Abstract
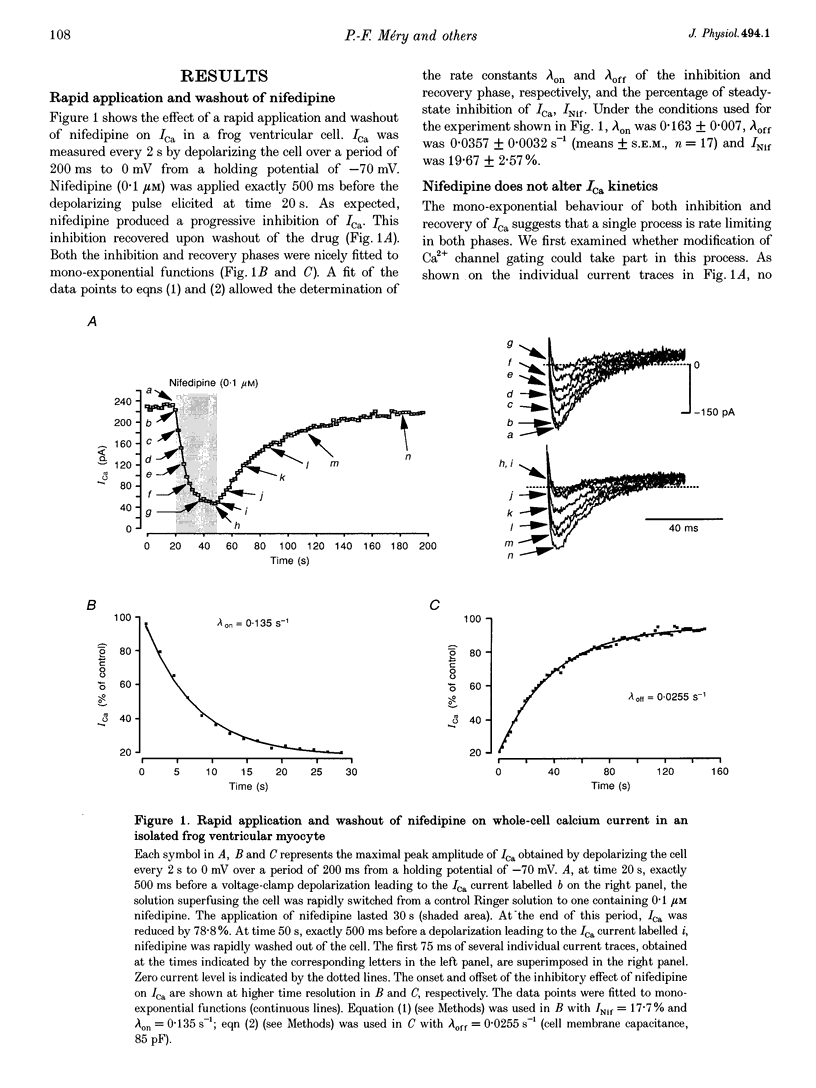
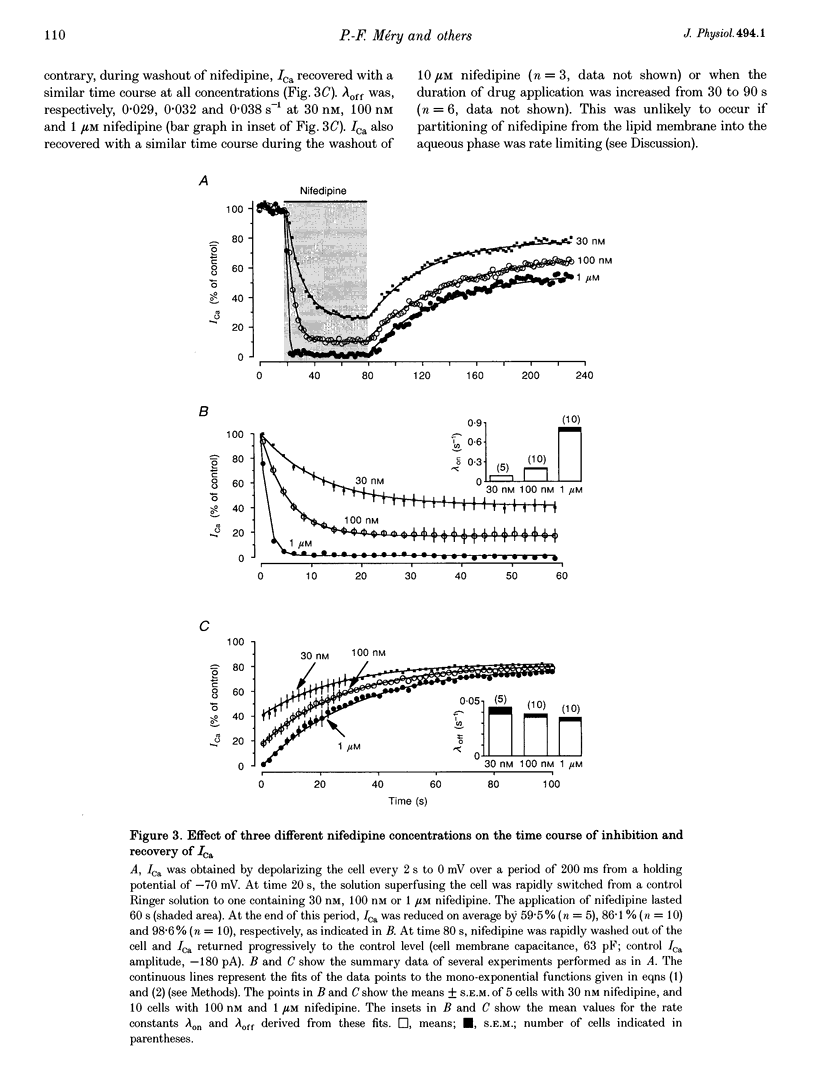
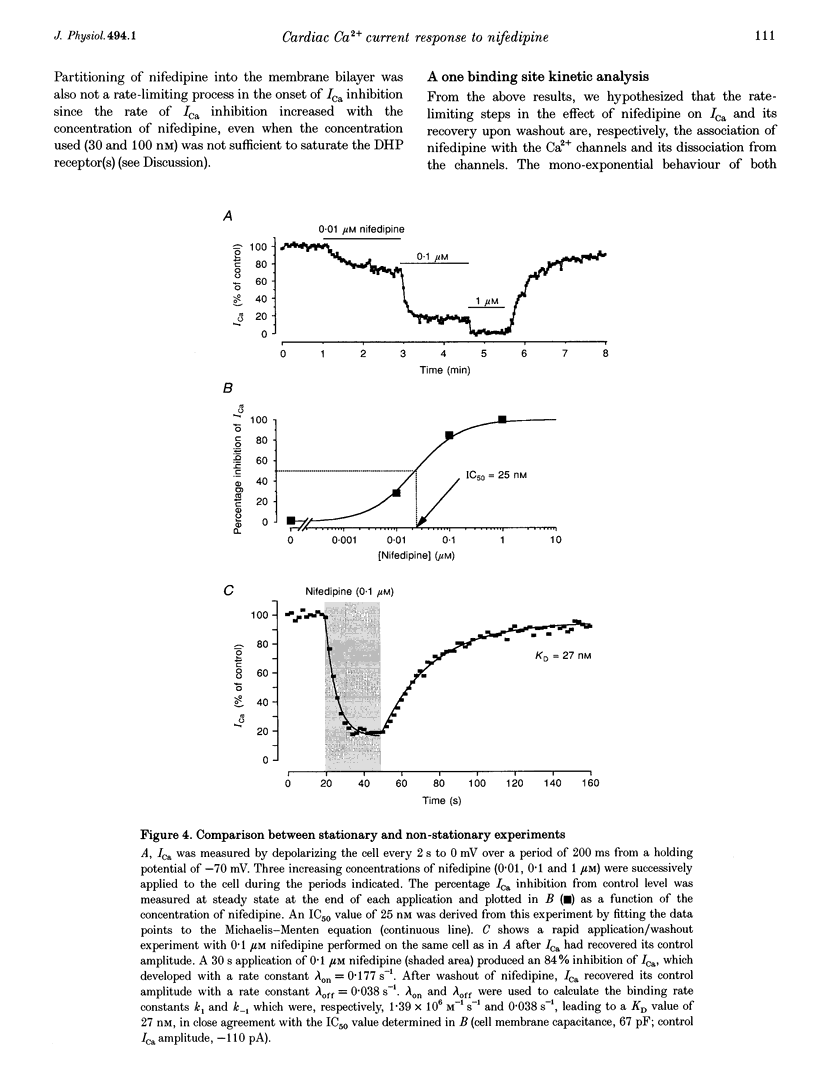
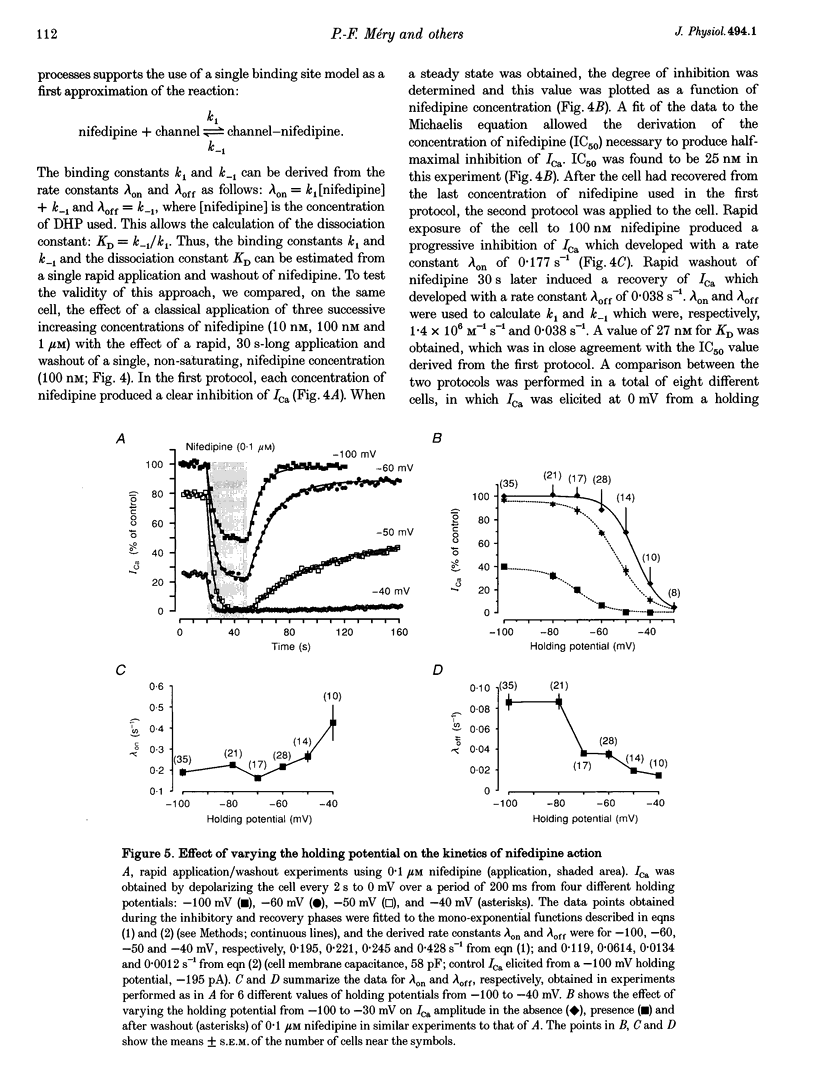
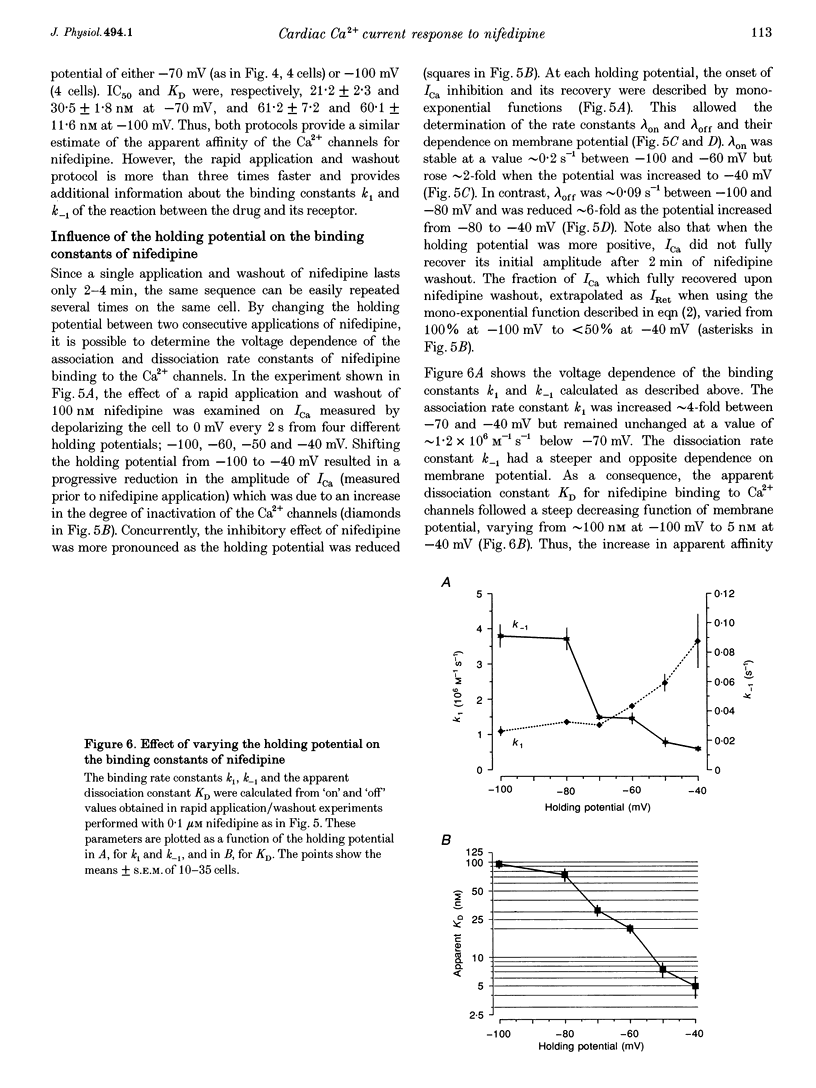
1. A fast perfusion system was used to analyse the kinetics of the response of L-type calcium current (ICa) to rapid applications and washouts of the dihydropyridine antagonist nifedipine in whole-cell patch-clamped frog ventricular myocytes. 2. Both the inhibition of ICa induced by nifedipine and the recovery from inhibition upon washout of the drug behaved as mono-exponential functions of time. 3. During application or washout of 100 nM nifedipine, only the peak amplitude of ICa varied but not its time course of activation or inactivation. 4. The rate constant of the onset of ICa inhibition increased with the concentration of nifedipine. However, the time course of the recovery from inhibition was independent of drug concentration. 5. Both rate constants were strongly sensitive to the holding potential but insensitive to the test potential. 6. Using simple rate equations and a one-binding-site analysis it was possible to determine the rate constants for association (k1) and dissociation (k-1) and the equilibrium dissociation constant (KD) of the reaction between nifedipine and Ca2+ channels. KD values for nifedipine were identical to IC50 values obtained from classical steady-state experiments. 