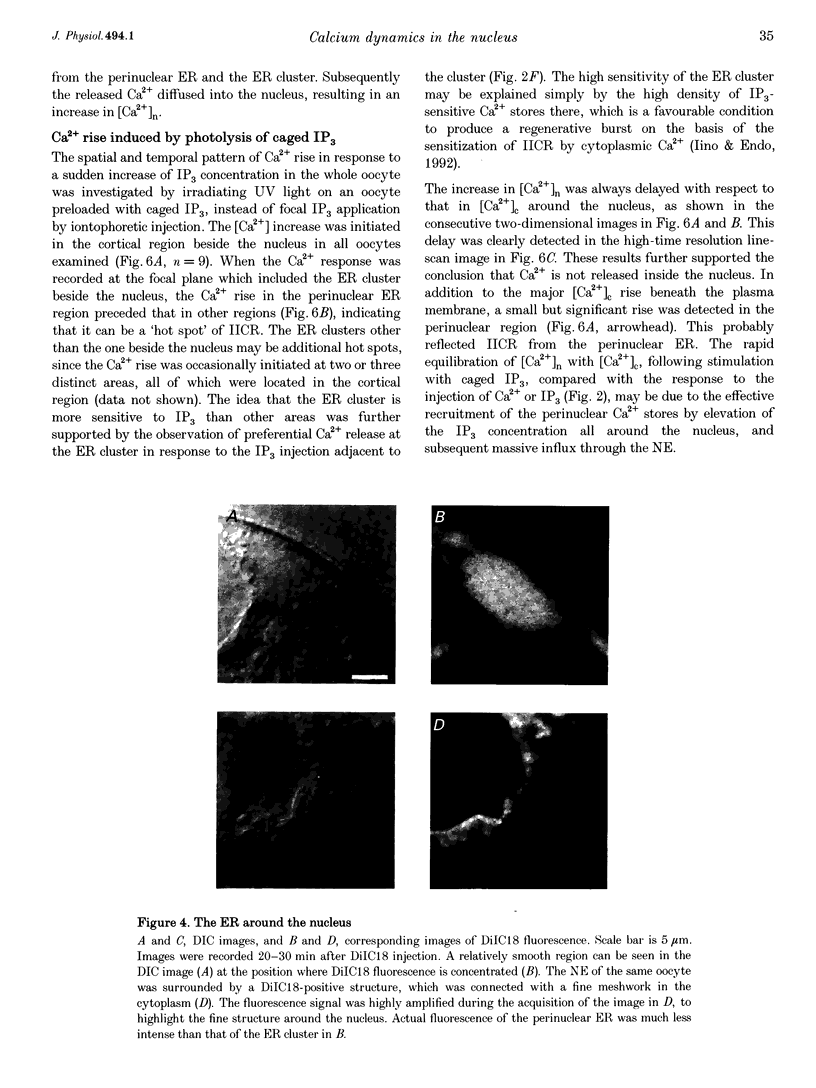
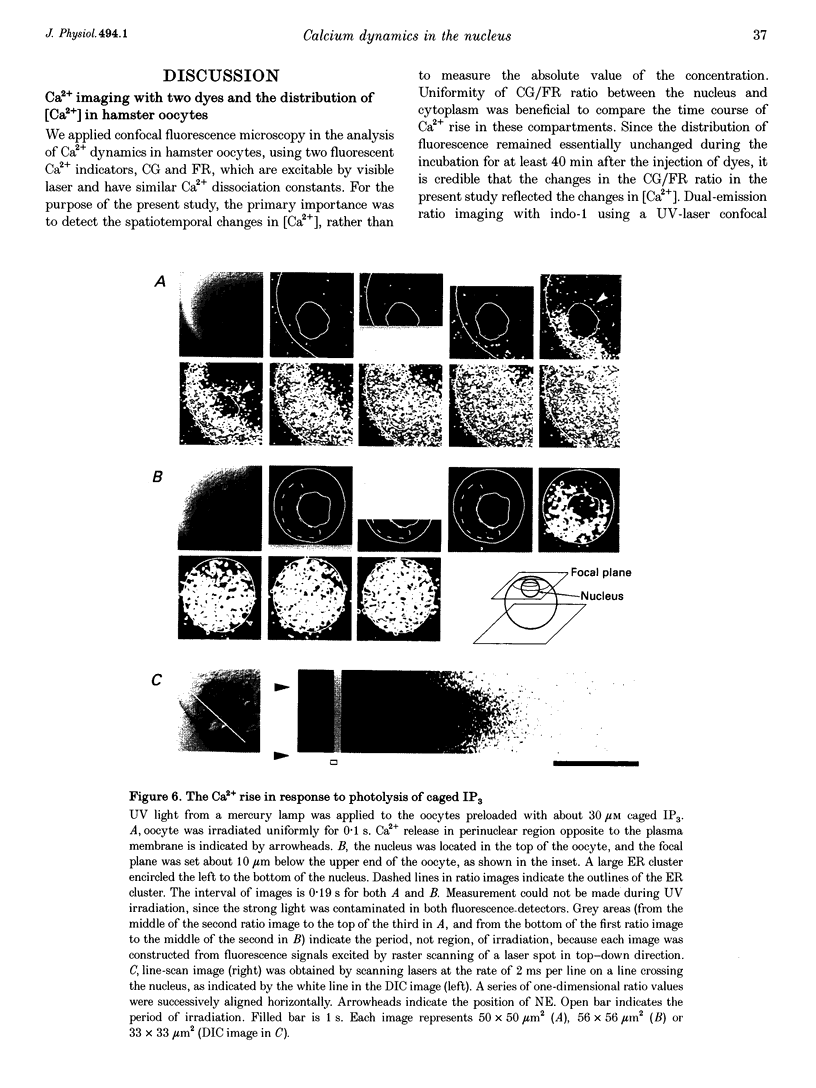
1. Subcellular Ca2+ dynamics inside and around the nucleus of immature hamster oocytes were analysed with confocal Ca2+ imaging. 2. The ratio value between emission intensity of two injected fluorescent Ca2+ indicators, Calcium Green and Fura Red, was almost uniform over the entire oocyte, suggesting that nucleoplasmic Ca2+ concentration ([Ca2+]n) is comparable to cytoplasmic Ca2+ concentration ([Ca2+]c) at the resting state. 3. When Ca2+ was iontophoretically injected into the nucleoplasm or the perinuclear cytoplasm, it diffused across the nuclear envelope (NE), and perinuclear [Ca2+]c and [Ca2+]n reached the same level within 2 s, although the NE worked as a weak but detectable barrier for Ca2+ diffusion. 4. Inositol 1,4,5-trisphosphate (IP3)-induced Ca2+ release from the NE through the inner membrane was not detected, even when a large amount of IP3 was delivered in close proximity to the inner nuclear membrane. 5. When an oocyte was uniformly stimulated by photolysis of caged IP3, a Ca2+ rise was initiated in the perinuclear cytoplasm. The [Ca2+]n rise was always delayed with respect to, but rapidly equilibrated with, the [Ca2+]c rise. 6. Clusters of the endoplasmic reticulum were located in the perinuclear cytoplasm and served as the trigger zone of IP3-induced Ca2+ release. 7. The results indicate that the [Ca2+]n rise occurs as the consequence of the influx of Ca2+ which was released in the perinuclear cytoplasm, not Ca2+ release from NE to the nucleoplasm.
Full text
PDF



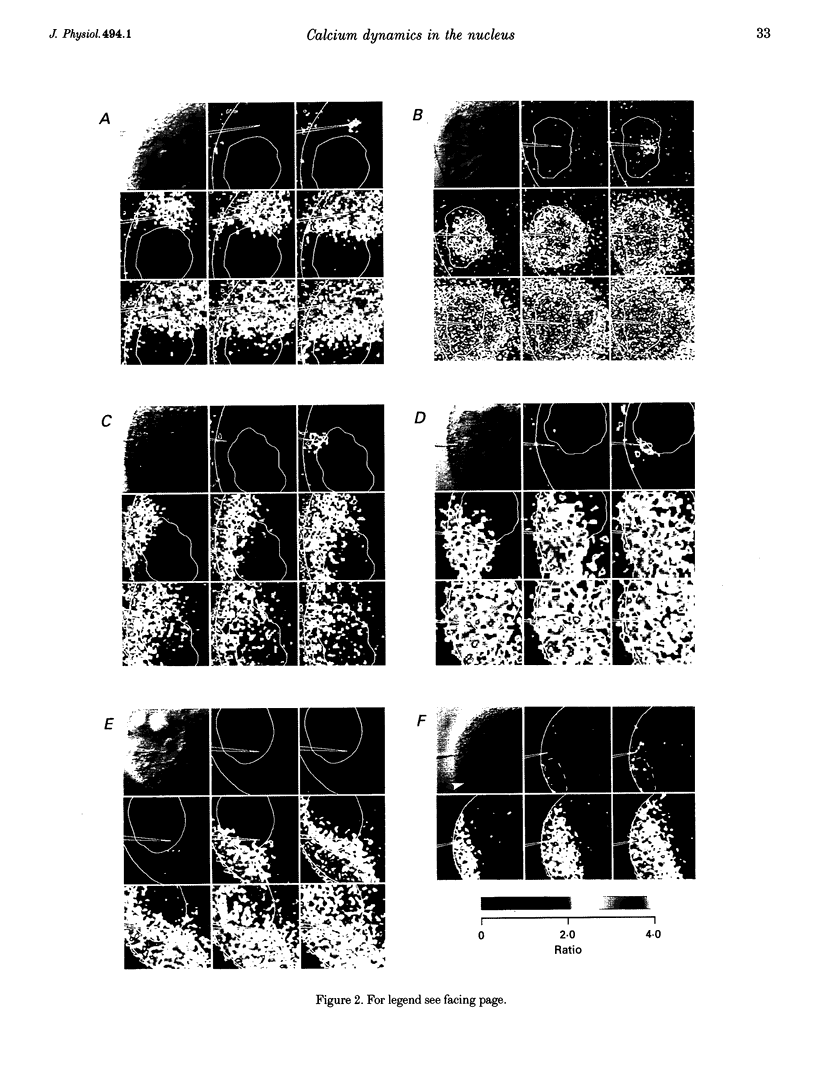
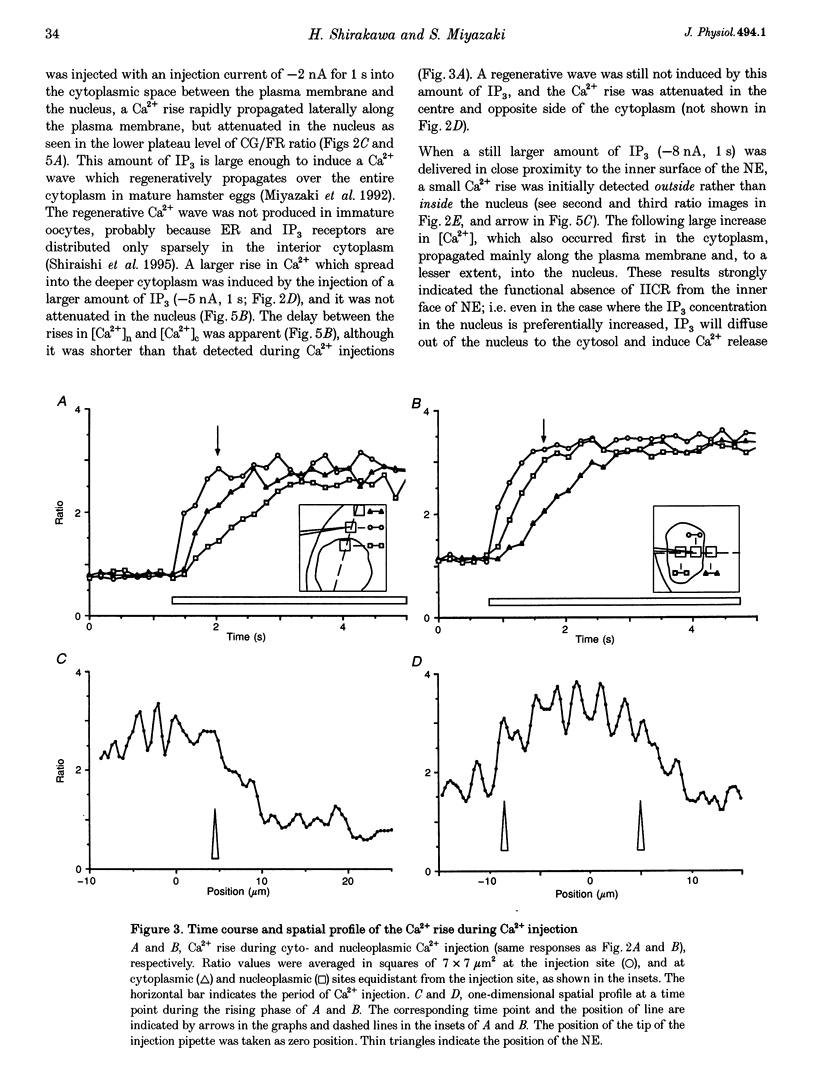
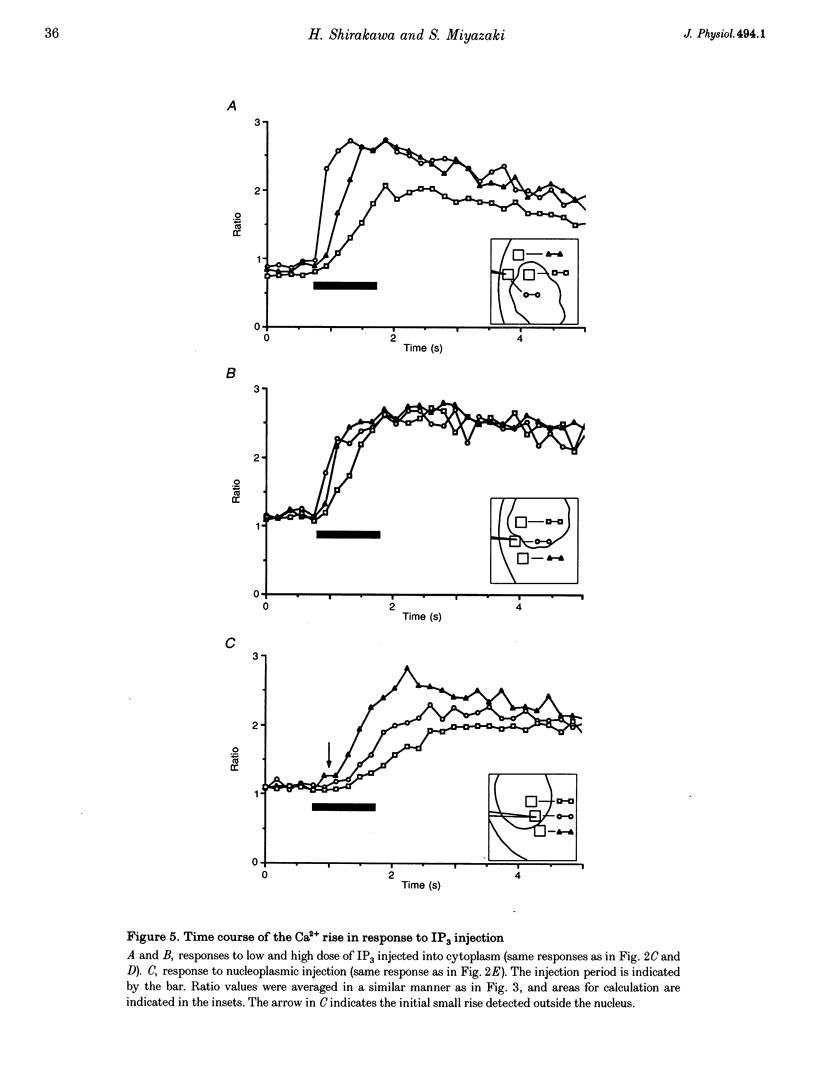



