Abstract
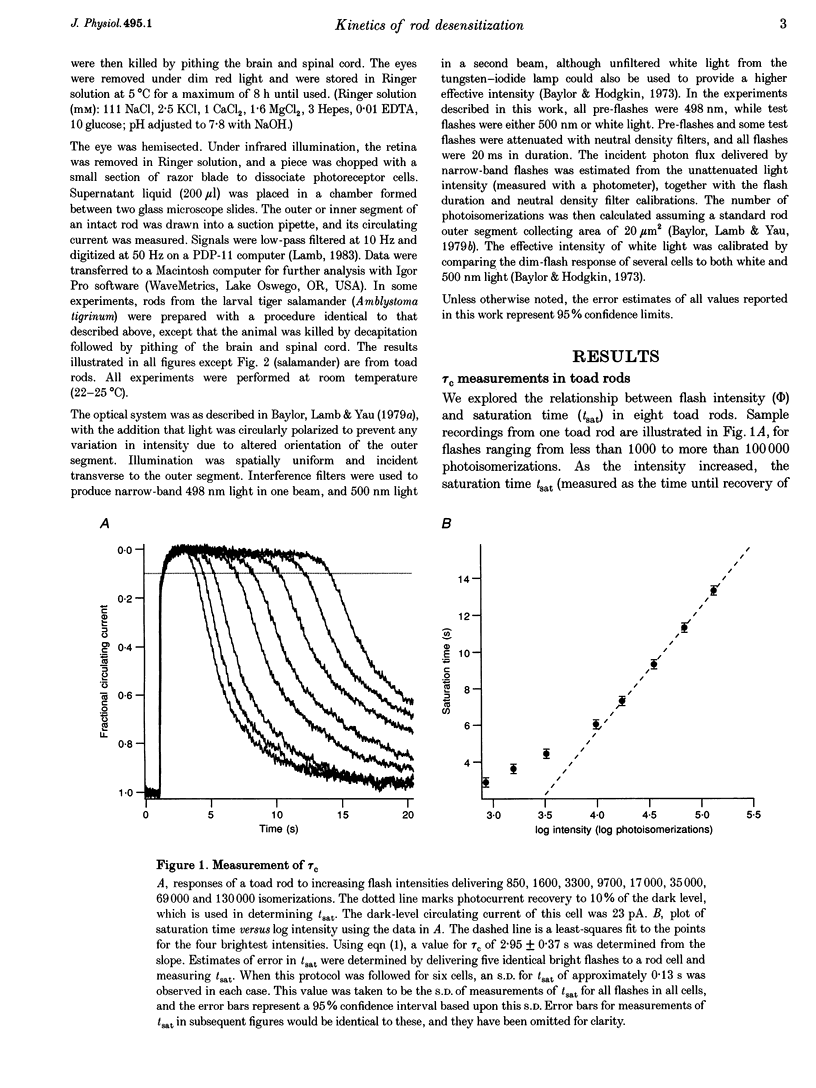
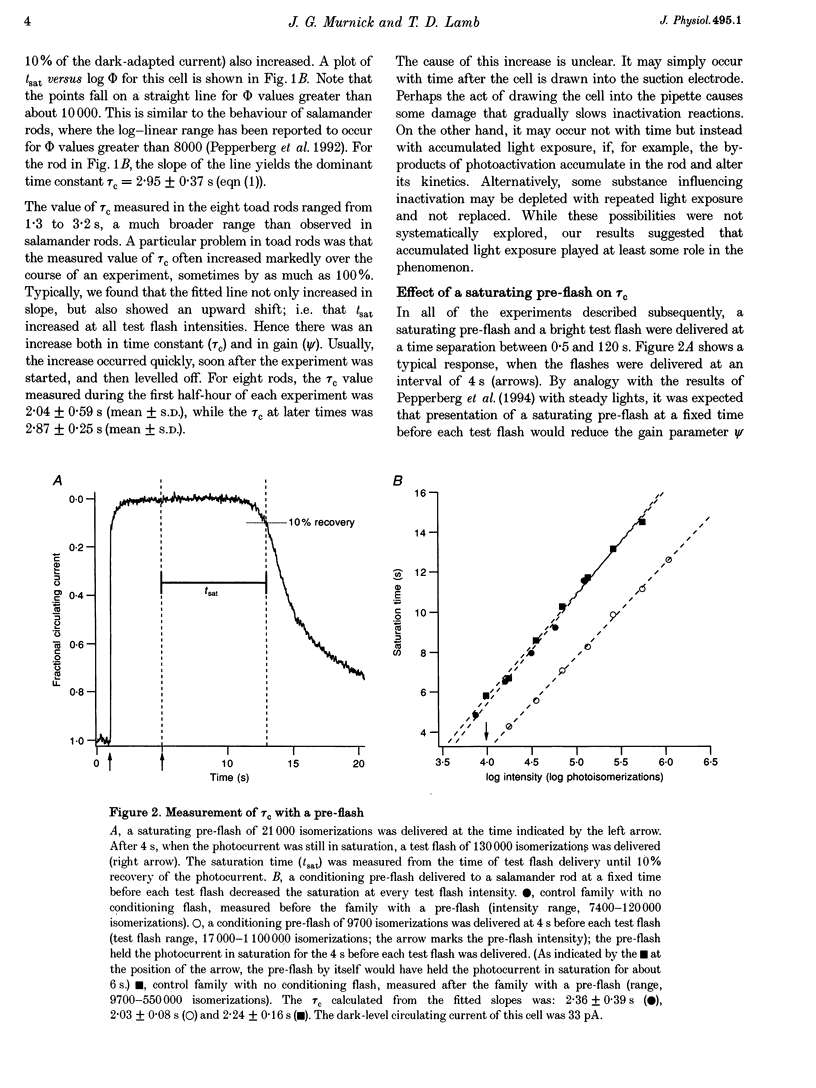
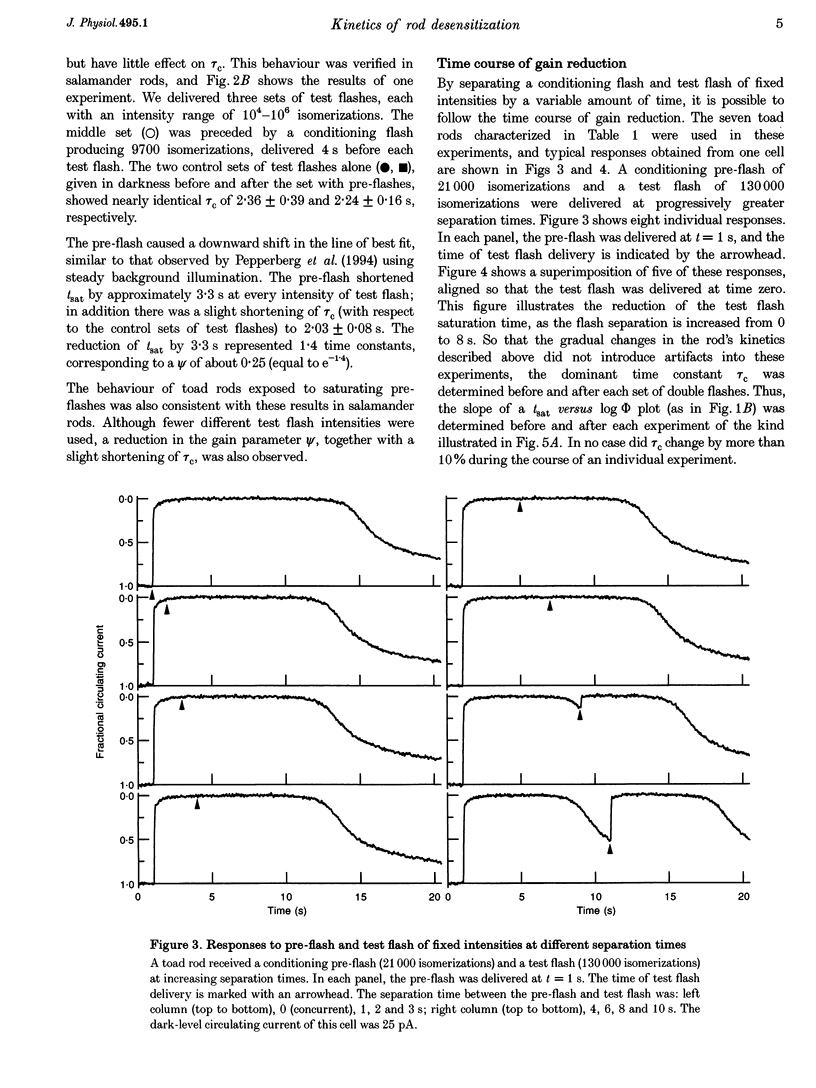
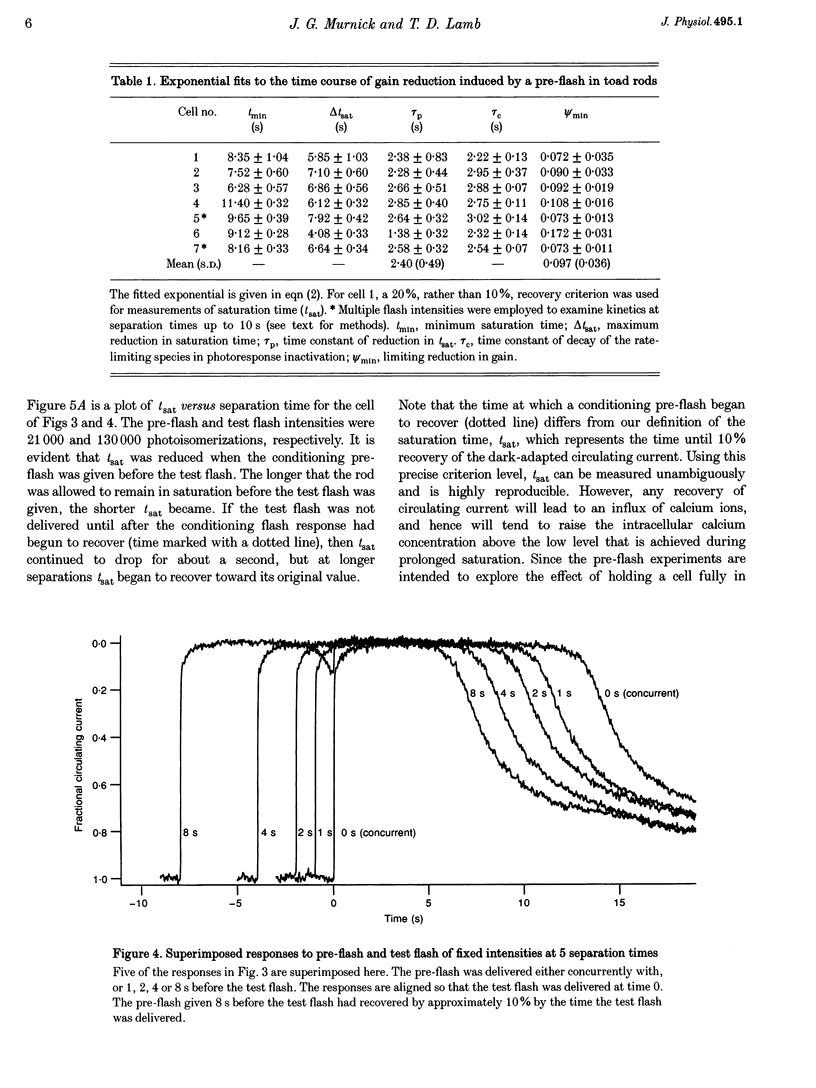
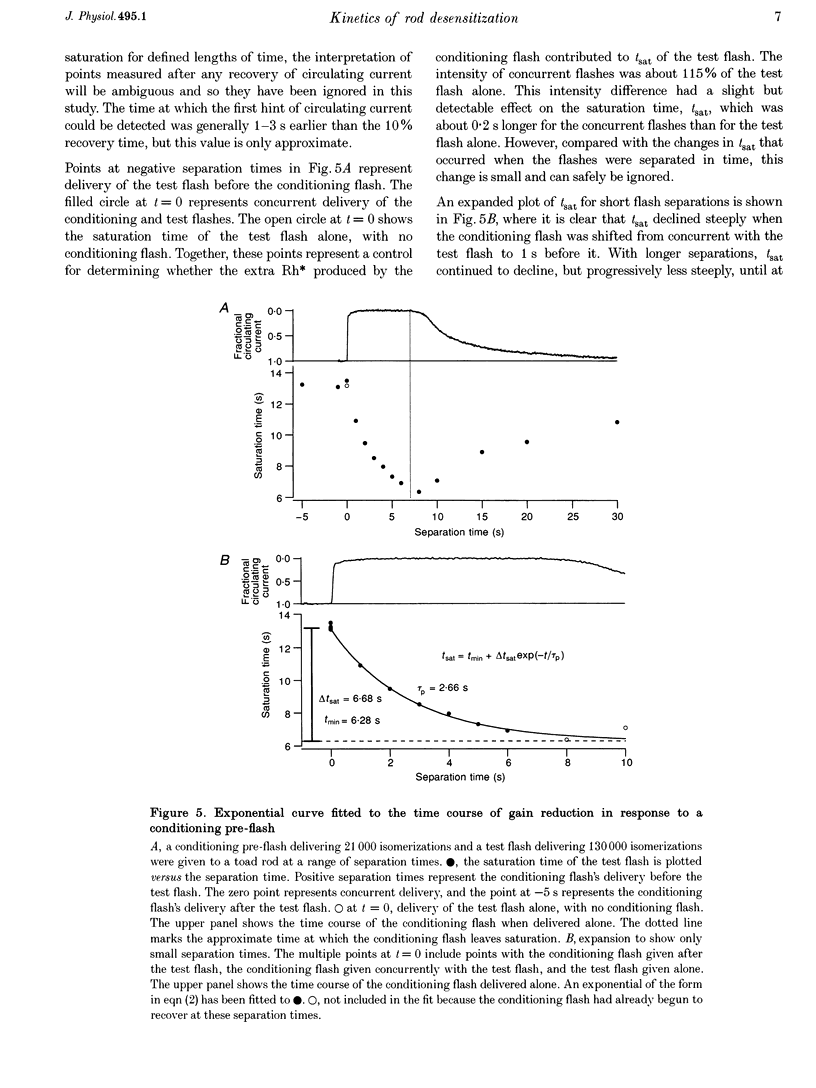
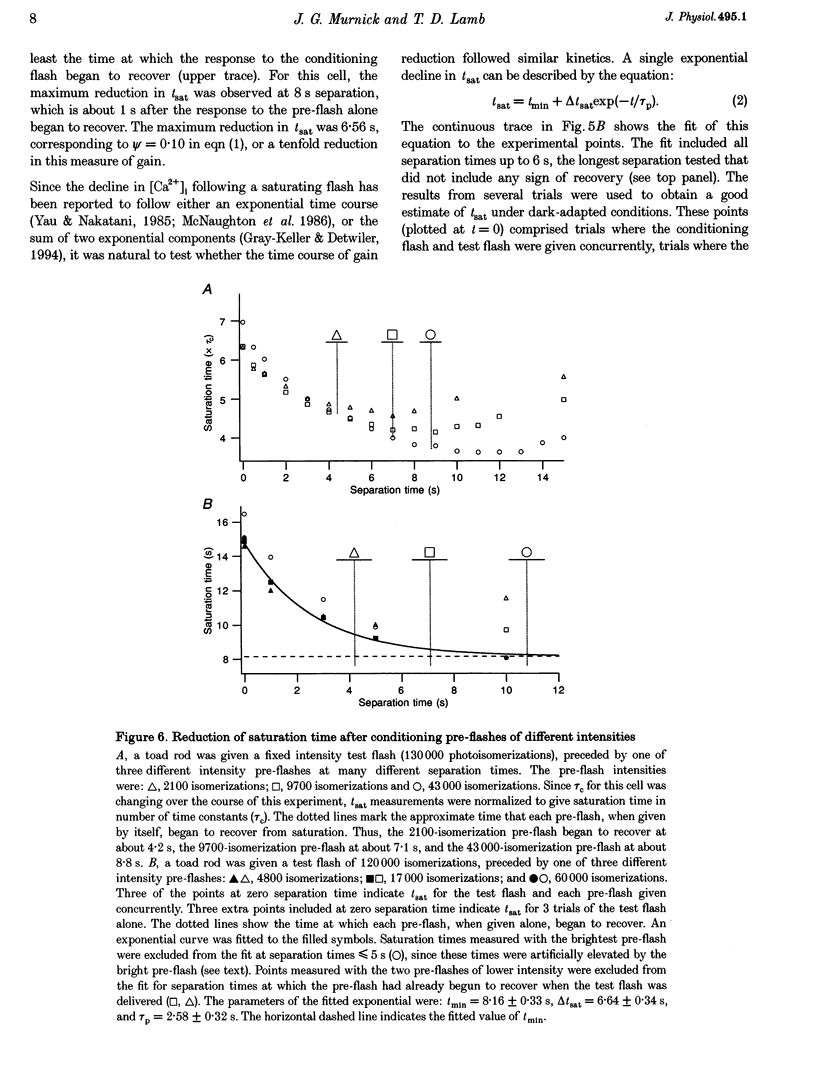
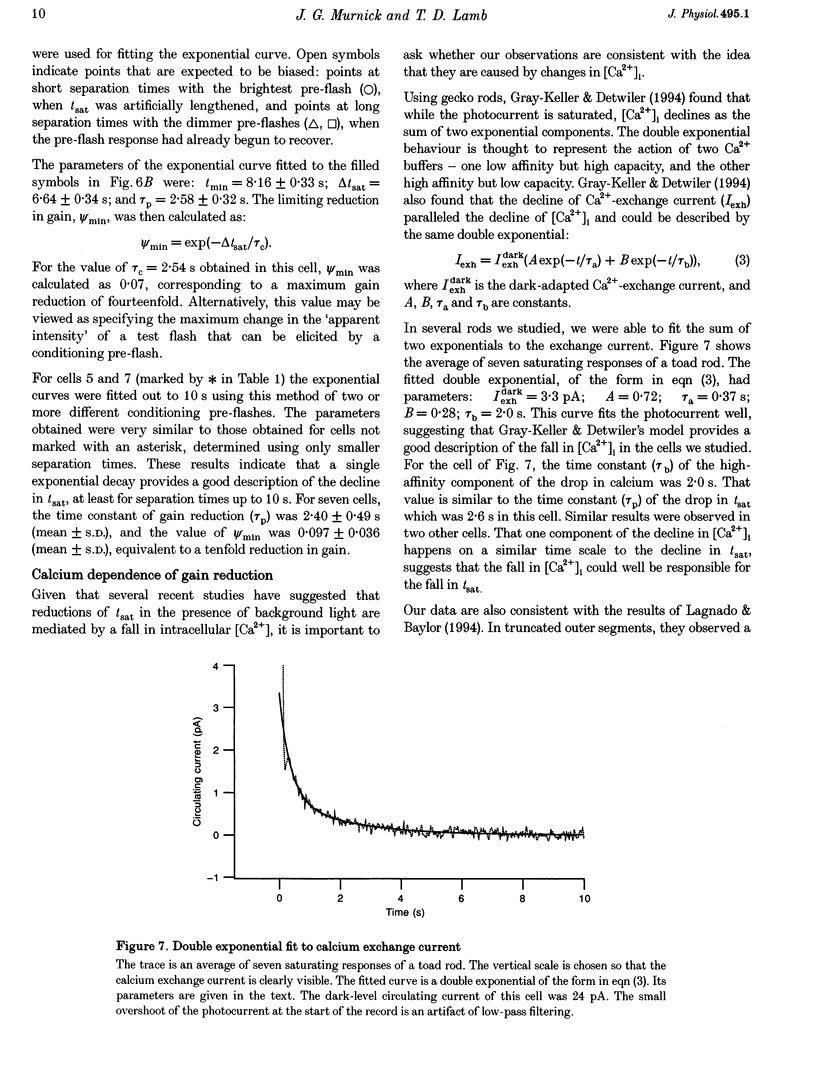
1. The suction pipette technique was used to examine the effect of a conditioning pre-flash on the saturation time (tsat) of a bright test flash (intensity 10,000-250,000 isomerizations) delivered to intact salamander or toad rod outer segments. The conditioning flash was delivered 0-60 s before the test flash; its intensity was typically between six and sixty times dimmer than the test flash, and it was sufficient by itself to fully saturate the photocurrent. 2. A saturating pre-flash delivered before a saturating test flash reduced the tsat of the test flash. This was equivalent to a reduction in phototransduction gain (psi). 3. The pre-flash had little effect on tau zero the time constant of decay of the rate-limiting species in photoresponse inactivation (activated rhodopsin or the activated G-protein-phosphodiesterase complex). 