Abstract
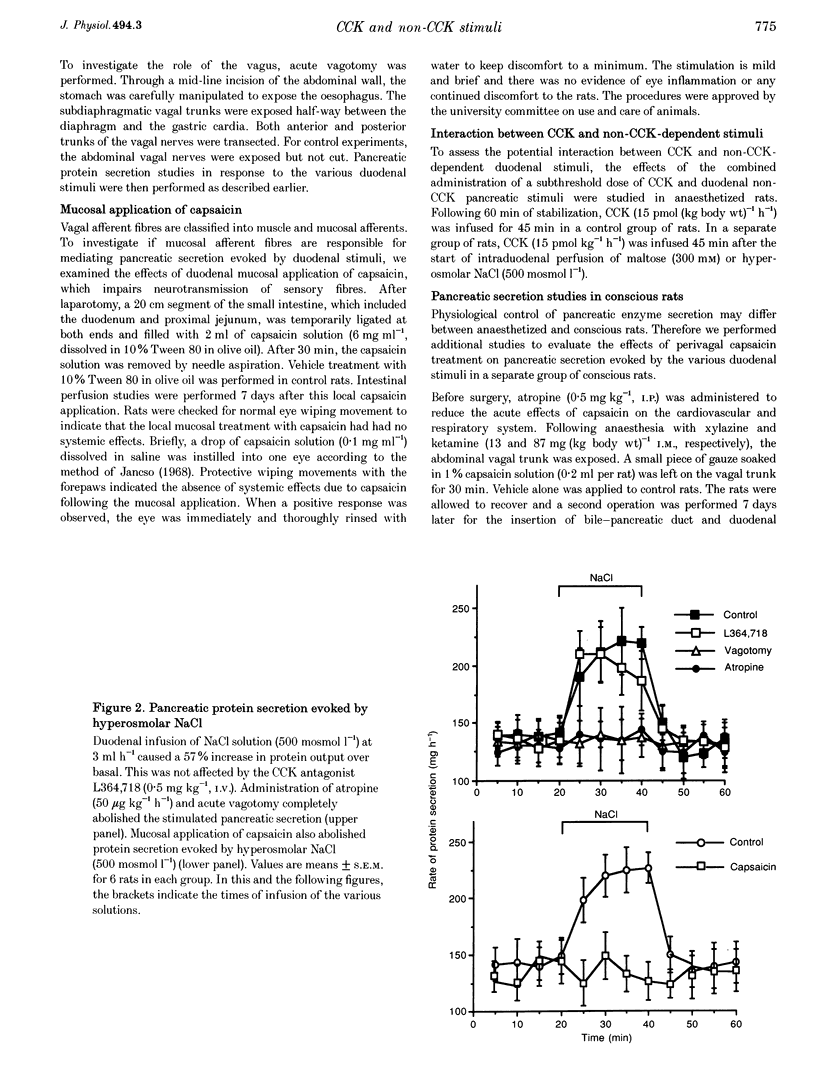
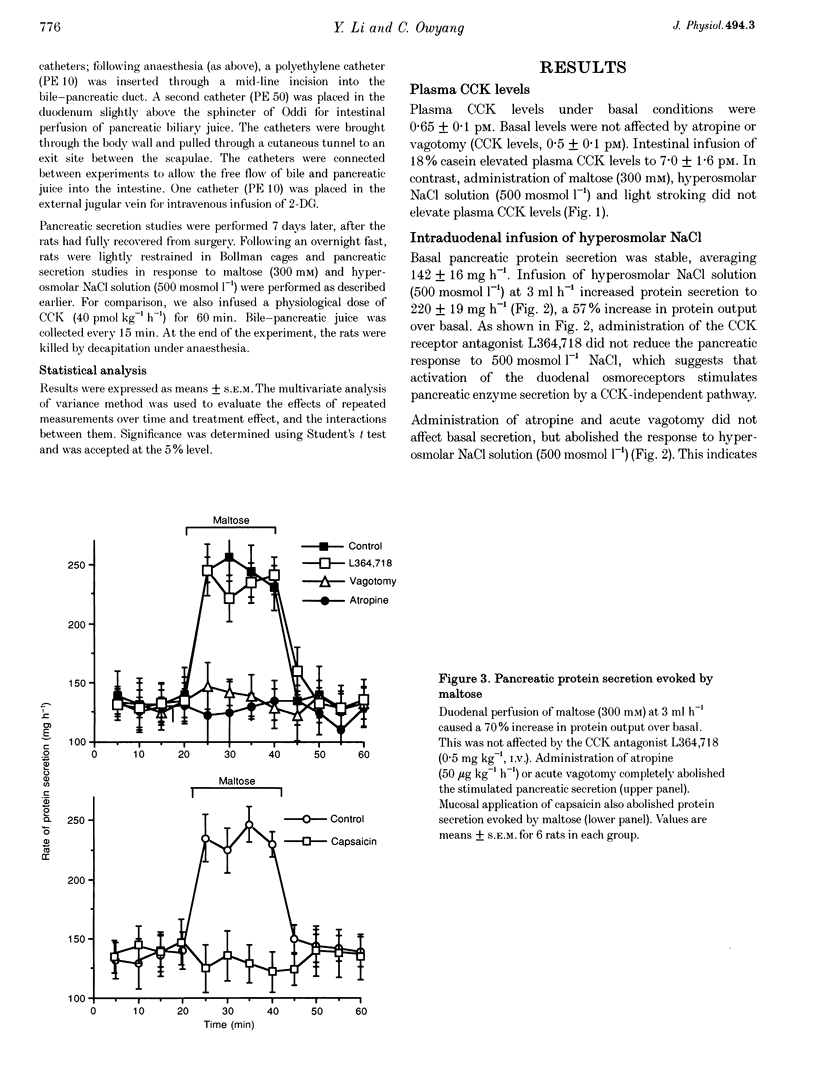
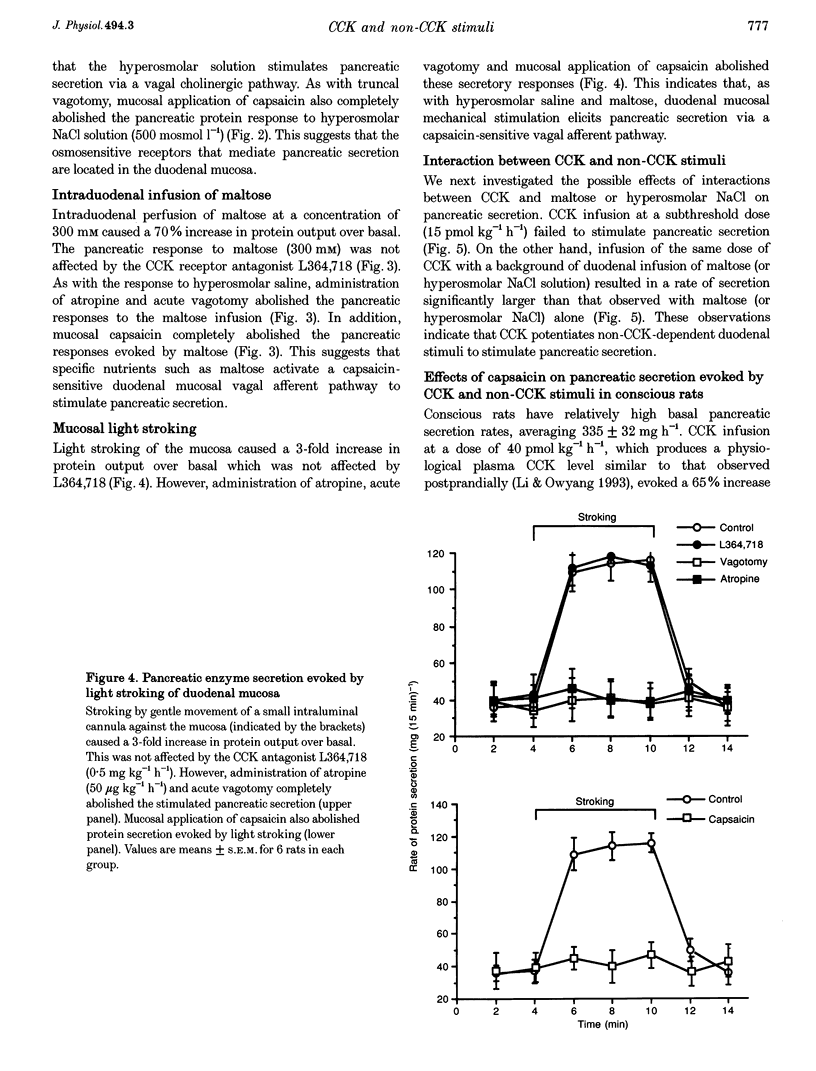
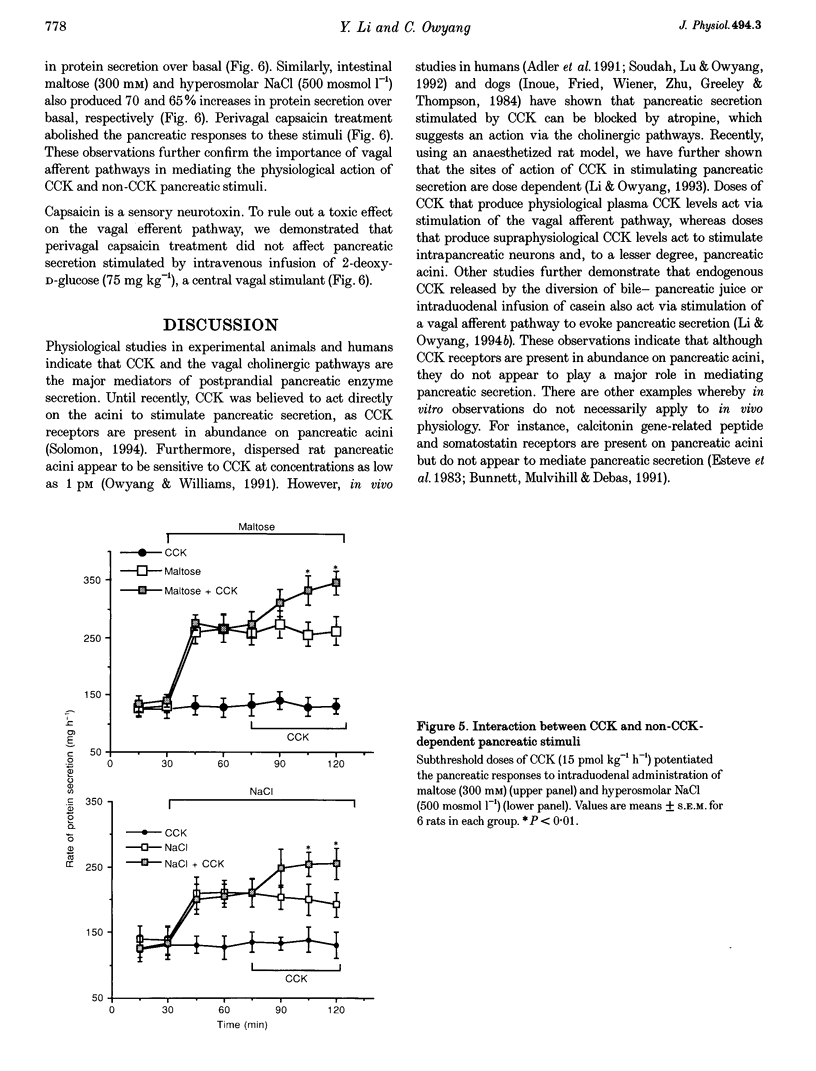
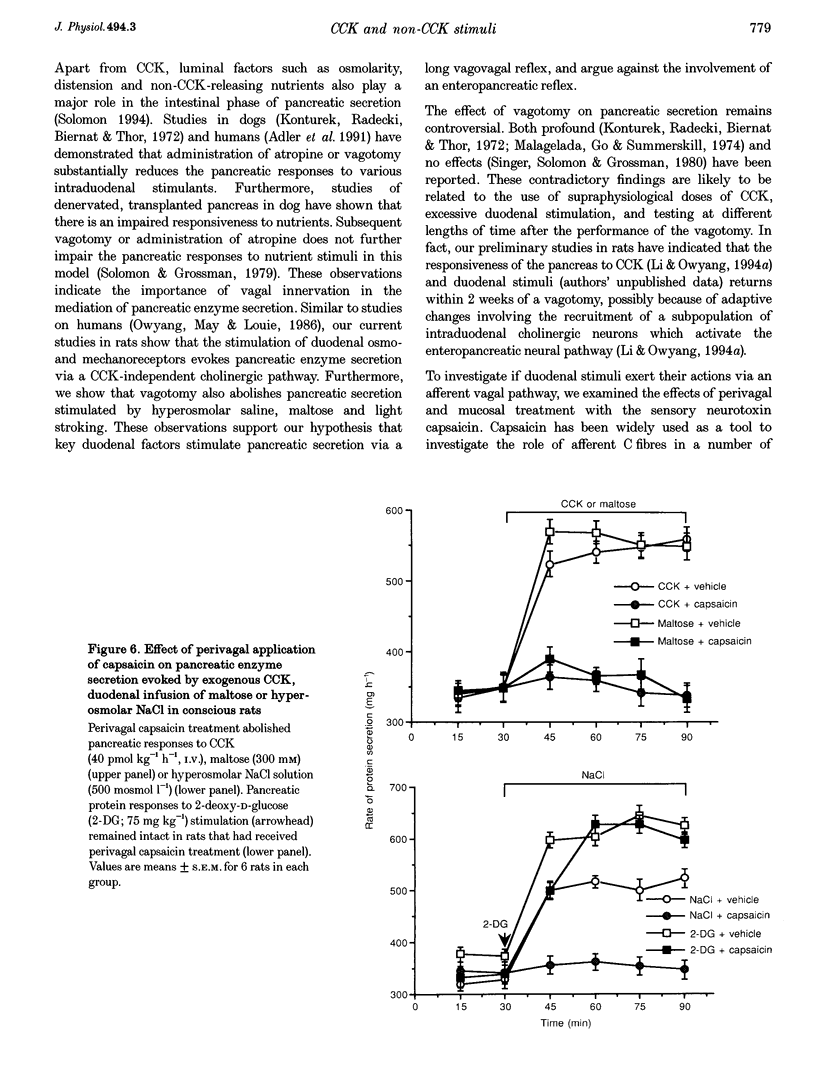
1. We have recently demonstrated that cholecystokinin (CCK) at physiological levels stimulates pancreatic enzyme secretion via gastroduodenal mucosal vagal afferent fibres in the rat. The present study was designed to investigate if non-CCK-mediated pancreatic stimuli which activate duodenal receptors also utilize similar vagal afferent pathways. 2. Intraduodenal administration of maltose (300 mM), hypertonic saline (500 mosmol l-1) and mucosal light stroking in anaesthetized rats evoked 70, 57 and 200% increases, respectively, in pancreatic protein secretion with no changes in plasma CCK concentration. Administration of the CCK receptor antagonist L364,718 did not affect pancreatic secretion evoked by these luminal stimuli. 3. Administration of atropine, acute vagotomy and duodenal mucosal application of capsaicin each completely abolished the pancreatic response to these stimuli. 4. Infusion of a subthreshold dose of the octapeptide of CCK (15 pmol (kg body wt)-1 h-1) potentiated the pancreatic response to duodenal infusion of maltose (300 mM) and hypertonic saline (500 mosmol l-1). 