Abstract
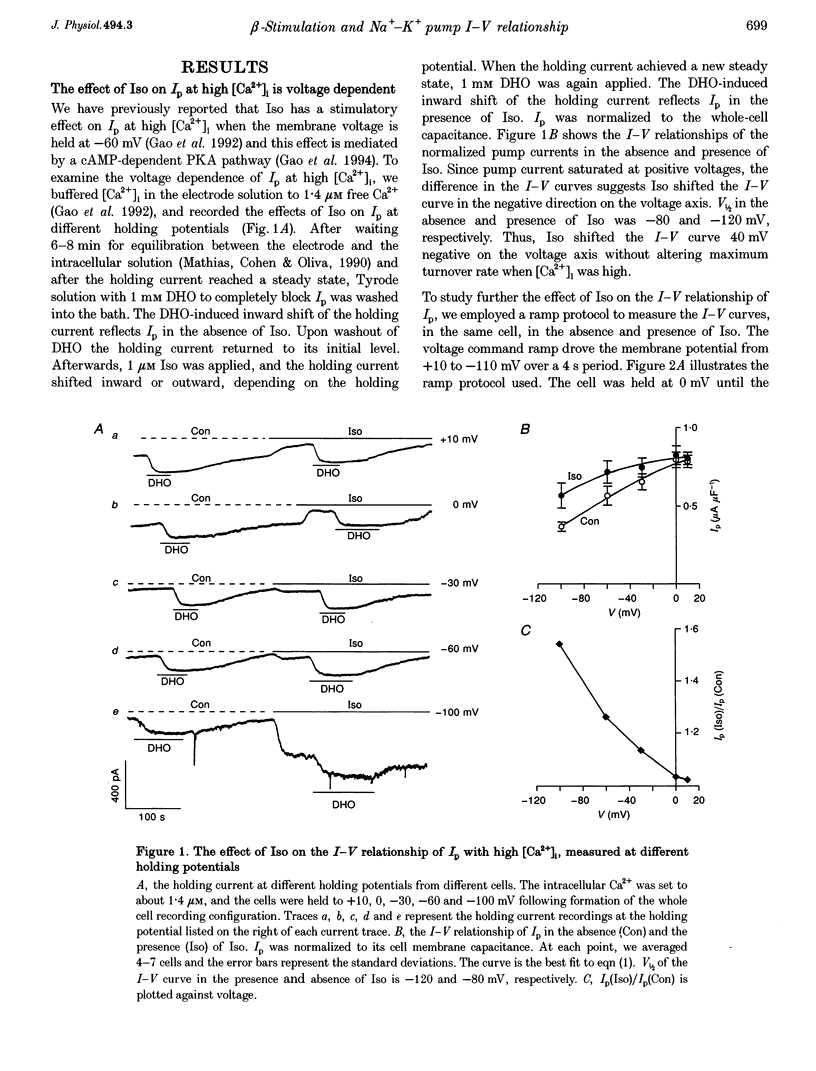
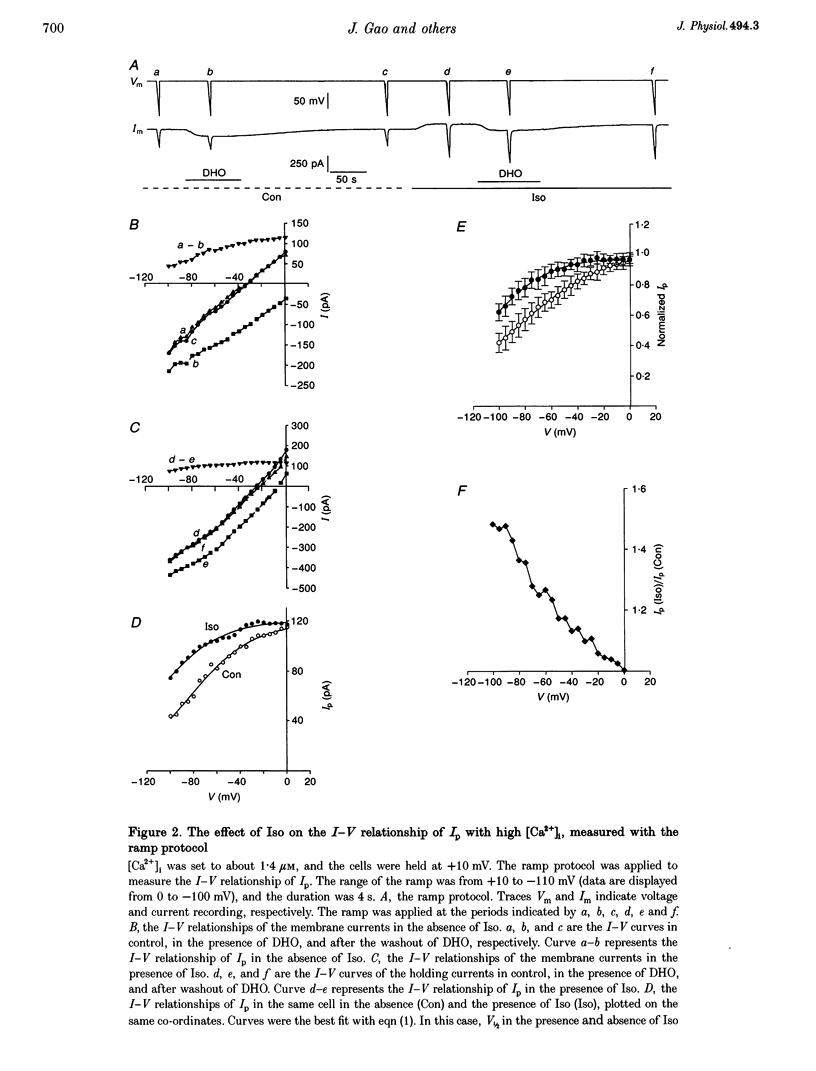
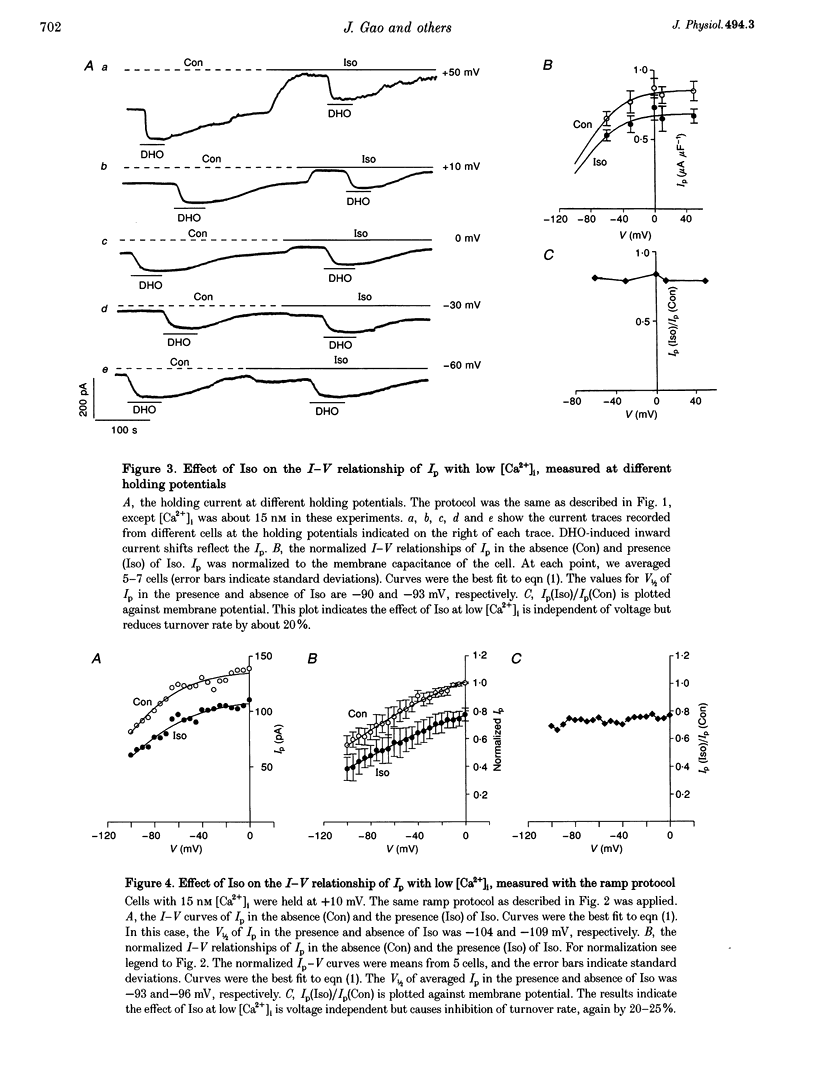
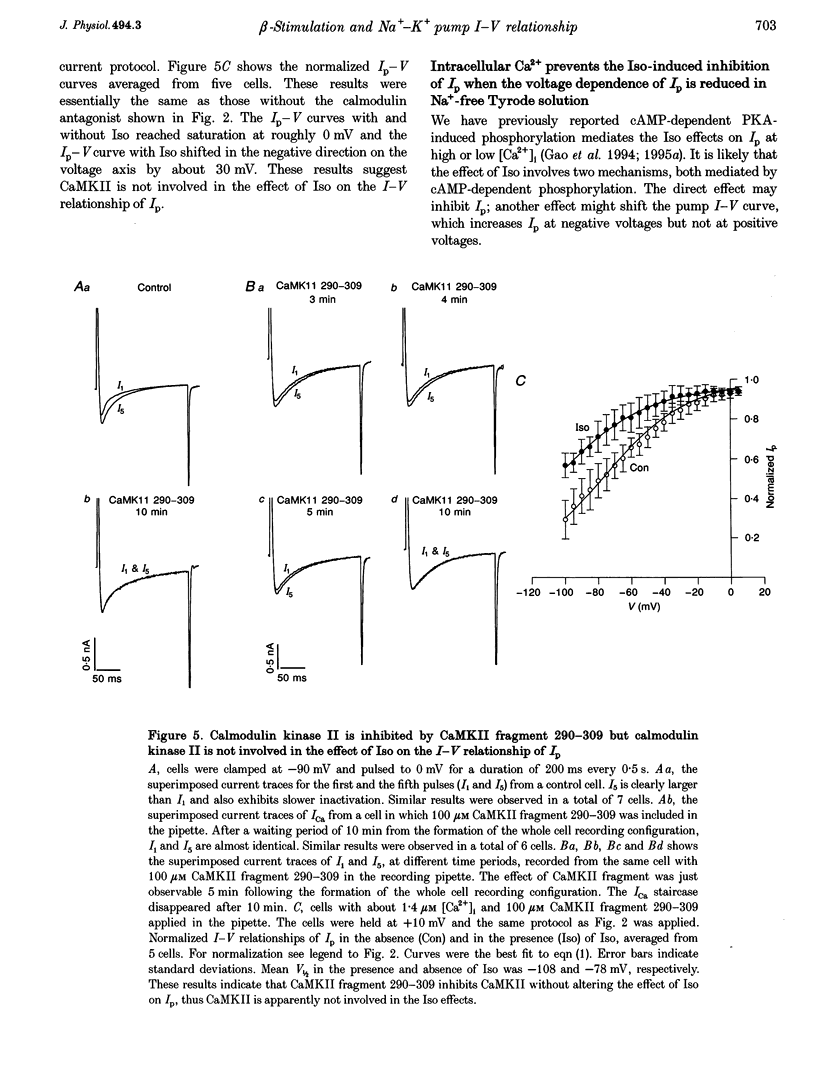
1. The whole cell patch clamp technique was used to study effects of the beta agonist isoprenaline (Iso) on the current-voltage (I-V) relationship of the Na(+)-K+ pump current (Ip) in acutely isolated guinea-pig ventricular myocytes. 2. The effect of Iso on Ip at high [Ca2+]i (1.4 microM) was voltage dependent. The I-V relationship of Ip in Iso shifted by approximately 30 mV in the negative direction on the voltage axis, increasing Ip at negative voltages but leaving Ip unchanged at positive voltages. 3. Intracellular application of the calmodulin antagonist, calmodulin-dependent protein kinase II fragment 290-309, did not eliminate or reduce the Iso-induced voltage shift, suggesting calmodulin-dependent protein kinase II was not involved. 4. The Iso inhibition of Ip at low [Ca2+]i (15 nM) was not voltage dependent. Ip was reduced by 20 to 30% in the presence of Iso at each holding potential. 5. When the voltage dependence of Ip was largely reduced by substitution of N-methyl-D-glucamine+ for external Na+, the magnitude of the low [Ca2+]i, Iso-induced inhibition of Ip was progressively eliminated by increasing the [Ca2+]i. At a [Ca2+]i of 1.4 microM, this inhibition disappeared. 6. At intermediate values of [Ca2+]i, the I-V curves in Na(+)-containing solution in the presence and the absence of Iso crossed over. The higher the [Ca2+]i, the more positive the voltage at which the two I-V curves intersected. 7. During beta-adrenergic activation our results suggest intracellular Ca2+ has two effects: (a) It prevents protein kinase A (PKA) phosphorylation-induced inhibition of Ip. (b) It causes a PKA phosphorylation-induced shift of the pump I-V relationship in the negative direction on the voltage axis. These effects may have important physiological significance in the regulation of heart rate and cardiac contractility.