7. With depolarized holding potentials, KD decreased strongly due to a large reduction in k-1 and a modest increase in k1. Assuming that these changes result from the distribution of Ca2+ channels between resting and inactivated states, a low-affinity binding to the resting state (R) and a high-affinity binding to the inactivated state (I) were obtained with the binding constants: k1R = 1.0 x 10(6) M-1 S-1, k-1R = 0.077 S-1, and KDR = 77 nM for the resting state; k1I = 4.47 x 10(6) M-1 S-1, k-1I = 7.7 x 10(-4) S-1, and KDI = 0.17 nM for the inactivated state. 8. Rapid application/washout experiments provide a unique way to determine, in an intact cell and in a relatively short period (2-4 min), the binding rate constants and the KD value of the reaction between a dihydropyridine antagonist and the Ca2+ channels.
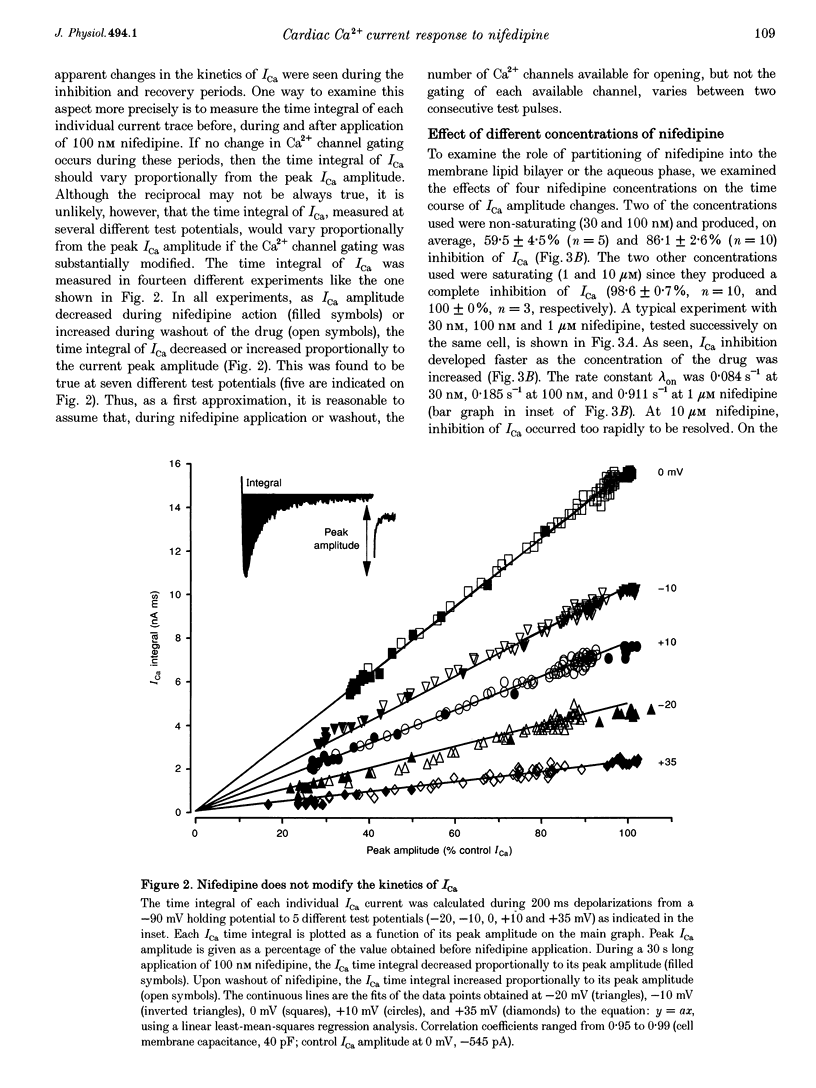
Full text
PDF


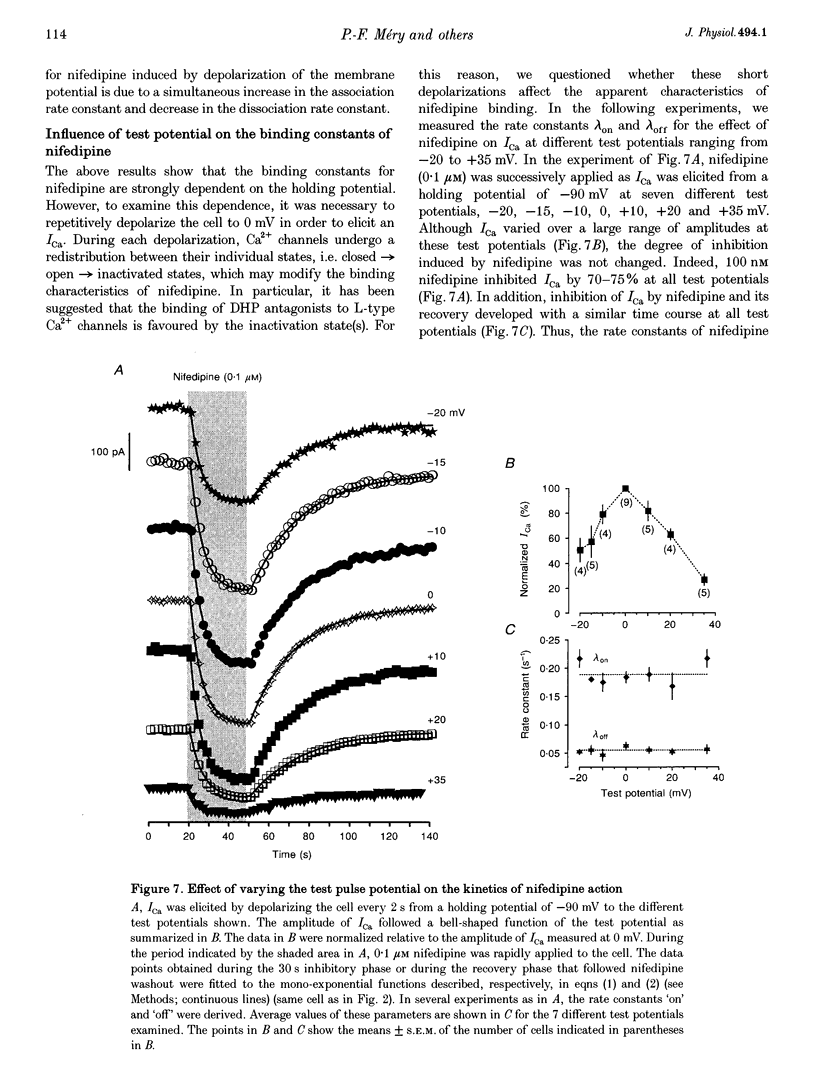
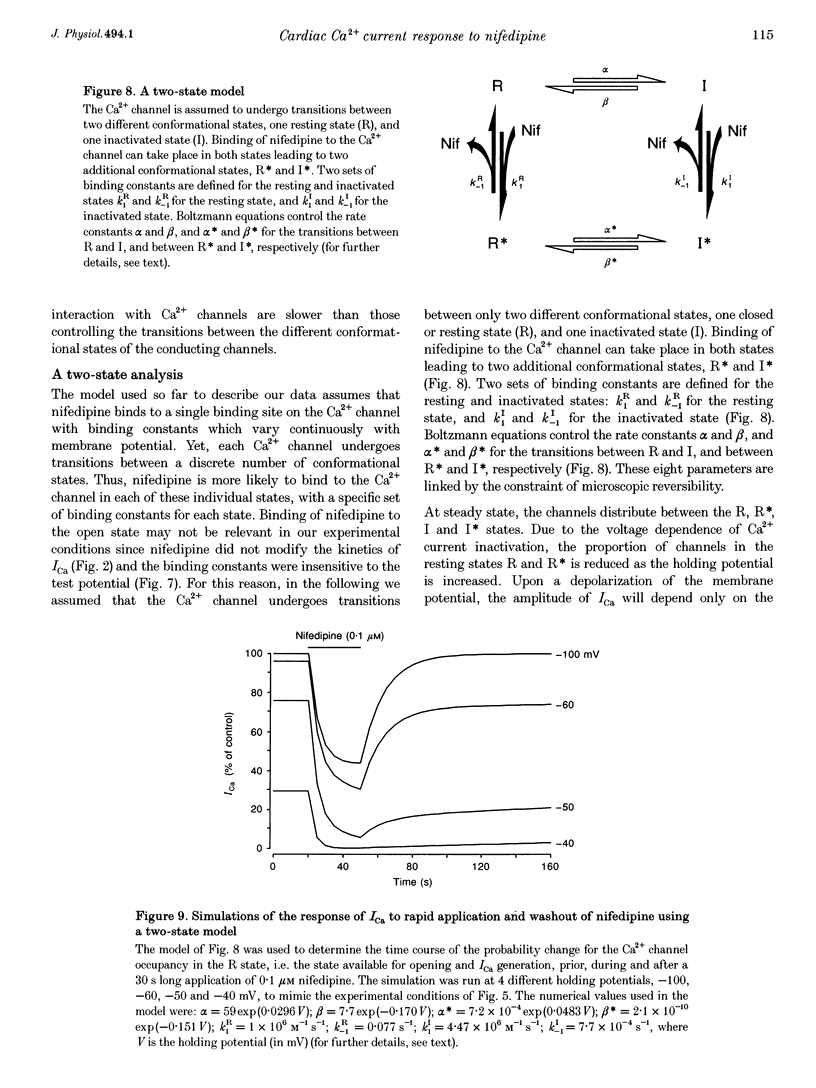





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechem M., Hoffmann H. The molecular mode of action of the Ca agonist (-) BAY K 8644 on the cardiac Ca channel. Pflugers Arch. 1993 Aug;424(3-4):343–353. doi: 10.1007/BF00384362. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Kunze D. L., Yatani A. Dual effects of dihydropyridines on whole cell and unitary calcium currents in single ventricular cells of guinea-pig. J Physiol. 1986 Oct;379:495–514. doi: 10.1113/jphysiol.1986.sp016266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Striessnig J. Receptor sites for Ca2+ channel antagonists. Trends Pharmacol Sci. 1992 Jun;13(6):256–262. doi: 10.1016/0165-6147(92)90079-l. [DOI] [PubMed] [Google Scholar]
