Abstract
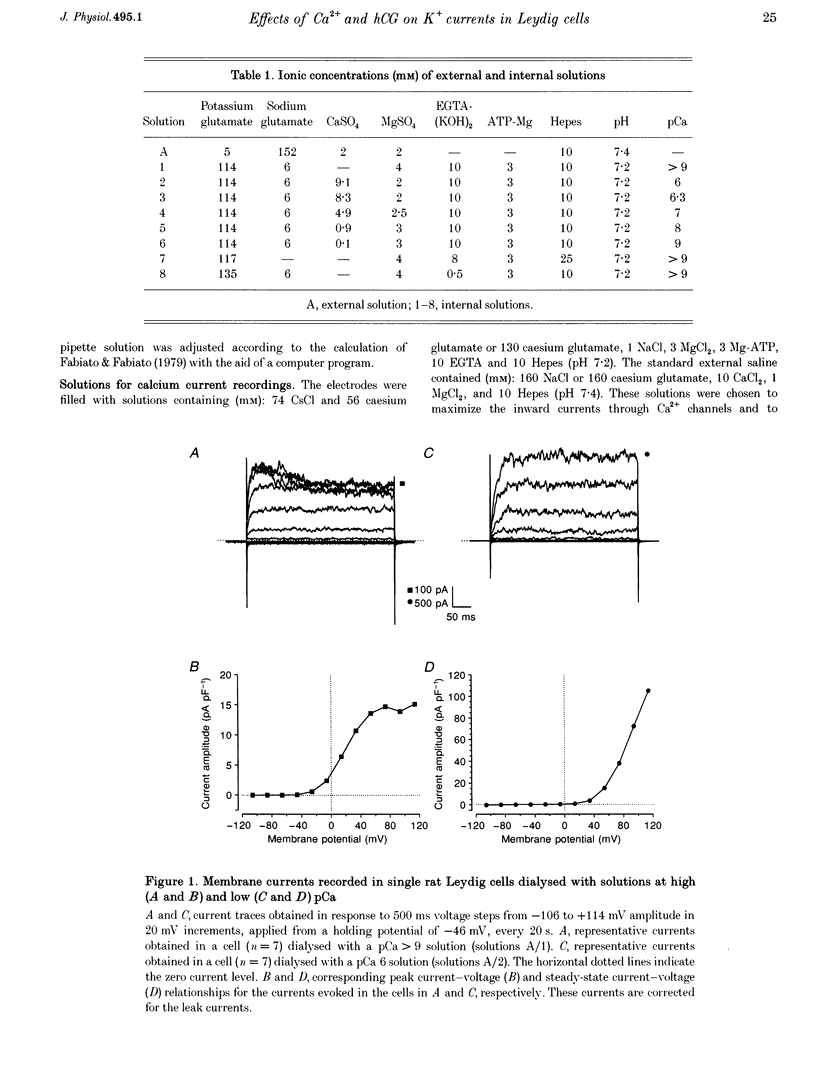