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baitinger C., Alderton J., Poenie M., Schulman H., Steinhardt R. A. Multifunctional Ca2+/calmodulin-dependent protein kinase is necessary for nuclear envelope breakdown. J Cell Biol. 1990 Nov;111(5 Pt 1):1763–1773. doi: 10.1083/jcb.111.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., MacManus J. P. Calmodulin stimulates DNA synthesis by rat liver cells. Biochem Biophys Res Commun. 1980 Jul 31;95(2):745–749. doi: 10.1016/0006-291x(80)90849-9. [DOI] [PubMed] [Google Scholar]
- Carroll J., Swann K. Spontaneous cytosolic calcium oscillations driven by inositol trisphosphate occur during in vitro maturation of mouse oocytes. J Biol Chem. 1992 Jun 5;267(16):11196–11201. [PubMed] [Google Scholar]
- Carroll J., Swann K., Whittingham D., Whitaker M. Spatiotemporal dynamics of intracellular [Ca2+]i oscillations during the growth and meiotic maturation of mouse oocytes. Development. 1994 Dec;120(12):3507–3517. doi: 10.1242/dev.120.12.3507. [DOI] [PubMed] [Google Scholar]
- Divecha N., Rhee S. G., Letcher A. J., Irvine R. F. Phosphoinositide signalling enzymes in rat liver nuclei: phosphoinositidase C isoform beta 1 is specifically, but not predominantly, located in the nucleus. Biochem J. 1993 Feb 1;289(Pt 3):617–620. doi: 10.1042/bj2890617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Scheer U., Krohne G., Jarasch E. D. The nuclear envelope and the architecture of the nuclear periphery. J Cell Biol. 1981 Dec;91(3 Pt 2):39s–50s. doi: 10.1083/jcb.91.3.39s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Nakada K., Shirakawa H., Miyazaki S. Development of inositol trisphosphate-induced calcium release mechanism during maturation of hamster oocytes. Dev Biol. 1993 Mar;156(1):69–79. doi: 10.1006/dbio.1993.1059. [DOI] [PubMed] [Google Scholar]
