Abstract
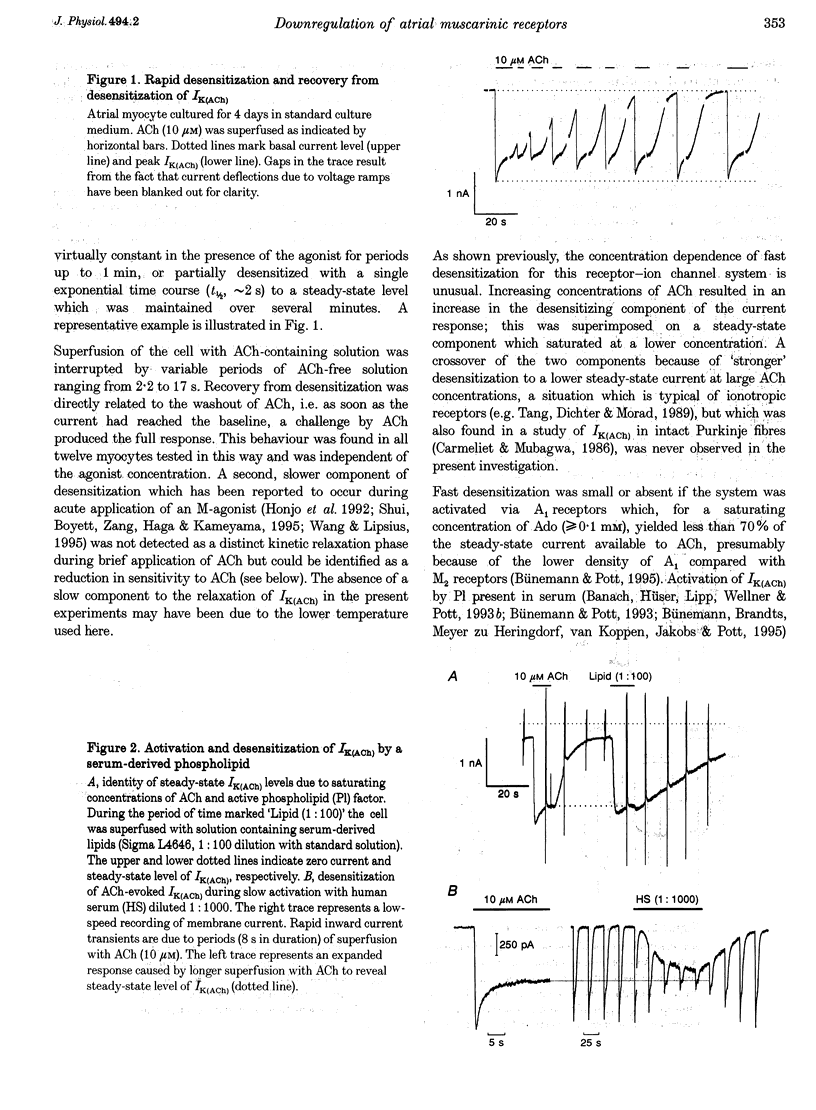
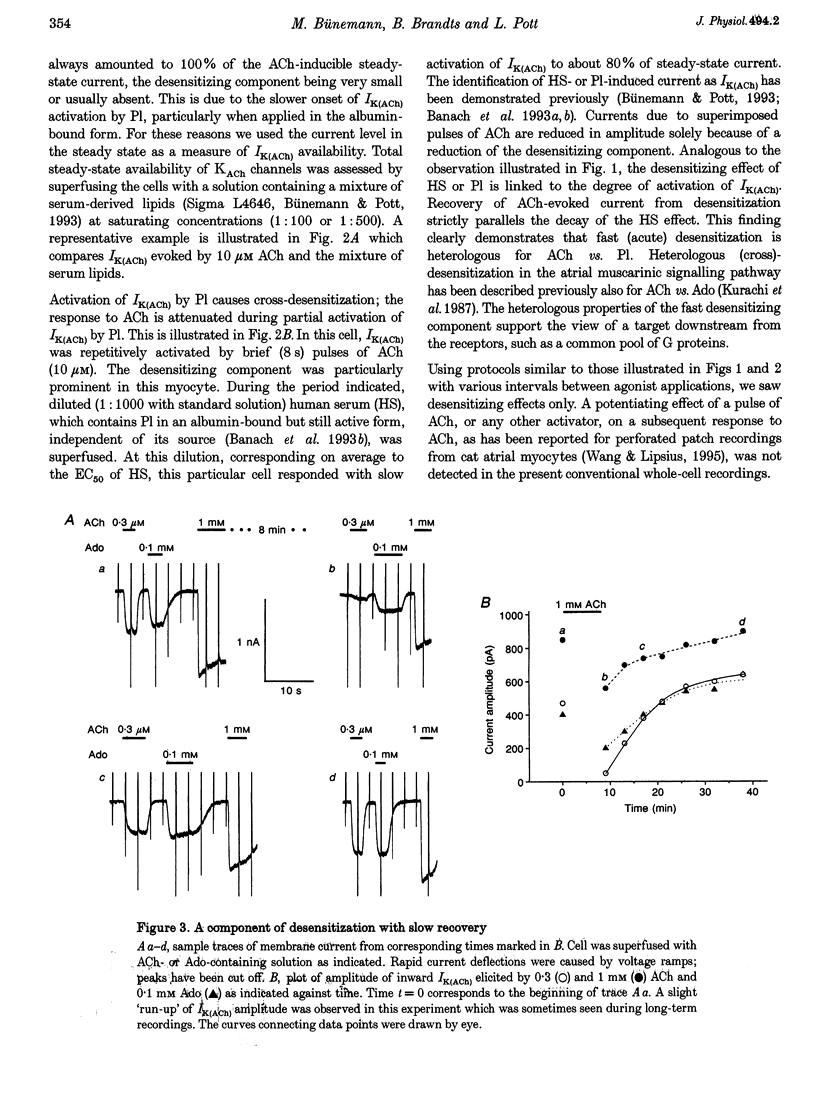
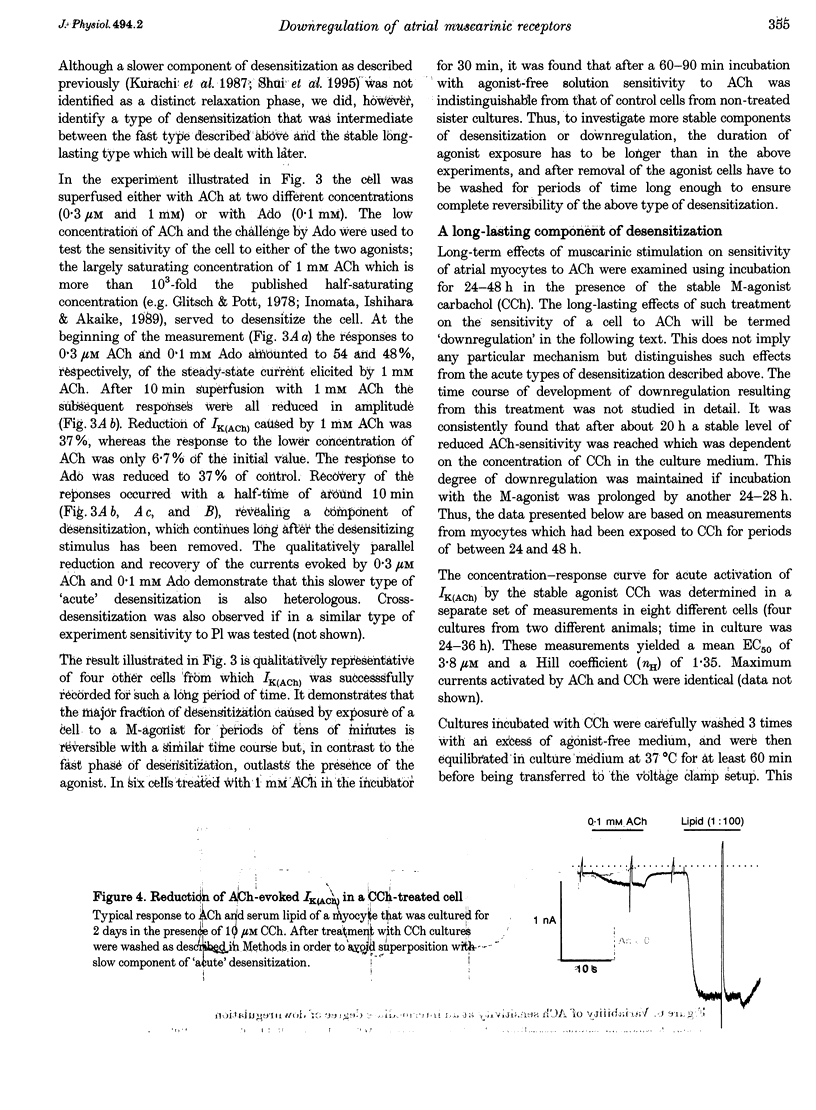
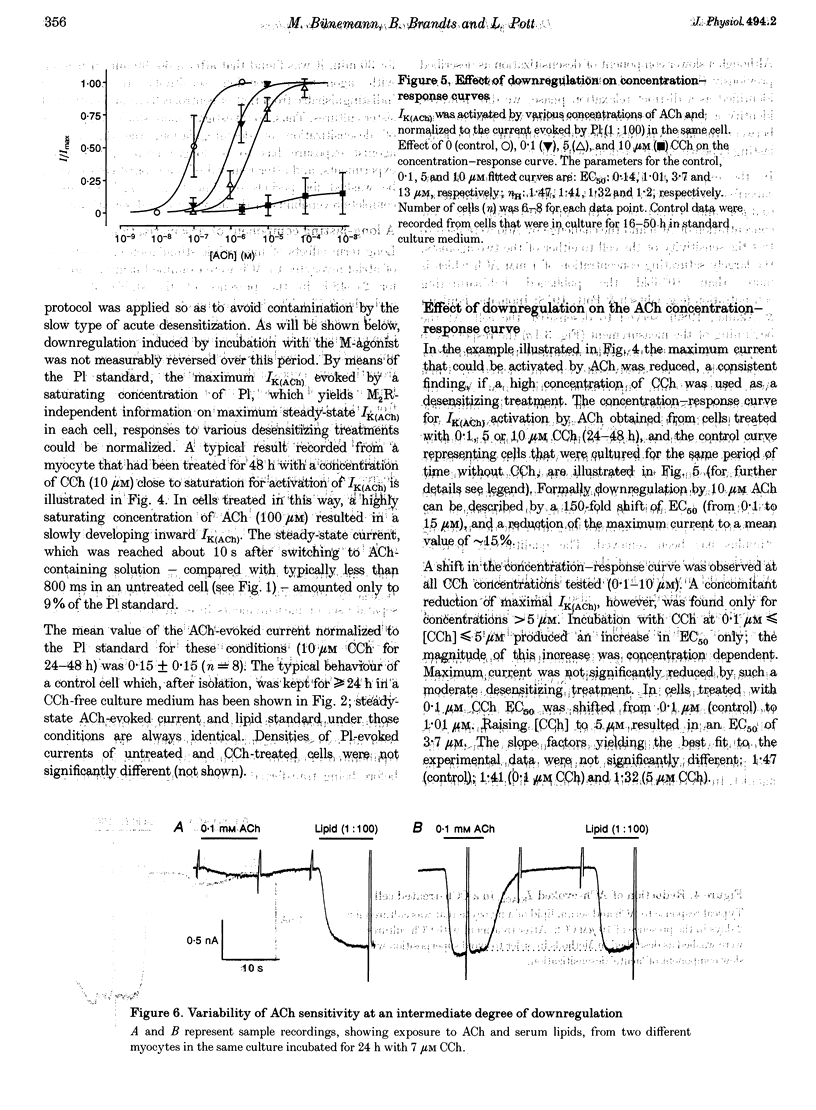
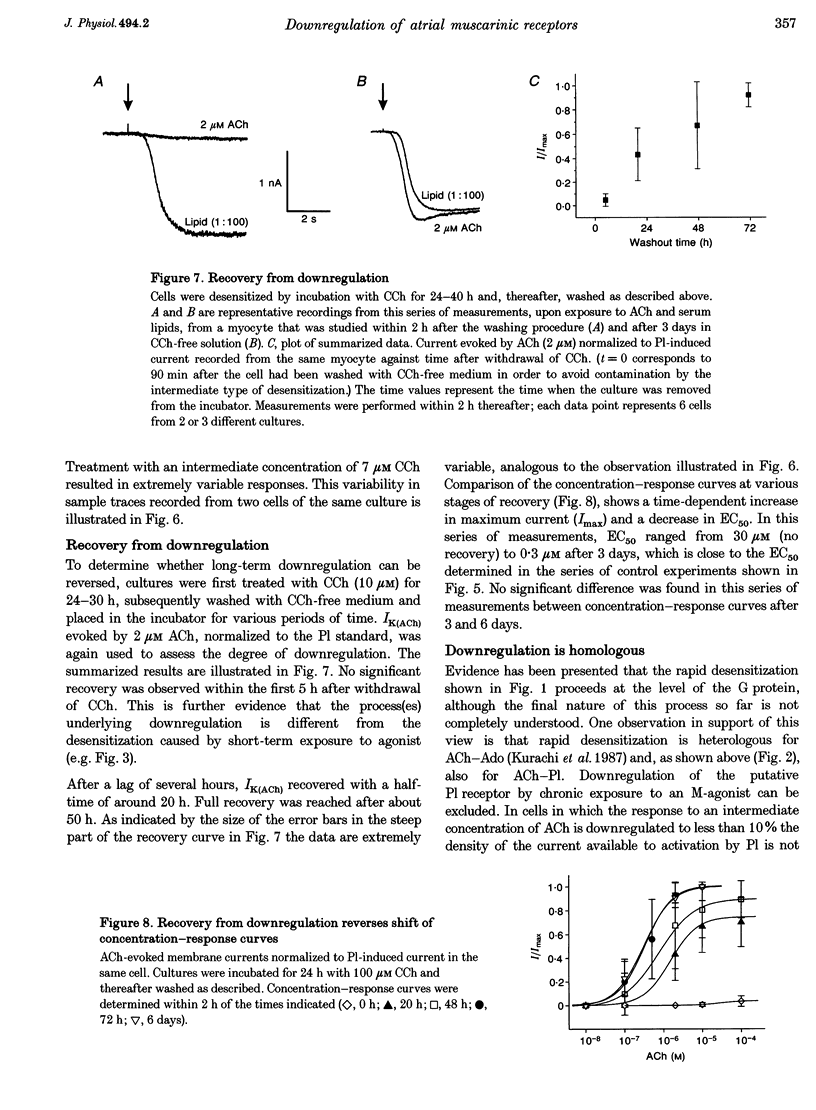
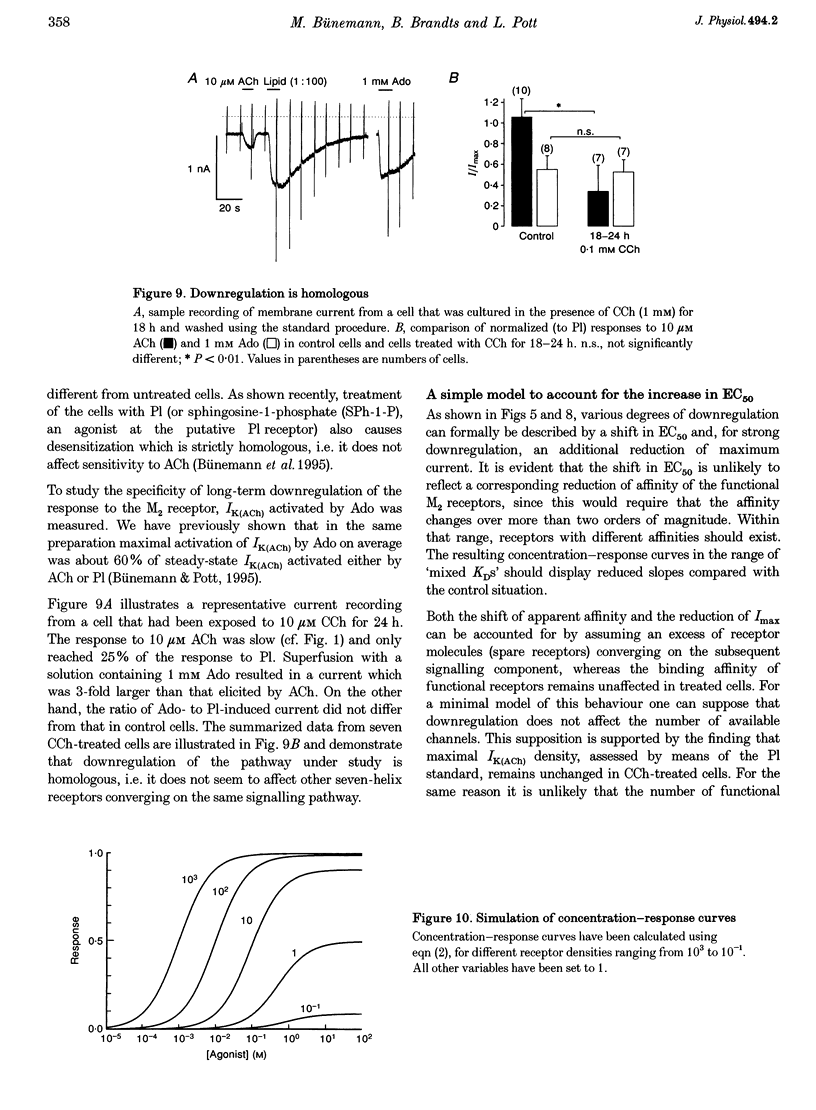
1. Desensitization of muscarinic K+ current (IK(ACh)) was studied in cultured atrial myocytes from guinea-pig hearts using whole-cell voltage clamp. 2. Three different types of desensitization could be identified. A fast component which upon rapid superfusion with ACh resulted in a partial relaxation of IK(ACh) within a few seconds to a plateau which was maintained in the presence of ACh. Recovery from this type of desensitization paralleled the decay of IK(ACh) after washout of the agonist. A second type of desensitization was observed within minutes. This was reversed around 10 min after washout of ACh. Both types were heterologous with regard to the A1 receptor and the novel phospholipid (Pl) receptor, both of which activate IK(ACh) via the same signalling pathway. 3. A third type of desensitization (downregulation) occurred upon exposure of the cultures for 24-48 h to the muscarinic agonist carbachol (CCh). The level of downregulation depended on the concentration of CCh (0.1 microM < or = [CCh] < or = 10 microM). No recovery was observed within 5 h after washout of CCh. Thereafter sensitivity to ACh slowly returned (half-time (t1/2), approximately 20 h). 