- Cognard C., Romey G., Galizzi J. P., Fosset M., Lazdunski M. Dihydropyridine-sensitive Ca2+ channels in mammalian skeletal muscle cells in culture: electrophysiological properties and interactions with Ca2+ channel activator (Bay K8644) and inhibitor (PN 200-110). Proc Natl Acad Sci U S A. 1986 Mar;83(5):1518–1522. doi: 10.1073/pnas.83.5.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J Physiol. 1986 Jul;376:183–202. doi: 10.1113/jphysiol.1986.sp016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A. M., Nerbonne J. M., Lester H. A. Photoinduced removal of nifedipine reveals mechanisms of calcium antagonist action on single heart cells. J Gen Physiol. 1985 Sep;86(3):353–379. doi: 10.1085/jgp.86.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton S. L., Yatani A., Brush K., Schwartz A., Brown A. M. A comparison between the binding and electrophysiological effects of dihydropyridines on cardiac membranes. Mol Pharmacol. 1987 Mar;31(3):221–231. [PubMed] [Google Scholar]
- Herbette L. G., Rhodes D. G., Mason R. P. New approaches to drug design and delivery based on drug-membrane interactions. Drug Des Deliv. 1991 Apr;7(2):75–118. [PubMed] [Google Scholar]
- Herbette L. G., Vant Erve Y. M., Rhodes D. G. Interaction of 1,4 dihydropyridine calcium channel antagonists with biological membranes: lipid bilayer partitioning could occur before drug binding to receptors. J Mol Cell Cardiol. 1989 Feb;21(2):187–201. doi: 10.1016/0022-2828(89)90861-4. [DOI] [PubMed] [Google Scholar]
- Hering S., Beech D. J., Bolton T. B., Lim S. P. Action of nifedipine or BAY K8644 is dependent on calcium channel state in single smooth muscle cells from rabbit ear artery. Pflugers Arch. 1988 May;411(5):590–592. doi: 10.1007/BF00582383. [DOI] [PubMed] [Google Scholar]
- Hering S., Hughes A. D., Timin E. N., Bolton T. B. Modulation of calcium channels in arterial smooth muscle cells by dihydropyridine enantiomers. J Gen Physiol. 1993 Mar;101(3):393–410. doi: 10.1085/jgp.101.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S., Lüllmann H., Sieg H. Frequency- and potential-dependency of the negative inotropic action of various dihydropyridine and non-dihydropyridine calcium antagonists. Pharmacol Toxicol. 1992 Sep;71(3 Pt 1):229–235. doi: 10.1111/j.1600-0773.1992.tb00552.x. [DOI] [PubMed] [Google Scholar]
- Hughes A. D., Wijetunge S. The action of amlodipine on voltage-operated calcium channels in vascular smooth muscle. Br J Pharmacol. 1993 May;109(1):120–125. doi: 10.1111/j.1476-5381.1993.tb13540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalasz H., Watanabe T., Yabana H., Itagaki K., Naito K., Nakayama H., Schwartz A., Vaghy P. L. Identification of 1,4-dihydropyridine binding domains within the primary structure of the alpha 1 subunit of the skeletal muscle L-type calcium channel. FEBS Lett. 1993 Sep 27;331(1-2):177–181. doi: 10.1016/0014-5793(93)80321-k. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Arena J. P., Chin S. Block of L-type calcium channels by charged dihydropyridines. Sensitivity to side of application and calcium. J Gen Physiol. 1991 Jul;98(1):63–75. doi: 10.1085/jgp.98.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Fleckenstein A. Inhibition of the slow inward current by nifedipine in mammalian ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1977 Jul;298(3):267–272. doi: 10.1007/BF00500899. [DOI] [PubMed] [Google Scholar]
- Kokubun S., Prod'hom B., Becker C., Porzig H., Reuter H. Studies on Ca channels in intact cardiac cells: voltage-dependent effects and cooperative interactions of dihydropyridine enantiomers. Mol Pharmacol. 1986 Dec;30(6):571–584. [PubMed] [Google Scholar]
- Kunze D. L., Hamilton S. L., Hawkes M. J., Brown A. M. Dihydropyridine binding and calcium channel function in clonal rat adrenal medullary tumor cells. Mol Pharmacol. 1987 Apr;31(4):401–409. [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Lee R. T., Smith T. W., Marsh J. D. Evidence for distinct calcium channel agonist and antagonist binding sites in intact cultured embryonic chick ventricular cells. Circ Res. 1987 May;60(5):683–691. doi: 10.1161/01.res.60.5.683. [DOI] [PubMed] [Google Scholar]