- Furuno T., Hamano T., Nakanishi M. Receptor-mediated calcium signal playing a nuclear third messenger in the activation of antigen-specific B cells. Biophys J. 1993 Mar;64(3):665–669. doi: 10.1016/S0006-3495(93)81425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko O. V., Gerasimenko J. V., Tepikin A. V., Petersen O. H. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell. 1995 Feb 10;80(3):439–444. doi: 10.1016/0092-8674(95)90494-8. [DOI] [PubMed] [Google Scholar]
- Gillot I., Whitaker M. Calcium signals in and around the nucleus in sea urchin eggs. Cell Calcium. 1994 Oct;16(4):269–278. doi: 10.1016/0143-4160(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Giovannardi S., Cesare P., Peres A. Rapid synchrony of nuclear and cytosolic Ca2+ signals activated by muscarinic stimulation in the human tumour line TE671/RD. Cell Calcium. 1994 Dec;16(6):491–499. doi: 10.1016/0143-4160(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Hennager D. J., Welsh M. J., DeLisle S. Changes in either cytosolic or nucleoplasmic inositol 1,4,5-trisphosphate levels can control nuclear Ca2+ concentration. J Biol Chem. 1995 Mar 10;270(10):4959–4962. doi: 10.1074/jbc.270.10.4959. [DOI] [PubMed] [Google Scholar]
- Himpens B., De Smedt H., Casteels R. Relationship between [Ca2+] changes in nucleus and cytosol. Cell Calcium. 1994 Oct;16(4):239–246. doi: 10.1016/0143-4160(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Homa S. T. Neomycin, an inhibitor of phosphoinositide hydrolysis, inhibits the resumption of bovine oocyte spontaneous meiotic maturation. J Exp Zool. 1991 Apr;258(1):95–103. doi: 10.1002/jez.1402580111. [DOI] [PubMed] [Google Scholar]
- Horowitz S. B. The permeability of the amphibian oocyte nucleus, in situ. J Cell Biol. 1972 Sep;54(3):609–625. doi: 10.1083/jcb.54.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Endo M. Calcium-dependent immediate feedback control of inositol 1,4,5-triphosphate-induced Ca2+ release. Nature. 1992 Nov 5;360(6399):76–78. doi: 10.1038/360076a0. [DOI] [PubMed] [Google Scholar]