4. Downregulation by CCh (0.1-5 microM) was characterized by an increase in EC50 for ACh with no reduction in maximum IK(ACh). With 5 microM CCh, EC50 was increased from 0.1 to 3.7 microM. At 10 microM CCh EC50 was increased to 15 microM and maximal current that could be evoked by ACh was reduced to 15%. 5. Downregulation by CCh was homologous with regard to A1 and Pl receptors. Maximum IK(ACh), assayed by a saturating concentration of Pl, was not reduced in downregulated cells, suggesting a mechanism localized at the M2 receptor. 6. The changes in the concentration-response curves can be accounted for by assuming an excess of M2 receptors relative to the subsequent component of the signalling pathway. 7. As the intact heart is under tonic vagal control, downregulation is likely to contribute to controlling the sensitivity of the heart to vagal activity in situ.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banach K., Bünemann M., Hüser J., Pott L. Serum contains a potent factor that decreases beta-adrenergic receptor-stimulated L-type Ca2+ current in cardiac myocytes. Pflugers Arch. 1993 May;423(3-4):245–250. doi: 10.1007/BF00374402. [DOI] [PubMed] [Google Scholar]
- Banach K., Hüser J., Lipp P., Wellner M. C., Pott L. Activation of muscarinic K+ current in guinea-pig atrial myocytes by a serum factor. J Physiol. 1993 Feb;461:263–281. doi: 10.1113/jphysiol.1993.sp019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünemann M., Brandts B., zu Heringdorf D. M., van Koppen C. J., Jakobs K. H., Pott L. Activation of muscarinic K+ current in guinea-pig atrial myocytes by sphingosine-1-phosphate. J Physiol. 