- Liu J., Bangalore R., Rutledge A., Triggle D. J. Modulation of L-type Ca2+ channels in clonal rat pituitary cells by membrane depolarization. Mol Pharmacol. 1994 Jun;45(6):1198–1206. [PubMed] [Google Scholar]
- Marsh J. D., Loh E., Lachance D., Barry W. H., Smith T. W. Relationship of binding of a calcium channel blocker to inhibition of contraction in intact cultured embryonic chick ventricular cells. Circ Res. 1983 Oct;53(4):539–543. doi: 10.1161/01.res.53.4.539. [DOI] [PubMed] [Google Scholar]
- Mason R. P., Campbell S. F., Wang S. D., Herbette L. G. Comparison of location and binding for the positively charged 1,4-dihydropyridine calcium channel antagonist amlodipine with uncharged drugs of this class in cardiac membranes. Mol Pharmacol. 1989 Oct;36(4):634–640. [PubMed] [Google Scholar]
- Mironneau J., Yamamoto T., Sayet I., Arnaudeau S., Rakotoarisoa L., Mironneau C. Effect of dihydropyridines on calcium channels in isolated smooth muscle cells from rat vena cava. Br J Pharmacol. 1992 Feb;105(2):321–328. doi: 10.1111/j.1476-5381.1992.tb14253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K., Miyagi Y., Araie E., Sasayama S. Effects of diltiazem and verapamil on (+)-PN 200-110 binding kinetics in dog cardiac membranes. Eur J Pharmacol. 1992 Apr 22;214(2-3):127–132. doi: 10.1016/0014-2999(92)90109-h. [DOI] [PubMed] [Google Scholar]
- Méry P. F., Lechêne P., Fischmeister R. A loudspeaker-driven system for rapid and multiple solution exchanges in patch-clamp experiments. Pflugers Arch. 1992 Apr;420(5-6):529–535. doi: 10.1007/BF00374629. [DOI] [PubMed] [Google Scholar]