4. The tsat declined exponentially as the separation time between a fixed intensity pre-flash and test flash was increased. The time constant (tau p) of decline in tsat was approximately 2.4s. The maximum reduction in tsat corresponded to a reduction in the apparent gain of phototransduction to approximately 0.10 of its original level. This exponential decline is consistent with a [Ca2+]i-mediated effect. 5. We conclude that the rate-limiting step in response inactivation and the step responsible for light-induced gain reduction constitute separate and distinct steps of the phototransduction cascade.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Corson D. W., Cornwall M. C., Pepperberg D. R. Evidence for the prolonged photoactivated lifetime of an analogue visual pigment containing 11-cis 9-desmethylretinal. Vis Neurosci. 1994 Jan-Feb;11(1):91–98. doi: 10.1017/s0952523800011135. [DOI] [PubMed] [Google Scholar]
- Gray-Keller M. P., Detwiler P. B. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994 Oct;13(4):849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Koch K. W., Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988 Jul 7;334(6177):64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Koutalos Y., Nakatani K., Tamura T., Yau K. W. Characterization of guanylate cyclase activity in single retinal rod outer segments. J Gen Physiol. 1995 Nov;106(5):863–890. doi: 10.1085/jgp.106.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutalos Y., Nakatani K., Yau K. W. The cGMP-phosphodiesterase and its contribution to sensitivity regulation in retinal rods. J Gen Physiol. 1995 Nov;106(5):891–921. doi: 10.1085/jgp.106.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L., Baylor D. A. Calcium controls light-triggered formation of catalytically active rhodopsin. Nature. 1994 Jan 20;367(6460):273–277. doi: 10.1038/367273a0. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Cervetto L., McNaughton P. A. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J Physiol. 1992 Sep;455:111–142. doi: 10.1113/jphysiol.1992.sp019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Matthews H. R., Torre V. Incorporation of calcium buffers into salamander retinal rods: a rejection of the calcium hypothesis of phototransduction. J Physiol. 