1995 Dec 15;489(Pt 3):701–707. doi: 10.1113/jphysiol.1995.sp021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünemann M., Pott L. Down-regulation of A1 adenosine receptors coupled to muscarinic K+ current in cultured guinea-pig atrial myocytes. J Physiol. 1995 Jan 1;482(Pt 1):81–92. doi: 10.1113/jphysiol.1995.sp020501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünemann M., Pott L. Membrane-delimited activation of muscarinic K current by an albumin-associated factor in guinea-pig atrial myocytes. Pflugers Arch. 1993 Nov;425(3-4):329–334. doi: 10.1007/BF00374183. [DOI] [PubMed] [Google Scholar]
- Carmeliet E., Mubagwa K. Desensitization of the acetylcholine-induced increase of potassium conductance in rabbit cardiac Purkinje fibres. J Physiol. 1986 Feb;371:239–255. doi: 10.1113/jphysiol.1986.sp015971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chern Y., Lai H. L., Fong J. C., Liang Y. Multiple mechanisms for desensitization of A2a adenosine receptor-mediated cAMP elevation in rat pheochromocytoma PC12 cells. Mol Pharmacol. 1993 Nov;44(5):950–958. [PubMed] [Google Scholar]
- Clapham D. E. Direct G protein activation of ion channels? Annu Rev Neurosci. 1994;17:441–464. doi: 10.1146/annurev.ne.17.030194.002301. [DOI] [PubMed] [Google Scholar]