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertorello A. M., Aperia A., Walaas S. I., Nairn A. C., Greengard P. Phosphorylation of the catalytic subunit of Na+,K(+)-ATPase inhibits the activity of the enzyme. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11359–11362. doi: 10.1073/pnas.88.24.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Osterrieder W., Trautwein W. Beta-adrenergic increase in the calcium conductance of cardiac myocytes studied with the patch clamp. Pflugers Arch. 1984 Jun;401(2):111–118. doi: 10.1007/BF00583870. [DOI] [PubMed] [Google Scholar]
- Chibalin A. V., Vasilets L. A., Hennekes H., Pralong D., Geering K. Phosphorylation of Na,K-ATPase alpha-subunits in microsomes and in homogenates of Xenopus oocytes resulting from the stimulation of protein kinase A and protein kinase C. J Biol Chem. 1992 Nov 5;267(31):22378–22384. [PubMed] [Google Scholar]
- Cohen I. S., Datyner N. B., Gintant G. A., Mulrine N. K., Pennefather P. Properties of an electrogenic sodium-potassium pump in isolated canine Purkinje myocytes. J Physiol. 1987 Feb;383:251–267. doi: 10.1113/jphysiol.1987.sp016407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J. Inhibition of the sodium pump in guinea-pig ventricular muscle by dihydro-ouabain: effects of external potassium and sodium. J Physiol. 1983 Jun;339:643–662. doi: 10.1113/jphysiol.1983.sp014740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weer P., Gadsby D. C., Rakowski R. F. Voltage dependence of the Na-K pump. Annu Rev Physiol. 1988;50:225–241. doi: 10.1146/annurev.ph.50.030188.001301. [DOI] [PubMed] [Google Scholar]