1986 Mar;372:315–349. doi: 10.1113/jphysiol.1986.sp016011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Pugh E. N., Jr A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol. 1992 Apr;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubarsky A., Nikonov S., Pugh E. N., Jr The kinetics of inactivation of the rod phototransduction cascade with constant Ca2+i. J Gen Physiol. 1996 Jan;107(1):19–34. doi: 10.1085/jgp.107.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R. Effects of lowered cytoplasmic calcium concentration and light on the responses of salamander rod photoreceptors. J Physiol. 1995 Apr 15;484(Pt 2):267–286. doi: 10.1113/jphysiol.1995.sp020664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R., Fain G. L., Murphy R. L., Lamb T. D. Light adaptation in cone photoreceptors of the salamander: a role for cytoplasmic calcium. J Physiol. 1990 Jan;420:447–469. doi: 10.1113/jphysiol.1990.sp017922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R. Incorporation of chelator into guinea-pig rods shows that calcium mediates mammalian photoreceptor light adaptation. J Physiol. 1991 May;436:93–105. doi: 10.1113/jphysiol.1991.sp018541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R., Murphy R. L., Fain G. L., Lamb T. D. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 1988 Jul 7;334(6177):67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- Matthews H. R. Static and dynamic actions of cytoplasmic Ca2+ in the adaptation of responses to saturating flashes in salamander rods. J Physiol. 1996 Jan 1;490(Pt 1):1–15. doi: 10.1113/jphysiol.1996.sp021123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy S. T., Younger J. P., Owen W. G. Free calcium concentrations in bullfrog rods determined in the presence of multiple forms of Fura-2. Biophys J. 1994 Nov;67(5):2076–2089. doi: 10.1016/S0006-3495(94)80691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and light adaptation in retinal rods and cones. Nature. 1988 Jul 7;334(6177):69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- Pepperberg D. R., Cornwall M. C., Kahlert M., Hofmann K. P., Jin J., Jones G. J., Ripps H. Light-dependent delay in the falling phase of the retinal rod photoresponse. Vis Neurosci. 1992 Jan;8(1):9–18. doi: 10.1017/s0952523800006441. [DOI] [PubMed] [Google Scholar]
- Pepperberg D. R., Jin J., Jones G. J. Modulation of transduction gain in light adaptation of retinal rods. Vis Neurosci. 1994 Jan-Feb;11(1):53–62. doi: 10.1017/s095252380001110x. [DOI] [PubMed] [Google Scholar]
- Pulvermüller A., Palczewski K., Hofmann K. P. Interaction between photoactivated rhodopsin and its kinase: stability and kinetics of complex formation. Biochemistry. 1993 Dec 28;32(51):14082–14088. doi: 10.1021/bi00214a002. [DOI] [PubMed] [Google Scholar]
- Ratto G. M., Payne R., Owen W. G., Tsien R. Y. The concentration of cytosolic free calcium in vertebrate rod outer segments measured with fura-2. J Neurosci. 1988 Sep;8(9):3240–3246. doi: 10.1523/JNEUROSCI.08-09-03240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Nakatani K., Yau K. W. Calcium feedback and sensitivity regulation in primate rods. J Gen Physiol. 1991 Jul;98(1):95–130. doi: 10.1085/jgp.98.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre V., Matthews H. R., Lamb T. D. Role of calcium in regulating the cyclic GMP cascade of phototransduction in retinal rods. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7109–7113. doi: 10.1073/pnas.83.18.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]