- Glitsch H. G., Pott L. Effects of acetylcholine and parasympathetic nerve stimulation on membrane potential in quiescent guinea-pig atria. J Physiol. 1978 Jun;279:655–668. doi: 10.1113/jphysiol.1978.sp012367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., TRAUTWEIN W. Effect of vagal stimulation on the sinus venosus of the frog's heart. Nature. 1955 Sep 10;176(4480):512–513. doi: 10.1038/176512a0. [DOI] [PubMed] [Google Scholar]
- Habecker B. A., Nathanson N. M. Regulation of muscarinic acetylcholine receptor mRNA expression by activation of homologous and heterologous receptors. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5035–5038. doi: 10.1073/pnas.89.11.5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga T., Haga K., Kameyama K. G protein--coupled receptor kinases. J Neurochem. 1994 Aug;63(2):400–412. doi: 10.1046/j.1471-4159.1994.63020400.x. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Huganir R. L., Greengard P. Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron. 1990 Nov;5(5):555–567. doi: 10.1016/0896-6273(90)90211-w. [DOI] [PubMed] [Google Scholar]
- Inomata N., Ishihara T., Akaike N. Activation kinetics of the acetylcholine-gated potassium current in isolated atrial cells. Am J Physiol. 1989 Oct;257(4 Pt 1):C646–C650. doi: 10.1152/ajpcell.1989.257.4.C646. [DOI] [PubMed] [Google Scholar]
- Kim D. Mechanism of rapid desensitization of muscarinic K+ current in adult rat and guinea pig atrial cells. Circ Res. 1993 Jul;73(1):89–97. doi: 10.1161/01.res.73.1.89. [DOI] [PubMed] [Google Scholar]