- Porzig H. Pharmacological modulation of voltage-dependent calcium channels in intact cells. Rev Physiol Biochem Pharmacol. 1990;114:209–262. doi: 10.1007/BFb0031020. [DOI] [PubMed] [Google Scholar]
- Salomone S., Godfraind T. Radioligand and functional estimates of the interaction of the 1,4-dihydropyridines, isradipine and lacidipine, with calcium channels in smooth muscle. Br J Pharmacol. 1993 May;109(1):100–106. doi: 10.1111/j.1476-5381.1993.tb13537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M. C., Kass R. S. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res. 1984 Sep;55(3):336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- Sarmiento J. G., Janis R. A., Colvin R. A., Triggle D. J., Katz A. M. Binding of the calcium channel blocker nitrendipine to its receptor in purified sarcolemma from canine cardiac ventricle. J Mol Cell Cardiol. 1983 Feb;15(2):135–137. doi: 10.1016/0022-2828(83)90289-4. [DOI] [PubMed] [Google Scholar]
- Strübing C., Hering S., Glossmann H. Evidence for an external location of the dihydropyridine agonist receptor site on smooth muscle and skeletal muscle calcium channels. Br J Pharmacol. 1993 Apr;108(4):884–891. doi: 10.1111/j.1476-5381.1993.tb13482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Yatani A., Bahinski A., Mori Y., Schwartz A. Molecular localization of regions in the L-type calcium channel critical for dihydropyridine action. Neuron. 1993 Dec;11(6):1013–1021. doi: 10.1016/0896-6273(93)90215-d. [DOI] [PubMed] [Google Scholar]