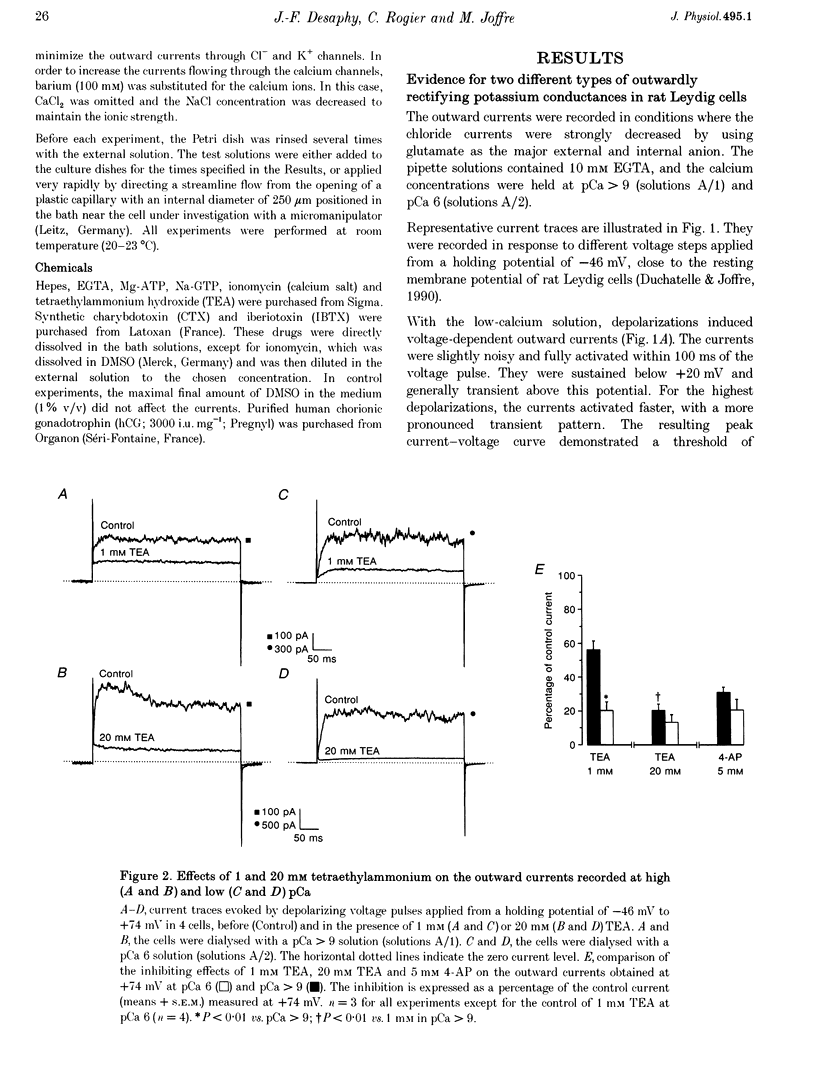
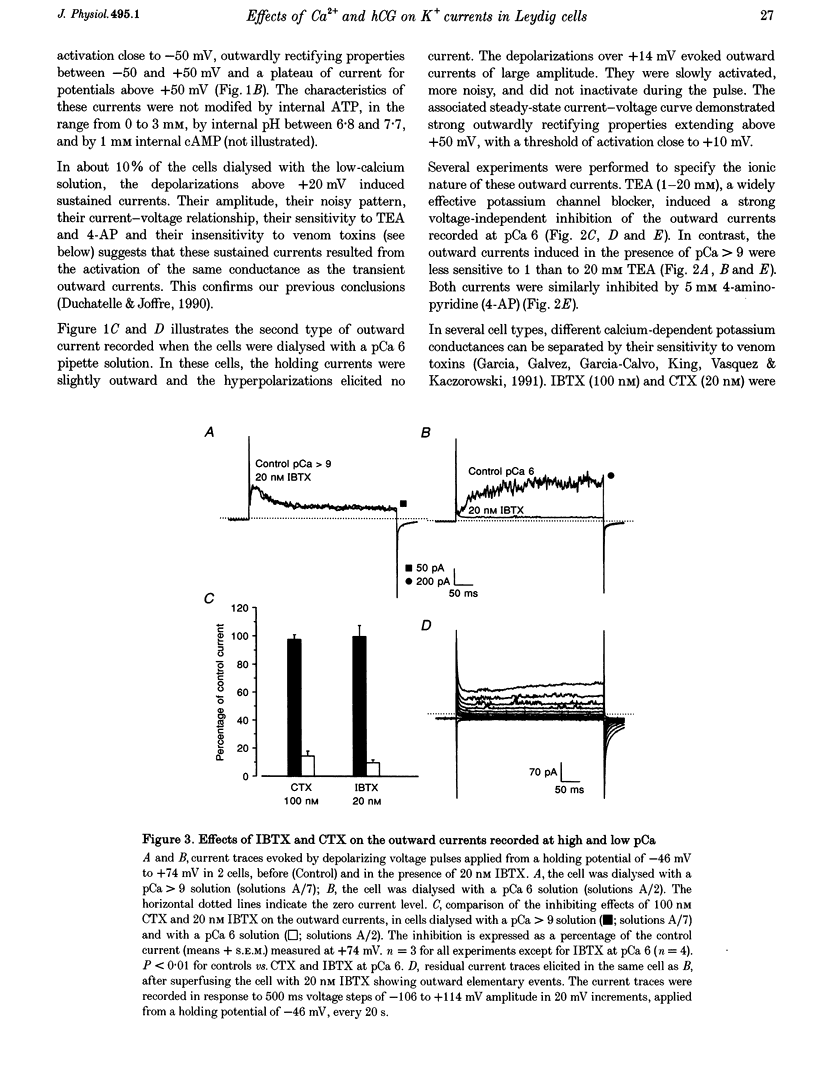