5. In conscious rats, perivagal application of capsaicin abolished the pancreatic response evoked by physiological doses of CCK and intraduodenal administration of maltose or hypertonic saline, confirming the physiological relevance of the observations in anaesthetized rats. 6. These results suggest that like CCK, non-CCK-mediated luminal stimuli evoke pancreatic enzyme secretion via stimulation of a vagal afferent pathway originating from the duodenal mucosa.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler G., Beglinger C., Braun U., Reinshagen M., Koop I., Schafmayer A., Rovati L., Arnold R. Interaction of the cholinergic system and cholecystokinin in the regulation of endogenous and exogenous stimulation of pancreatic secretion in humans. Gastroenterology. 1991 Feb;100(2):537–543. doi: 10.1016/0016-5085(91)90227-c. [DOI] [PubMed] [Google Scholar]
- Blackshaw L. A., Grundy D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J Auton Nerv Syst. 1990 Dec;31(3):191–201. doi: 10.1016/0165-1838(90)90185-l. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buck S. H., Burks T. F. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986 Sep;38(3):179–226. [PubMed] [Google Scholar]
- Bunnett N. W., Mulvihill S. J., Debas H. T. Calcitonin gene-related peptide inhibits exocrine secretion from the rat pancreas by a neurally mediated mechanism. Exp Physiol. 1991 Jan;76(1):115–123. doi: 10.1113/expphysiol.1991.sp003473. [DOI] [PubMed] [Google Scholar]
- Clarke G. D., Davison J. S. Mucosal receptors in the gastric antrum and small intestine of the rat with afferent fibres in the cervical vagus. J Physiol. 