- Katagiri S., Takamatsu T., Minamikawa T., Fujita S. Secretagogue-induced calcium wave shows higher and prolonged transients of nuclear calcium concentration in mast cells. FEBS Lett. 1993 Nov 22;334(3):343–346. doi: 10.1016/0014-5793(93)80708-3. [DOI] [PubMed] [Google Scholar]
- Kaufman M. L., Homa S. T. Defining a role for calcium in the resumption and progression of meiosis in the pig oocyte. J Exp Zool. 1993 Jan 1;265(1):69–76. doi: 10.1002/jez.1402650110. [DOI] [PubMed] [Google Scholar]
- Lanini L., Bachs O., Carafoli E. The calcium pump of the liver nuclear membrane is identical to that of endoplasmic reticulum. J Biol Chem. 1992 Jun 5;267(16):11548–11552. [PubMed] [Google Scholar]
- Lefèvre B., Pesty A., Testart J. Cytoplasmic and nucleic calcium oscillations in immature mouse oocytes: evidence of wave polarization by confocal imaging. Exp Cell Res. 1995 May;218(1):166–173. doi: 10.1006/excr.1995.1144. [DOI] [PubMed] [Google Scholar]
- Lin C., Hajnóczky G., Thomas A. P. Propagation of cytosolic calcium waves into the nuclei of hepatocytes. Cell Calcium. 1994 Oct;16(4):247–258. doi: 10.1016/0143-4160(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Ratiometric confocal Ca(2+)-measurements with visible wavelength indicators in isolated cardiac myocytes. Cell Calcium. 1993 May;14(5):359–372. doi: 10.1016/0143-4160(93)90040-d. [DOI] [PubMed] [Google Scholar]
- Malviya A. N. The nuclear inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate receptors. Cell Calcium. 1994 Oct;16(4):301–313. doi: 10.1016/0143-4160(94)90094-9. [DOI] [PubMed] [Google Scholar]
- McCray J. A., Trentham D. R. Properties and uses of photoreactive caged compounds. Annu Rev Biophys Biophys Chem. 1989;18:239–270. doi: 10.1146/annurev.bb.18.060189.001323. [DOI] [PubMed] [Google Scholar]