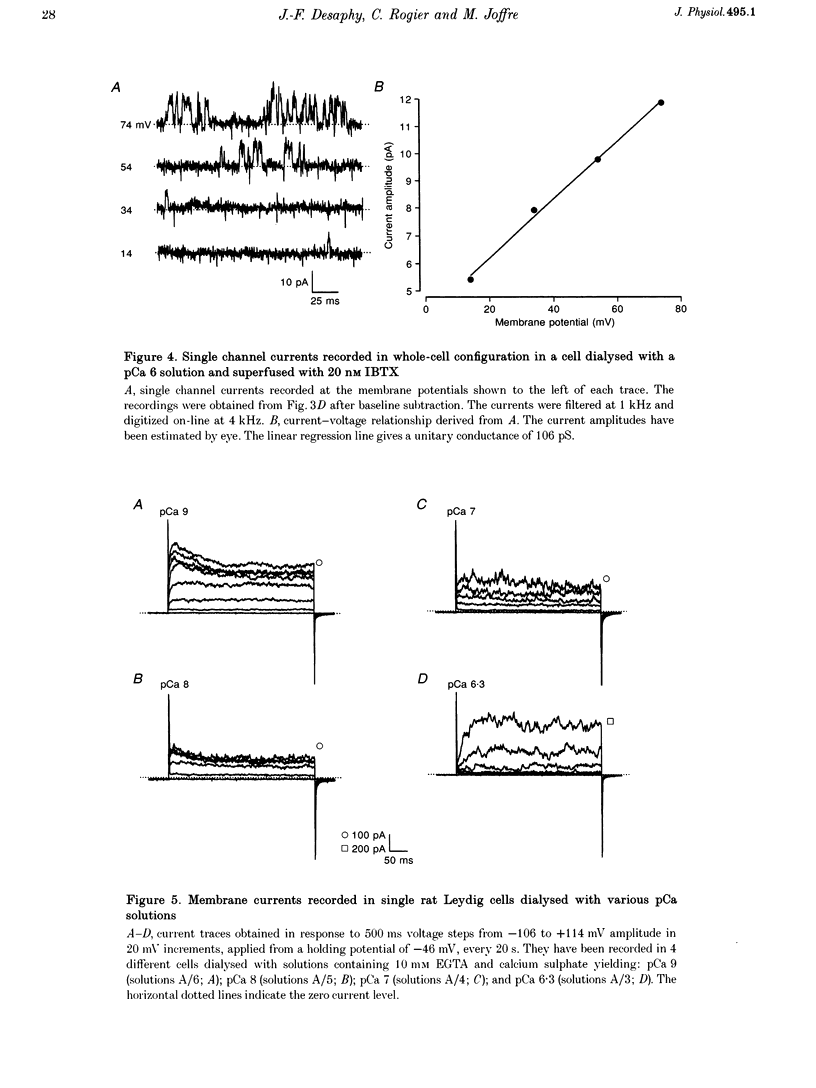