1978 Nov;284:55–67. doi: 10.1113/jphysiol.1978.sp012527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell D. F., Iggo A. Tension receptors with vagal afferent fibres in the proximal duodenum and pyloric sphincter of sheep. J Physiol. 1984 Sep;354:457–475. doi: 10.1113/jphysiol.1984.sp015388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison J. S. Response of single vagal afferent fibres to mechanical and chemical stimulation of the gastric and duodenal mucosa in cats. Q J Exp Physiol Cogn Med Sci. 1972 Oct;57(4):405–416. doi: 10.1113/expphysiol.1972.sp002176. [DOI] [PubMed] [Google Scholar]
- Esteve J. P., Vaysse N., Susini C., Kunsch J. M., Fourmy D., Pradayrol L., Wunsch E., Moroder L., Ribet A. Bimodal regulation of pancreatic exocrine function in vitro by somatostatin-28. Am J Physiol. 1983 Aug;245(2):G208–G216. doi: 10.1152/ajpgi.1983.245.2.G208. [DOI] [PubMed] [Google Scholar]
- Gamse R., Petsche U., Lembeck F., Jancsò G. Capsaicin applied to peripheral nerve inhibits axoplasmic transport of substance P and somatostatin. Brain Res. 1982 May 13;239(2):447–462. doi: 10.1016/0006-8993(82)90521-2. [DOI] [PubMed] [Google Scholar]
- Inoue K., Fried G. M., Wiener I., Zhu X. G., Greeley G. H., Jr, Thompson J. C. Colonic inhibition of cholecystokinin release and pancreatic protein secretion in dogs. Surg Gynecol Obstet. 1984 Nov;159(5):423–428. [PubMed] [Google Scholar]
- Jeanningros R. Vagal unitary responses to intestinal amino acid infusions in the anesthetized cat: a putative signal for protein induced satiety. Physiol Behav. 1982 Jan;28(1):9–21. doi: 10.1016/0031-9384(82)90094-4. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Radecki T., Biernat J., Thor P. Effect of vagotomy on pancreatic secretion evoked by endogenous and exogenous cholecystokinin and caerulein. Gastroenterology. 1972 Aug;63(2):273–278. [PubMed] [Google Scholar]
- Konturek S. J., Tasler J., Cieszkowski M., Szewczyk K., Hladij M. Effect of cholecystokinin receptor antagonist on pancreatic responses to exogenous gastrin and cholecystokinin and to meal stimuli. Gastroenterology. 1988 Apr;94(4):1014–1023. doi: 10.1016/0016-5085(88)90561-6. [DOI] [PubMed] [Google Scholar]
- Lew W. Y., Longhurst J. C. Substance P, 5-hydroxytryptamine, and bradykinin stimulate abdominal visceral afferents. Am J Physiol. 1986 Mar;250(3 Pt 2):R465–R473. doi: 10.1152/ajpregu.1986.250.3.R465. [DOI] [PubMed] [Google Scholar]
- Li Y., Owyang C. Endogenous cholecystokinin stimulates pancreatic enzyme secretion via vagal afferent pathway in rats. Gastroenterology. 1994 Aug;107(2):525–531. doi: 10.1016/0016-5085(94)90180-5. [DOI] [PubMed] [Google Scholar]
- Li Y., Owyang C. Vagal afferent pathway mediates physiological action of cholecystokinin on pancreatic enzyme secretion. J Clin Invest. 1993 Jul;92(1):418–424. doi: 10.1172/JCI116583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie D. S., May D., Miller P., Owyang C. Cholecystokinin mediates feedback regulation of pancreatic enzyme secretion in rats. Am J Physiol. 1986 Feb;250(2 Pt 1):G252–G259. doi: 10.1152/ajpgi.1986.250.2.G252. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Brodin E., Hua X., Saria A. Vascular permeability changes and smooth muscle contraction in relation to capsaicin-sensitive substance P afferents in the guinea-pig. Acta Physiol Scand. 1984 Feb;120(2):217–227. doi: 10.1111/j.1748-1716.1984.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Malagelada J. R., Go V. L., Summerskill W. H. Altered pancreatic and biliary function after vagotomy and pyloroplasty. Gastroenterology. 1974 Jan;66(1):22–27. [PubMed] [Google Scholar]
- Mei N. Intestinal chemosensitivity. Physiol Rev. 1985 Apr;65(2):211–237. doi: 10.1152/physrev.1985.65.2.211. [DOI] [PubMed] [Google Scholar]
- Mei N. Vagal glucoreceptors in the small intestine of the cat. J Physiol. 1978 Sep;282:485–506. doi: 10.1113/jphysiol.1978.sp012477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mélone J. Vagal receptors sensitive to lipids in the small intestine of the cat. J Auton Nerv Syst. 1986 Nov;17(3):231–241. doi: 10.1016/0165-1838(86)90060-3. [DOI] [PubMed] [Google Scholar]
- O'Rourke M. F., Reidelberger R. D., Solomon T. E. Effects of atropine on pancreatic response to bethanechol, cholecystokinin, and food intake in rats. Am J Physiol. 1991 Nov;261(5 Pt 1):G735–G741. doi: 10.1152/ajpgi.1991.261.5.G735. [DOI] [PubMed] [Google Scholar]
- Owyang C., May D., Louie D. S. Trypsin suppression of pancreatic enzyme secretion. Differential effect on cholecystokinin release and the enteropancreatic reflex. Gastroenterology. 1986 Sep;91(3):637–643. doi: 10.1016/0016-5085(86)90633-5. [DOI] [PubMed] [Google Scholar]
