Abstract
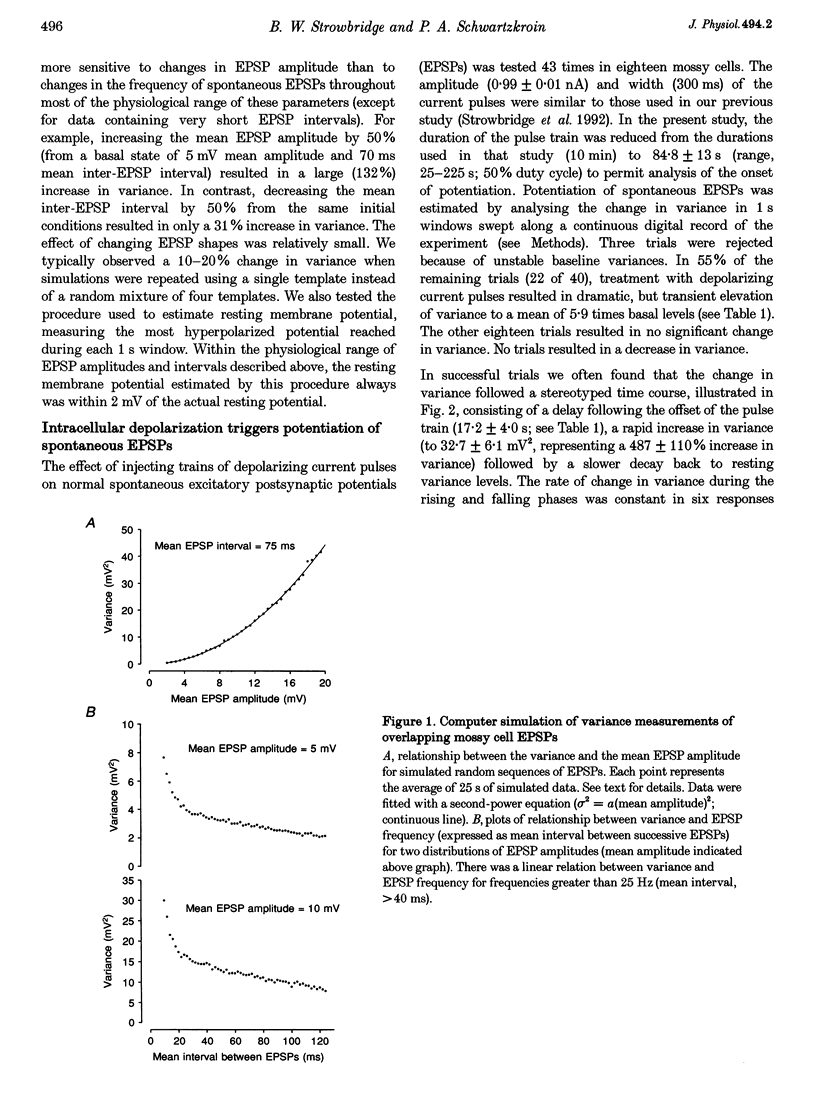
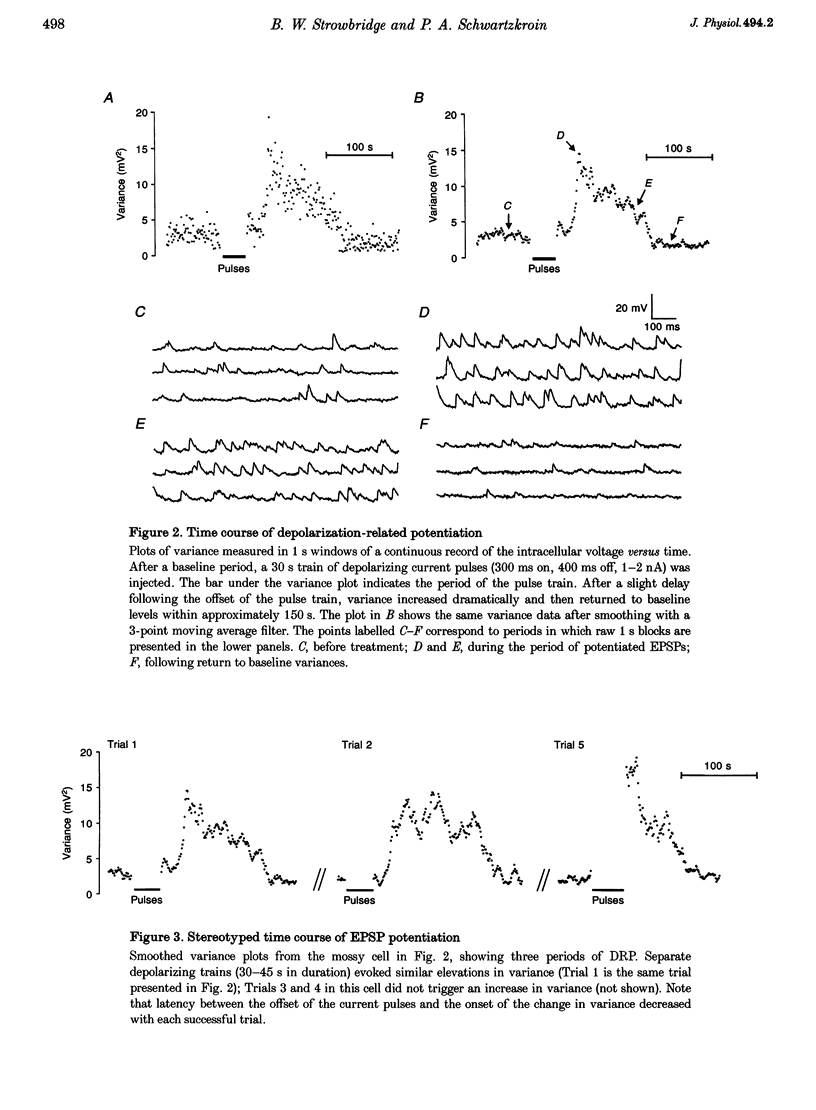
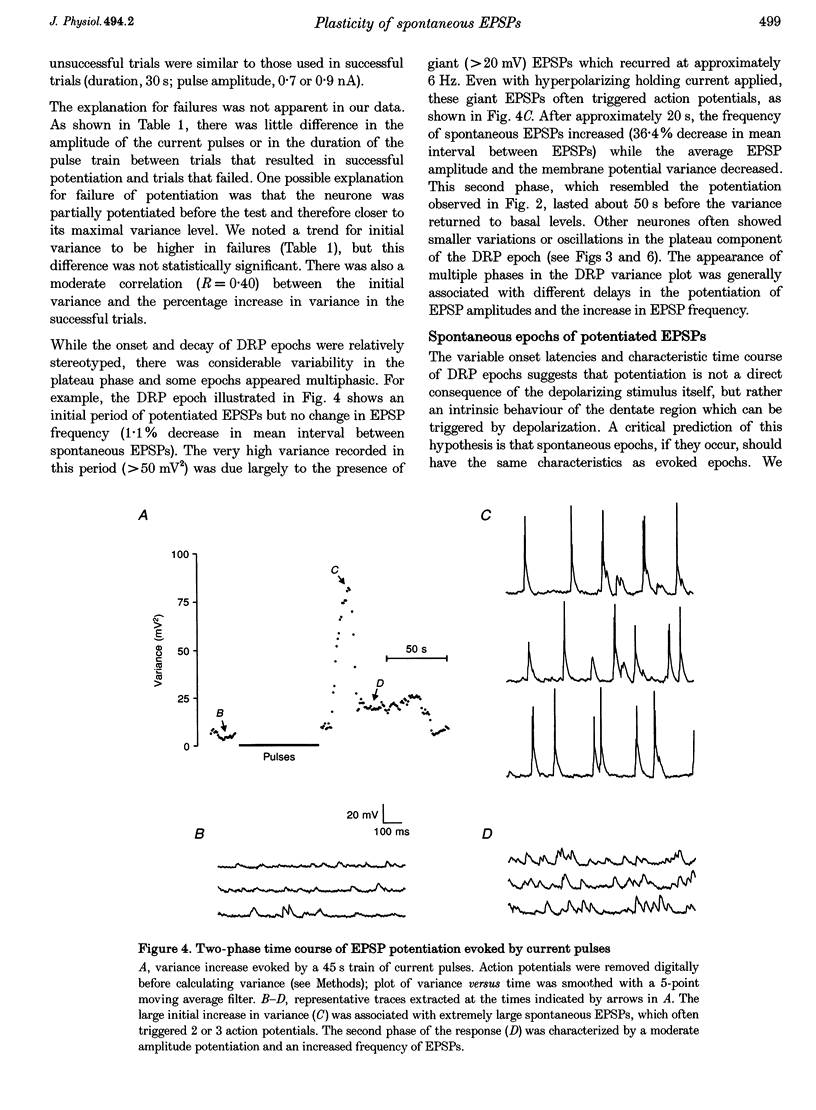
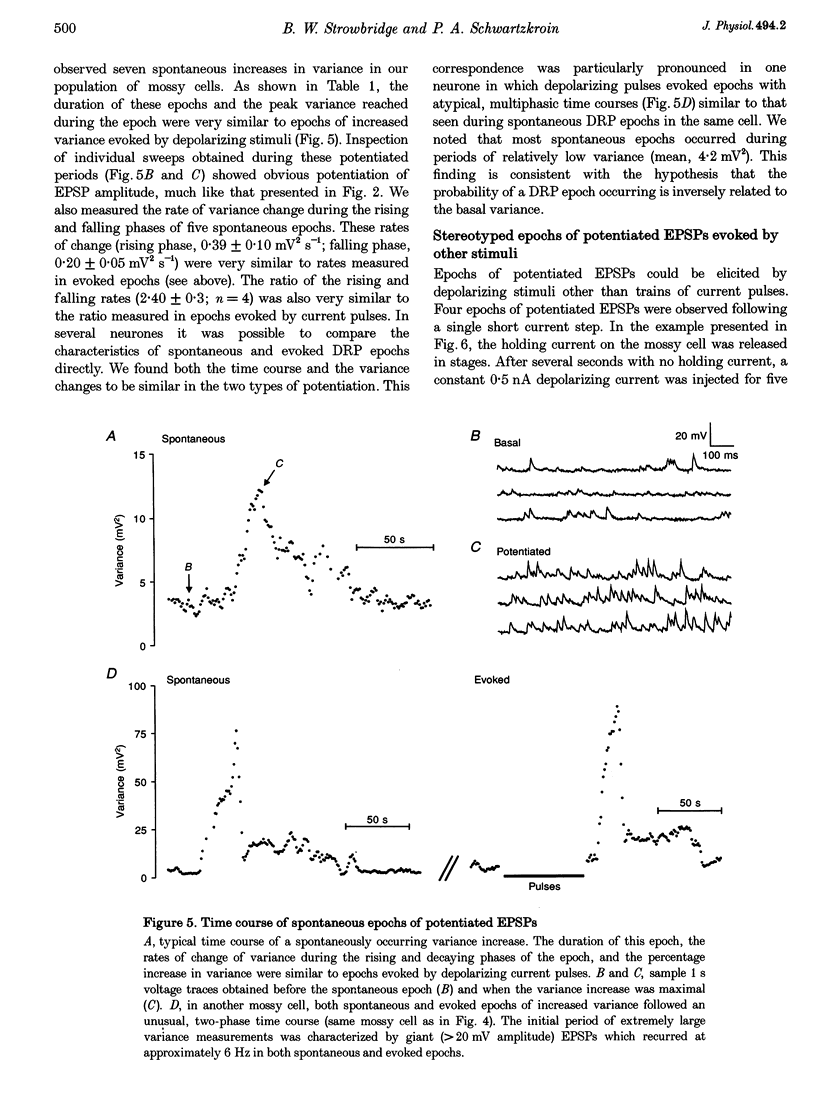
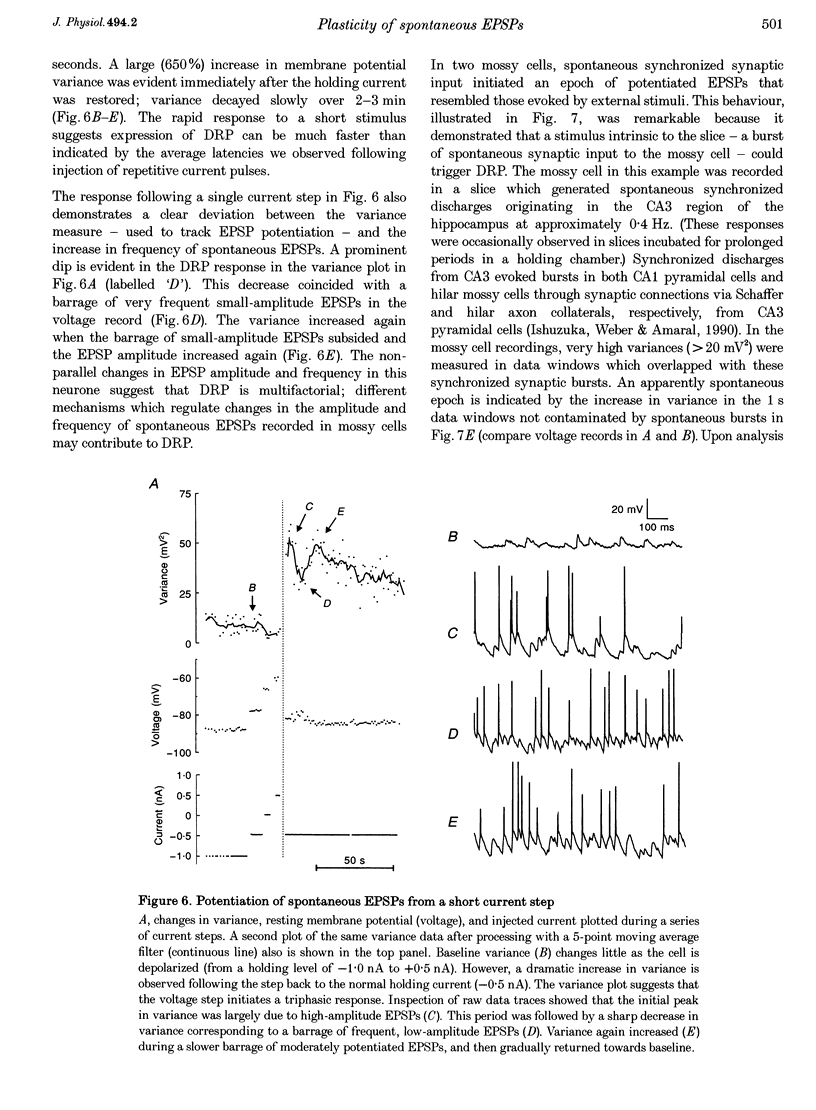
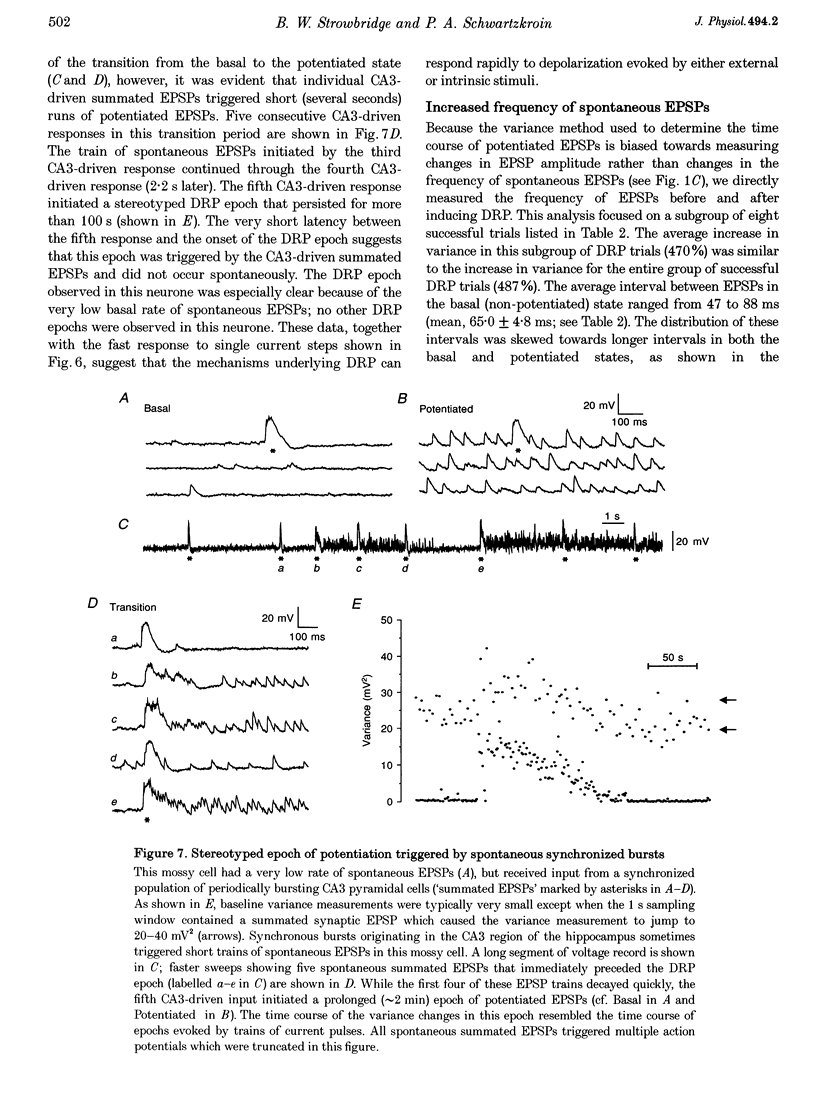
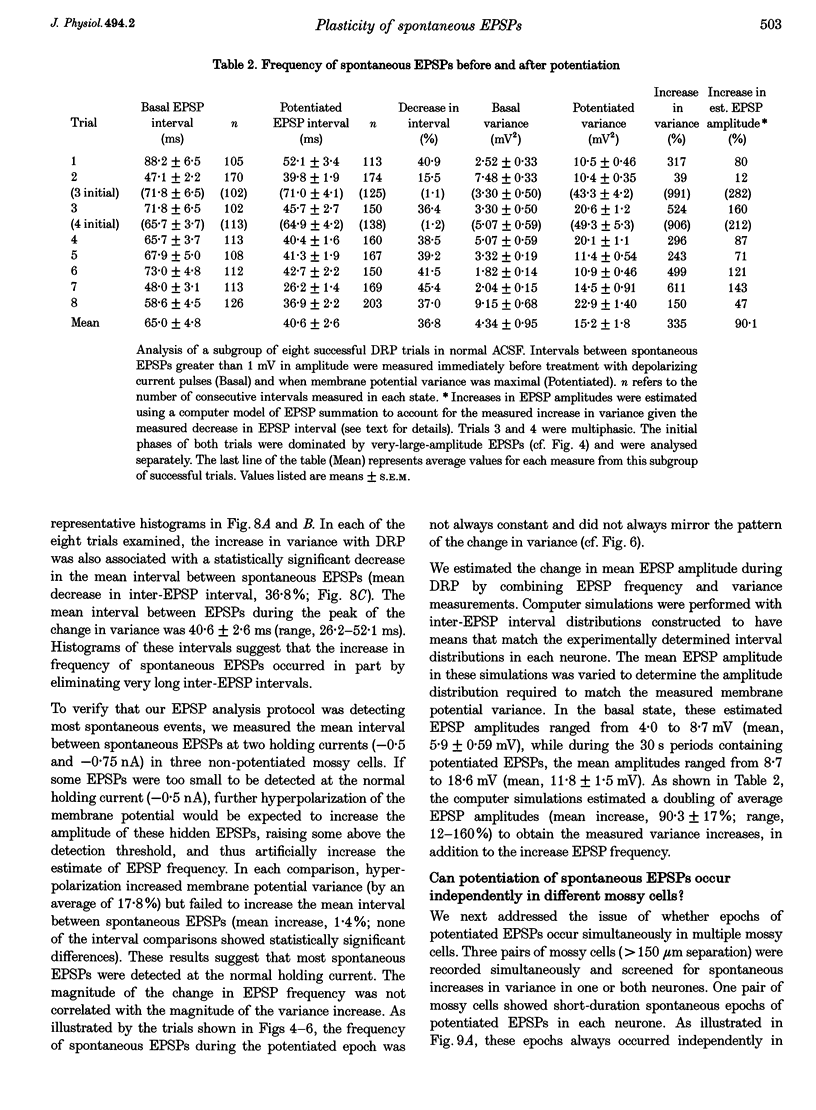
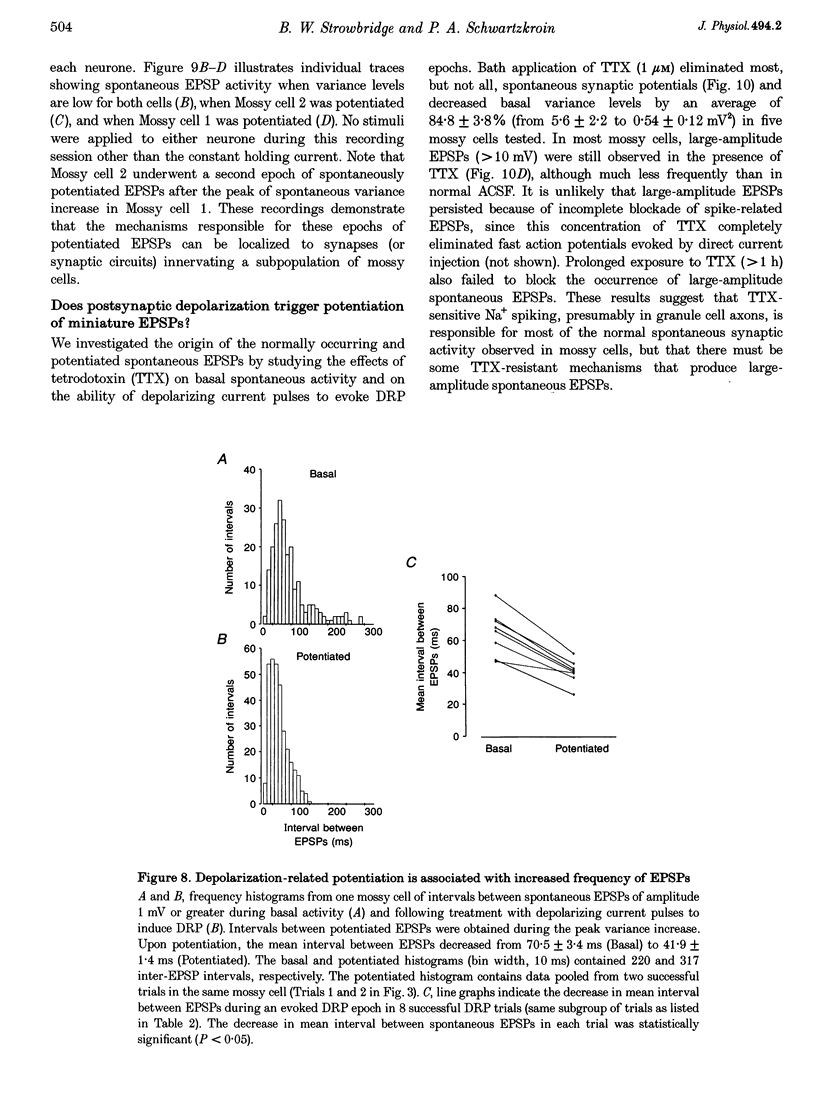
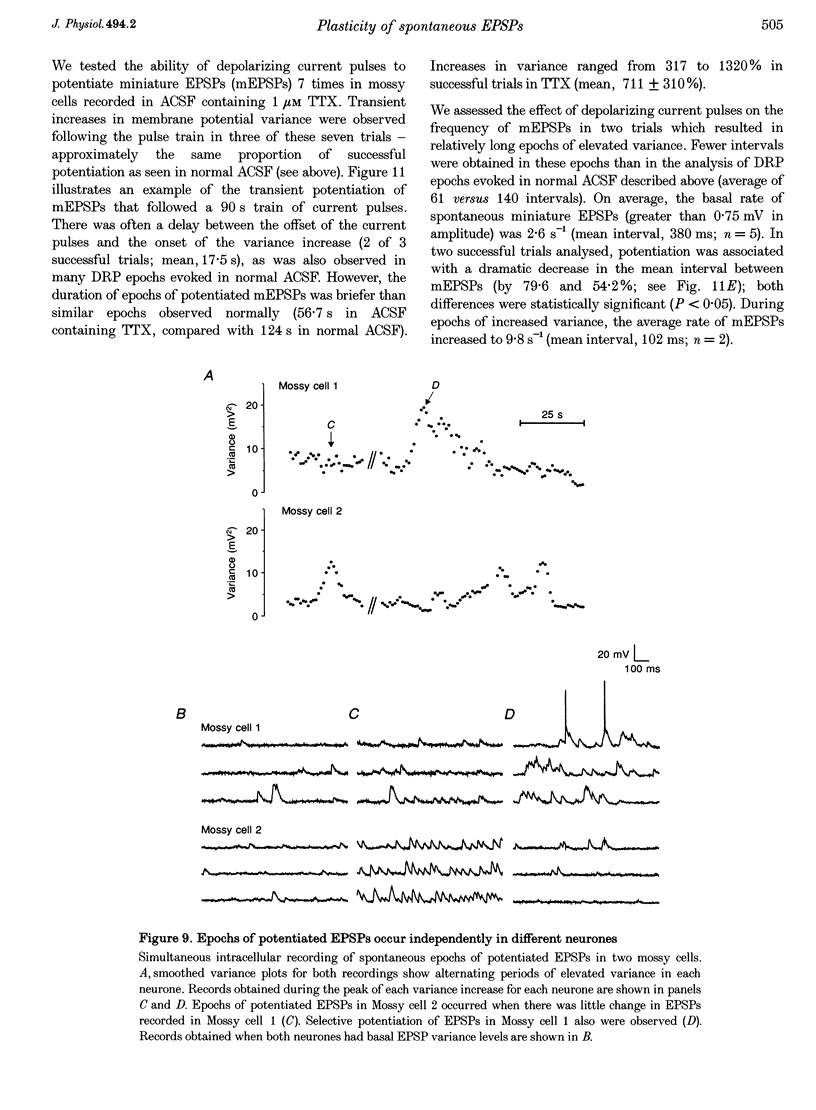
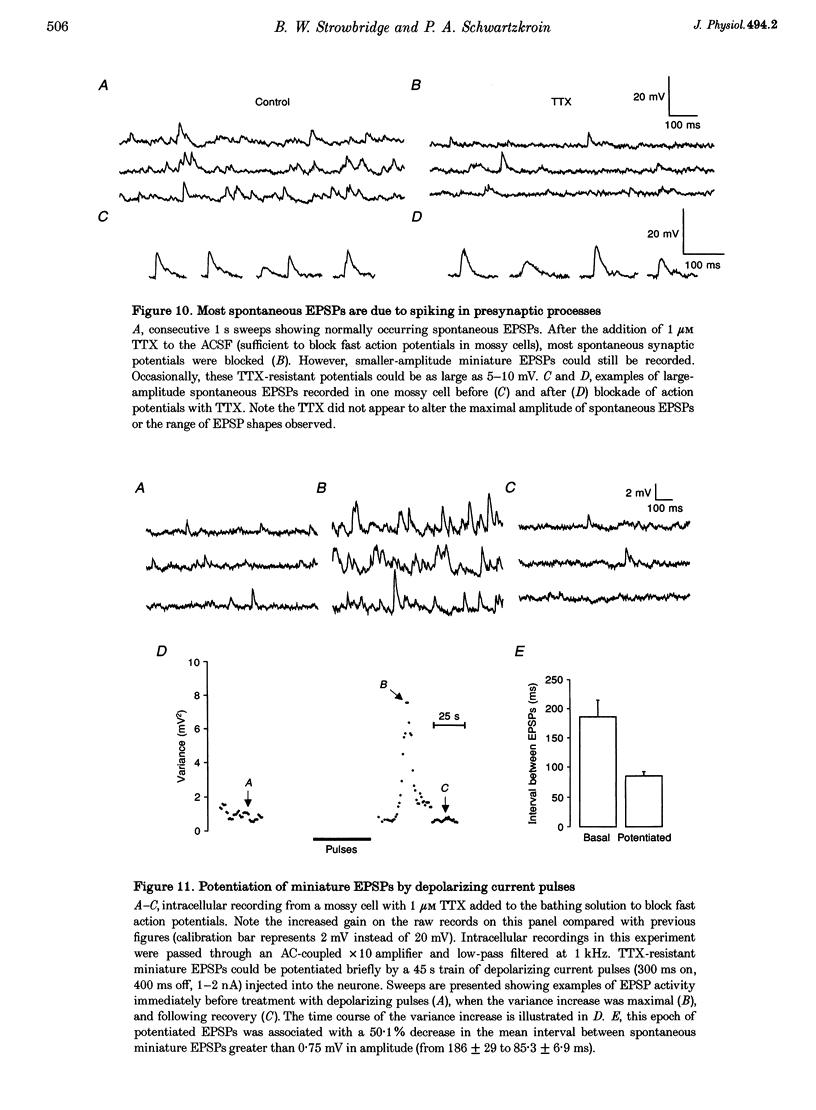
1. The amplitude and frequency of spontaneously occurring EPSPs recorded intracellularly in rat mossy cells was estimated by measuring membrane potential variance in short segments of a continuous voltage record. Changes in variance reflected changes in the amplitude and/or the frequency of spontaneous EPSPs. 2. Short trains of depolarizing current pulses evoked a delayed increase in membrane potential variance in 55% of trials. Variance increased by 487% during these responses and remained elevated for 124 +/- 16 s. Increases in variance were not associated with large changes in the intrinsic properties of the mossy cell such as resting membrane potential and input resistance. We termed this phenomenon depolarization-related potentiation (DRP). 