- Désilets M., Baumgarten C. M. Isoproterenol directly stimulates the Na+-K+ pump in isolated cardiac myocytes. Am J Physiol. 1986 Jul;251(1 Pt 2):H218–H225. doi: 10.1152/ajpheart.1986.251.1.H218. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Falk R. T., Cohen I. S. Membrane current following activity in canine cardiac Purkinje fibers. J Gen Physiol. 1984 May;83(5):771–799. doi: 10.1085/jgp.83.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisone G., Cheng S. X., Nairn A. C., Czernik A. J., Hemmings H. C., Jr, Hög J. O., Bertorello A. M., Kaiser R., Bergman T., Jörnvall H. Identification of the phosphorylation site for cAMP-dependent protein kinase on Na+,K(+)-ATPase and effects of site-directed mutagenesis. J Biol Chem. 1994 Mar 25;269(12):9368–9373. [PubMed] [Google Scholar]
- Gadsby D. C. Beta-adrenoceptor agonists increase membrane K+ conductance in cardiac Purkinje fibres. Nature. 1983 Dec 15;306(5944):691–693. doi: 10.1038/306691a0. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C., Nakao M. Steady-state current-voltage relationship of the Na/K pump in guinea pig ventricular myocytes. J Gen Physiol. 1989 Sep;94(3):511–537. doi: 10.1085/jgp.94.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C. The Na/K pump of cardiac cells. Annu Rev Biophys Bioeng. 1984;13:373–398. doi: 10.1146/annurev.bb.13.060184.002105. [DOI] [PubMed] [Google Scholar]
- Gao J., Cohen I. S., Mathias R. T., Baldo G. J. Regulation of the beta-stimulation of the Na(+)-K+ pump current in guinea-pig ventricular myocytes by a cAMP-dependent PKA pathway. J Physiol. 1994 Jun 15;477(Pt 3):373–380. doi: 10.1113/jphysiol.1994.sp020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Mathias R. T., Cohen I. S., Baldo G. J. Isoprenaline, Ca2+ and the Na(+)-K+ pump in guinea-pig ventricular myocytes. J Physiol. 1992 Apr;449:689–704. doi: 10.1113/jphysiol.1992.sp019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Krahn T., Pusch H., Suleymanian M. Effect of isoprenaline on active Na transport in sheep cardiac Purkinje fibres. Pflugers Arch. 1989 Oct;415(1):88–94. doi: 10.1007/BF00373145. [DOI] [PubMed] [Google Scholar]