- Kofuji P., Davidson N., Lester H. A. Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by G beta gamma subunits and function as heteromultimers. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6542–6546. doi: 10.1073/pnas.92.14.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G., Gordon E. A., Wickman K., Velimirović B., Krapivinsky L., Clapham D. E. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature. 1995 Mar 9;374(6518):135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Kubo Y., Reuveny E., Slesinger P. A., Jan Y. N., Jan L. Y. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 1993 Aug 26;364(6440):802–806. doi: 10.1038/364802a0. [DOI] [PubMed] [Google Scholar]
- Kurachi Y. G protein regulation of cardiac muscarinic potassium channel. Am J Physiol. 1995 Oct;269(4 Pt 1):C821–C830. doi: 10.1152/ajpcell.1995.269.4.C821. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986 Sep;407(3):264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Short-term desensitization of muscarinic K+ channel current in isolated atrial myocytes and possible role of GTP-binding proteins. Pflugers Arch. 1987 Oct;410(3):227–233. doi: 10.1007/BF00580270. [DOI] [PubMed] [Google Scholar]
- Kwatra M. M., Benovic J. L., Caron M. G., Lefkowitz R. J., Hosey M. M. Phosphorylation of chick heart muscarinic cholinergic receptors by the beta-adrenergic receptor kinase. Biochemistry. 1989 May 30;28(11):4543–4547. doi: 10.1021/bi00437a005. [DOI] [PubMed] [Google Scholar]