3. Epochs of elevated variance were associated with an increase in both the average amplitude and frequency of spontaneous EPSPs. During the peak of the response, the mean interval between spontaneous EPSPs decreased by 36.8%. Computer-generated voltage records with randomly distributed EPSP amplitudes and inter-EPSP intervals suggested that this decrease in inter-EPSP intervals was not sufficient to account for the magnitude of the variance increase observed. Based on this model, we estimated that a 90% increase in the average amplitude of spontaneous EPSPs, in addition to the experimentally measured decrease in the average inter-EPSP interval, was required to reproduce the magnitude of the change in variance observed. In the potentiated state, the amplitude of spontaneous EPSPs often exceeded 10 mV. 4. We also observed epochs of increased variance that occurred spontaneously. These spontaneous epochs closely resembled epochs evoked by depolarizing stimuli, suggesting that the stimulus was acting as a trigger for a spontaneously occurring behaviour. Additional evidence supporting this hypothesis was provided by the observation that stereotyped patterns of increased variance could be evoked by brief stimuli, such as a single 5 s depolarizing step. Dual intracellular recordings from two mossy cells demonstrated that spontaneous epochs of increased variance occurred independently in different neurones. This result makes it unlikely that these variance increases were due to a global change in the slice environment such as a propagating wave of potassium ions. 5. Bath application of the Na+ channel blocker TTX eliminated most, but not all, of the normal on-going spontaneous EPSPs in mossy cells. Treatment with depolarizing current pulses was effective in potentiating TTX-resistant spontaneous EPSPs in three of seven trials. Potentiation also decreased the mean interval between TTX-resistant miniature EPSPs (by an average of 66.9%) in two trials examined. 6. These results suggest that DRP results from the activation of an intrinsic phenomenon within the dentate gyrus by strong depolarization of a single mossy cell. Our data suggest that several mechanisms are involved in the