- Raybould H. E., Taché Y. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathway in rats. Am J Physiol. 1988 Aug;255(2 Pt 1):G242–G246. doi: 10.1152/ajpgi.1988.255.2.G242. [DOI] [PubMed] [Google Scholar]
- Schmidt W. E., Creutzfeldt W., Schleser A., Choudhury A. R., Nustede R., Höcker M., Nitsche R., Sostmann H., Rovati L. C., Fölsch U. R. Role of CCK in regulation of pancreaticobiliary functions and GI motility in humans: effects of loxiglumide. Am J Physiol. 1991 Feb;260(2 Pt 1):G197–G206. doi: 10.1152/ajpgi.1991.260.2.G197. [DOI] [PubMed] [Google Scholar]
- Solomon T. E., Grossman M. I. Effect of atropine and vagotomy on response of transplanted pancreas. Am J Physiol. 1979 Feb;236(2):E186–E190. doi: 10.1152/ajpendo.1979.236.2.E186. [DOI] [PubMed] [Google Scholar]
- Soudah H. C., Lu Y., Hasler W. L., Owyang C. Cholecystokinin at physiological levels evokes pancreatic enzyme secretion via a cholinergic pathway. Am J Physiol. 1992 Jul;263(1 Pt 1):G102–G107. doi: 10.1152/ajpgi.1992.263.1.G102. [DOI] [PubMed] [Google Scholar]
- South E. H., Ritter R. C. Capsaicin application to central or peripheral vagal fibers attenuates CCK satiety. Peptides. 1988 May-Jun;9(3):601–612. doi: 10.1016/0196-9781(88)90171-4. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Matsumoto J., Ueshima K., Okabe S. Role of capsaicin-sensitive afferent neurons in alkaline secretory response to luminal acid in the rat duodenum. Gastroenterology. 1991 Oct;101(4):954–961. doi: 10.1016/0016-5085(91)90721-v. [DOI] [PubMed] [Google Scholar]