- Uehara A., Hume J. R. Interactions of organic calcium channel antagonists with calcium channels in single frog atrial cells. J Gen Physiol. 1985 May;85(5):621–647. doi: 10.1085/jgp.85.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X. Y., Rutledge A., Triggle D. J. Voltage-dependent binding of 1,4-dihydropyridine Ca2+ channel antagonists and activators in cultured neonatal rat ventricular myocytes. Mol Pharmacol. 1989 Apr;35(4):541–552. [PubMed] [Google Scholar]
- Welling A., Kwan Y. W., Bosse E., Flockerzi V., Hofmann F., Kass R. S. Subunit-dependent modulation of recombinant L-type calcium channels. Molecular basis for dihydropyridine tissue selectivity. Circ Res. 1993 Nov;73(5):974–980. doi: 10.1161/01.res.73.5.974. [DOI] [PubMed] [Google Scholar]
- Yaney G. C., Stafford G. A., Henstenberg J. D., Sharp G. W., Weiland G. A. Binding of the dihydropyridine calcium channel blocker (+)-[3H] isopropyl-4-(2,1,3-benzoxadiazol-4-yl)-1,4-dihydro-5-methoxycarbonyl-2, 6-dimethyl-3-pyridinecarboxylate (PN200-110) to RINm5F membranes and cells: characterization and functional significance. J Pharmacol Exp Ther. 1991 Aug;258(2):652–662. [PubMed] [Google Scholar]
- Yatani A., Hamilton S. L., Brown A. M. Diphenylhydantoin blocks cardiac calcium channels and binds to the dihydropyridine receptor. Circ Res. 1986 Sep;59(3):356–361. doi: 10.1161/01.res.59.3.356. [DOI] [PubMed] [Google Scholar]