expression of DRP since changes in EPSP amplitude and frequency can occur with varying delays from the stimulus. The ability of depolarizing current pulses to potentiate TTX-resistant miniature EPSPs suggests that at least one component of this plasticity occurs at the granule cell-mossy cell synapse.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaral D. G. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978 Dec 15;182(4 Pt 2):851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- Buckmaster P. S., Schwartzkroin P. A. Interneurons and inhibition in the dentate gyrus of the rat in vivo. J Neurosci. 1995 Jan;15(1 Pt 2):774–789. doi: 10.1523/JNEUROSCI.15-01-00774.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster P. S., Strowbridge B. W., Kunkel D. D., Schmiege D. L., Schwartzkroin P. A. Mossy cell axonal projections to the dentate gyrus molecular layer in the rat hippocampal slice. Hippocampus. 1992 Oct;2(4):349–362. doi: 10.1002/hipo.450020403. [DOI] [PubMed] [Google Scholar]
- Buckmaster P. S., Strowbridge B. W., Schwartzkroin P. A. A comparison of rat hippocampal mossy cells and CA3c pyramidal cells. J Neurophysiol. 1993 Oct;70(4):1281–1299. doi: 10.1152/jn.1993.70.4.1281. [DOI] [PubMed] [Google Scholar]