- Hauswirth O., Noble D., Tsien R. W. Adrenaline: mechanism of action on the pacemaker potential in cardiac Purkinje fibers. Science. 1968 Nov 22;162(3856):916–917. doi: 10.1126/science.162.3856.916. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Trautwein W. The effect of dihydro-ouabain and lithium-ions on the outward current in cardiac Purkinje fibers. Evidence for electrogenicity of active transport. Pflugers Arch. 1974;350(1):41–54. doi: 10.1007/BF00586737. [DOI] [PubMed] [Google Scholar]
- Ishizuka N., Berlin J. R. Beta-adrenergic stimulation does not regulate Na pump function in voltage-clamped ventricular myocytes of the rat heart. Pflugers Arch. 1993 Aug;424(3-4):361–363. doi: 10.1007/BF00384364. [DOI] [PubMed] [Google Scholar]
- Lafaire A. V., Schwarz W. Voltage dependence of the rheogenic Na+/K+ ATPase in the membrane of oocytes of Xenopus laevis. J Membr Biol. 1986;91(1):43–51. doi: 10.1007/BF01870213. [DOI] [PubMed] [Google Scholar]
- Lingham R. B., Sen A. K. Regulation of rat brain (Na+ +K+)-ATPase activity by cyclic AMP. Biochim Biophys Acta. 1982 Jun 14;688(2):475–485. doi: 10.1016/0005-2736(82)90359-5. [DOI] [PubMed] [Google Scholar]
- Mathias R. T., Cohen I. S., Oliva C. Limitations of the whole cell patch clamp technique in the control of intracellular concentrations. Biophys J. 1990 Sep;58(3):759–770. doi: 10.1016/S0006-3495(90)82418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M., Gadsby D. C. [Na] and [K] dependence of the Na/K pump current-voltage relationship in guinea pig ventricular myocytes. J Gen Physiol. 1989 Sep;94(3):539–565. doi: 10.1085/jgp.94.