- Minamikawa T., Takahashi A., Fujita S. Differences in features of calcium transients between the nucleus and the cytosol in cultured heart muscle cells: analyzed by confocal microscopy. Cell Calcium. 1995 Mar;17(3):167–176. doi: 10.1016/0143-4160(95)90031-4. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Yuzaki M., Nakada K., Shirakawa H., Nakanishi S., Nakade S., Mikoshiba K. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science. 1992 Jul 10;257(5067):251–255. doi: 10.1126/science.1321497. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Orrenius S., Nilsson T., Berggren P. O. An inositol 1,4,5-trisphosphate-sensitive Ca2+ pool in liver nuclei. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6858–6862. doi: 10.1073/pnas.87.17.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., Zhivotovsky B., Orrenius S. Nuclear calcium transport and the role of calcium in apoptosis. Cell Calcium. 1994 Oct;16(4):279–288. doi: 10.1016/0143-4160(94)90091-4. [DOI] [PubMed] [Google Scholar]
- O'Malley D. M. Calcium permeability of the neuronal nuclear envelope: evaluation using confocal volumes and intracellular perfusion. J Neurosci. 1994 Oct;14(10):5741–5758. doi: 10.1523/JNEUROSCI.14-10-05741.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opas M., Dziak E., Fliegel L., Michalak M. Regulation of expression and intracellular distribution of calreticulin, a major calcium binding protein of nonmuscle cells. J Cell Physiol. 1991 Oct;149(1):160–171. doi: 10.1002/jcp.1041490120. [DOI] [PubMed] [Google Scholar]
- Roche E., Prentki M. Calcium regulation of immediate-early response genes. Cell Calcium. 1994 Oct;16(4):331–338. doi: 10.1016/0143-4160(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Shen S. S., Buck W. R. Sources of calcium in sea urchin eggs during the fertilization response. Dev Biol. 1993 May;157(1):157–169. doi: 10.1006/dbio.1993.1120. [DOI] [PubMed] [Google Scholar]