- Claiborne B. J., Xiang Z., Brown T. H. Hippocampal circuitry complicates analysis of long-term potentiation in mossy fiber synapses. Hippocampus. 1993 Apr;3(2):115–121. doi: 10.1002/hipo.450030202. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke R. A., Prince D. A. Electrophysiology of dentate gyrus granule cells. J Neurophysiol. 1984 Feb;51(2):195–209. doi: 10.1152/jn.1984.51.2.195. [DOI] [PubMed] [Google Scholar]
- Frotscher M., Misgeld U., Nitsch C. Ultrastructure of mossy fiber endings in in vitro hippocampal slices. Exp Brain Res. 1981;41(3-4):247–255. doi: 10.1007/BF00238881. [DOI] [PubMed] [Google Scholar]
- Frotscher M., Seress L., Schwerdtfeger W. K., Buhl E. The mossy cells of the fascia dentata: a comparative study of their fine structure and synaptic connections in rodents and primates. J Comp Neurol. 1991 Oct 1;312(1):145–163. doi: 10.1002/cne.903120111. [DOI] [PubMed] [Google Scholar]
- Ishizuka N., Weber J., Amaral D. G. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990 May 22;295(4):580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Kauer J. A., Malenka R. C., Nicoll R. A. NMDA application potentiates synaptic transmission in the hippocampus. Nature. 1988 Jul 21;334(6179):250–252. doi: 10.1038/334250a0. [DOI] [PubMed] [Google Scholar]
- Kullmann D. M., Perkel D. J., Manabe T., Nicoll R. A. Ca2+ entry via postsynaptic voltage-sensitive Ca2+ channels can transiently potentiate excitatory synaptic transmission in the hippocampus. Neuron. 1992 Dec;9(6):1175–1183. doi: 10.1016/0896-6273(92)90075-o. [DOI] [PubMed] [Google Scholar]
- Liao D., Hessler N. A., Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995 Jun 1;375(6530):400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C. Postsynaptic factors control the duration of synaptic enhancement in area CA1 of the hippocampus. Neuron. 1991 Jan;6(1):53–60. doi: 10.1016/0896-6273(91)90121-f. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985 Nov;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak C. E., Seress L., Amaral D. G. The development, ultrastructure and synaptic connections of the mossy cells of the dentate gyrus. J Neurocytol. 1985 Oct;14(5):835–857. doi: 10.1007/BF01170832. [DOI] [PubMed] [Google Scholar]