- Kwatra M. M., Leung E., Maan A. C., McMahon K. K., Ptasienski J., Green R. D., Hosey M. M. Correlation of agonist-induced phosphorylation of chick heart muscarinic receptors with receptor desensitization. J Biol Chem. 1987 Dec 5;262(34):16314–16321. [PubMed] [Google Scholar]
- Moro O., Lameh J., Sadée W. Serine- and threonine-rich domain regulates internalization of muscarinic cholinergic receptors. J Biol Chem. 1993 Apr 5;268(10):6862–6865. [PubMed] [Google Scholar]
- Mullaney I., Dodd M. W., Buckley N., Milligan G. Agonist activation of transfected human M1 muscarinic acetylcholine receptors in CHO cells results in down-regulation of both the receptor and the alpha subunit of the G-protein Gq. Biochem J. 1993 Jan 1;289(Pt 1):125–131. doi: 10.1042/bj2890125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T., Sugimoto T., Kurachi Y. Effects of anions on the G protein-mediated activation of the muscarinic K+ channel in the cardiac atrial cell membrane. Intracellular chloride inhibition of the GTPase activity of GK. J Gen Physiol. 1992 May;99(5):665–682. doi: 10.1085/jgp.99.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveny E., Slesinger P. A., Inglese J., Morales J. M., Iñiguez-Lluhi J. A., Lefkowitz R. J., Bourne H. R., Jan Y. N., Jan L. Y. Activation of the cloned muscarinic potassium channel by G protein beta gamma subunits. Nature. 1994 Jul 14;370(6485):143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- Richardson R. M., Kim C., Benovic J. L., Hosey M. M. Phosphorylation and desensitization of human m2 muscarinic cholinergic receptors by two isoforms of the beta-adrenergic receptor kinase. J Biol Chem. 1993 Jun 25;268(18):13650–13656. [PubMed] [Google Scholar]