- Shiraishi K., Okada A., Shirakawa H., Nakanishi S., Mikoshiba K., Miyazaki S. Developmental changes in the distribution of the endoplasmic reticulum and inositol 1,4,5-trisphosphate receptors and the spatial pattern of Ca2+ release during maturation of hamster oocytes. Dev Biol. 1995 Aug;170(2):594–606. doi: 10.1006/dbio.1995.1239. [DOI] [PubMed] [Google Scholar]
- Stricker S. A., Centonze V. E., Melendez R. F. Calcium dynamics during starfish oocyte maturation and fertilization. Dev Biol. 1994 Nov;166(1):34–58. doi: 10.1006/dbio.1994.1295. [DOI] [PubMed] [Google Scholar]
- Sullivan K. M., Busa W. B., Wilson K. L. Calcium mobilization is required for nuclear vesicle fusion in vitro: implications for membrane traffic and IP3 receptor function. Cell. 1993 Jul 2;73(7):1411–1422. doi: 10.1016/0092-8674(93)90366-x. [DOI] [PubMed] [Google Scholar]
- Terasaki M., Jaffe L. A. Organization of the sea urchin egg endoplasmic reticulum and its reorganization at fertilization. J Cell Biol. 1991 Sep;114(5):929–940. doi: 10.1083/jcb.114.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombes R. M., Simerly C., Borisy G. G., Schatten G. Meiosis, egg activation, and nuclear envelope breakdown are differentially reliant on Ca2+, whereas germinal vesicle breakdown is Ca2+ independent in the mouse oocyte. J Cell Biol. 1992 May;117(4):799–811. doi: 10.1083/jcb.117.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M., Patel R. Calcium and cell cycle control. Development. 1990 Apr;108(4):525–542. doi: 10.1242/dev.108.4.525. [DOI] [PubMed] [Google Scholar]
- al-Mohanna F. A., Caddy K. W., Bolsover S. R. The nucleus is insulated from large cytosolic calcium ion changes. Nature. 1994 Feb 24;367(6465):745–750. doi: 10.1038/367745a0. [DOI] [PubMed] [Google Scholar]