- Scharfman H. E. Blockade of excitation reveals inhibition of dentate spiny hilar neurons recorded in rat hippocampal slices. J Neurophysiol. 1992 Sep;68(3):978–984. doi: 10.1152/jn.1992.68.3.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H. E. Evidence from simultaneous intracellular recordings in rat hippocampal slices that area CA3 pyramidal cells innervate dentate hilar mossy cells. J Neurophysiol. 1994 Nov;72(5):2167–2180. doi: 10.1152/jn.1994.72.5.2167. [DOI] [PubMed] [Google Scholar]
- Scharfman H. E., Kunkel D. D., Schwartzkroin P. A. Synaptic connections of dentate granule cells and hilar neurons: results of paired intracellular recordings and intracellular horseradish peroxidase injections. Neuroscience. 1990;37(3):693–707. doi: 10.1016/0306-4522(90)90100-i. [DOI] [PubMed] [Google Scholar]
- Scharfman H. E., Schwartzkroin P. A. Electrophysiology of morphologically identified mossy cells of the dentate hilus recorded in guinea pig hippocampal slices. J Neurosci. 1988 Oct;8(10):3812–3821. doi: 10.1523/JNEUROSCI.08-10-03812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H. E., Schwartzkroin P. A. Responses of cells of the rat fascia dentata to prolonged stimulation of the perforant path: sensitivity of hilar cells and changes in granule cell excitability. Neuroscience. 1990;35(3):491–504. doi: 10.1016/0306-4522(90)90324-w. [DOI] [PubMed] [Google Scholar]
- Staley K. J., Otis T. S., Mody I. Membrane properties of dentate gyrus granule cells: comparison of sharp microelectrode and whole-cell recordings. J Neurophysiol. 1992 May;67(5):1346–1358. doi: 10.1152/jn.1992.67.5.1346. [DOI] [PubMed] [Google Scholar]
- Strowbridge B. W., Buckmaster P. S., Schwartzkroin P. A. Potentiation of spontaneous synaptic activity in rat mossy cells. Neurosci Lett. 1992 Aug 17;142(2):205–210. doi: 10.1016/0304-3940(92)90374-g. [DOI] [PubMed] [Google Scholar]
- Wyllie D. J., Manabe T., Nicoll R. A. A rise in postsynaptic Ca2+ potentiates miniature excitatory postsynaptic currents and AMPA responses in hippocampal neurons. Neuron. 1994 Jan;12(1):127–138. doi: 10.1016/0896-6273(94)90158-9. [DOI] [PubMed] [Google Scholar]