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne M. E., Fong Y. L., Ono T., Colbran R. J., Kemp B. E., Soderling T. R., Means A. R. Calcium/calmodulin-dependent protein kinase II. Characterization of distinct calmodulin binding and inhibitory domains. J Biol Chem. 1988 May 25;263(15):7190–7195. [PubMed] [Google Scholar]
- Rakowski R. F., Gadsby D. C., De Weer P. Stoichiometry and voltage dependence of the sodium pump in voltage-clamped, internally dialyzed squid giant axon. J Gen Physiol. 1989 May;93(5):903–941. doi: 10.1085/jgp.93.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Cohen H. T., Katz A. I. Intracellular signaling in the regulation of renal Na-K-ATPase. I. Role of cyclic AMP and phospholipase A2. J Clin Invest. 1992 May;89(5):1496–1500. doi: 10.1172/JCI115740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung P., Pai G., Johnson D. G., Punzalan R., Levin S. R. Relationships between adenylate cyclase and Na+, K(+)-ATPase in rat pancreatic islets. J Biol Chem. 1990 Mar 5;265(7):3936–3939. [PubMed] [Google Scholar]
- Vasilets L. A., Schwarz W. Regulation of endogenous and expressed Na+/K+ pumps in Xenopus oocytes by membrane potential and stimulation of protein kinases. J Membr Biol. 1992 Jan;125(2):119–132. doi: 10.1007/BF00233352. [DOI] [PubMed] [Google Scholar]
- Vassalle M., Barnabei O. Norepinephrine and potassium fluxes in cardiac Purkinje fibers. Pflugers Arch. 1971;322(4):287–303. doi: 10.1007/BF00587747. [DOI] [PubMed] [Google Scholar]
- Yingst D. R. Modulation of the Na,K-ATPase by Ca and intracellular proteins. Annu Rev Physiol. 1988;50:291–303. doi: 10.1146/annurev.ph.50.030188.001451. [DOI] [PubMed] [Google Scholar]
- Yuan W., Bers D. M. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am J Physiol. 1994 Sep;267(3 Pt 2):H982–H993. doi: 10.1152/ajpheart.1994.267.3.H982. [DOI] [PubMed] [Google Scholar]