- Shui Z., Boyett M. R., Zang W. J., Haga T., Kameyama K. Receptor kinase-dependent desensitization of the muscarinic K+ current in rat atrial cells. J Physiol. 1995 Sep 1;487(Pt 2):359–366. doi: 10.1113/jphysiol.1995.sp020885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. M., Dichter M., Morad M. Quisqualate activates a rapidly inactivating high conductance ionic channel in hippocampal neurons. Science. 1989 Mar 17;243(4897):1474–1477. doi: 10.1126/science.2467378. [DOI] [PubMed] [Google Scholar]
- Wang Y. G., Lipsius S. L. Acetylcholine potentiates acetylcholine-induced increases in K+ current in cat atrial myocytes. Am J Physiol. 1995 Mar;268(3 Pt 2):H1313–H1321. doi: 10.1152/ajpheart.1995.268.3.H1313. [DOI] [PubMed] [Google Scholar]
- Wei H. B., Yamamura H. I., Roeske W. R. Down-regulation and desensitization of the muscarinic M1 and M2 receptors in transfected fibroblast B82 cells. Eur J Pharmacol. 1994 Aug 16;268(3):381–391. doi: 10.1016/0922-4106(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Zang W. J., Yu X. J., Honjo H., Kirby M. S., Boyett M. R. On the role of G protein activation and phosphorylation in desensitization to acetylcholine in guinea-pig atrial cells. J Physiol. 1993 May;464:649–679. doi: 10.1113/jphysiol.1993.sp019656. [DOI] [PMC free article] [PubMed] [Google Scholar]