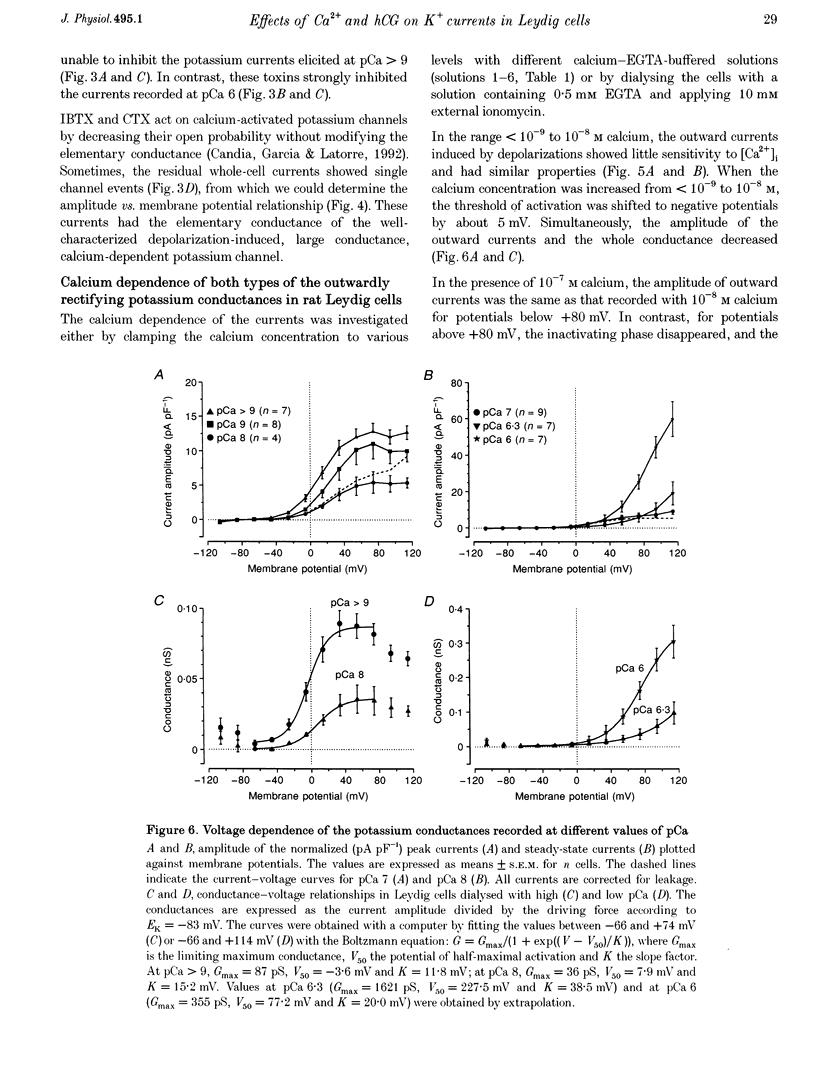
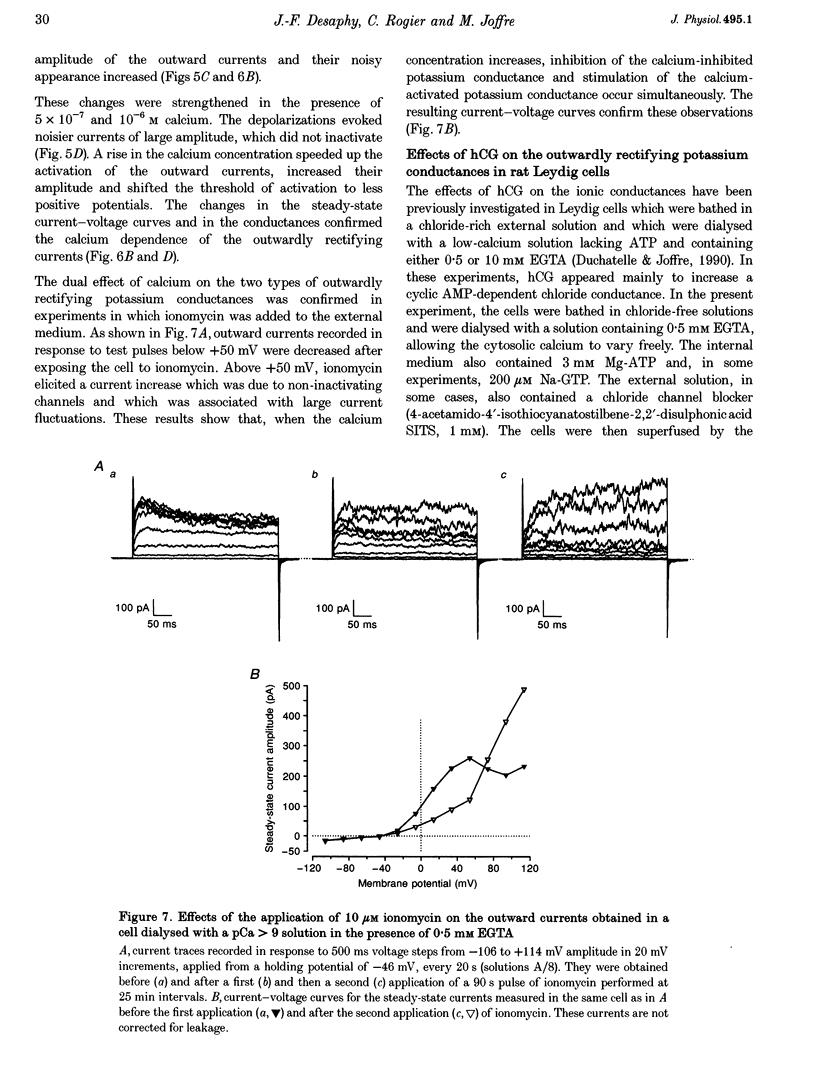
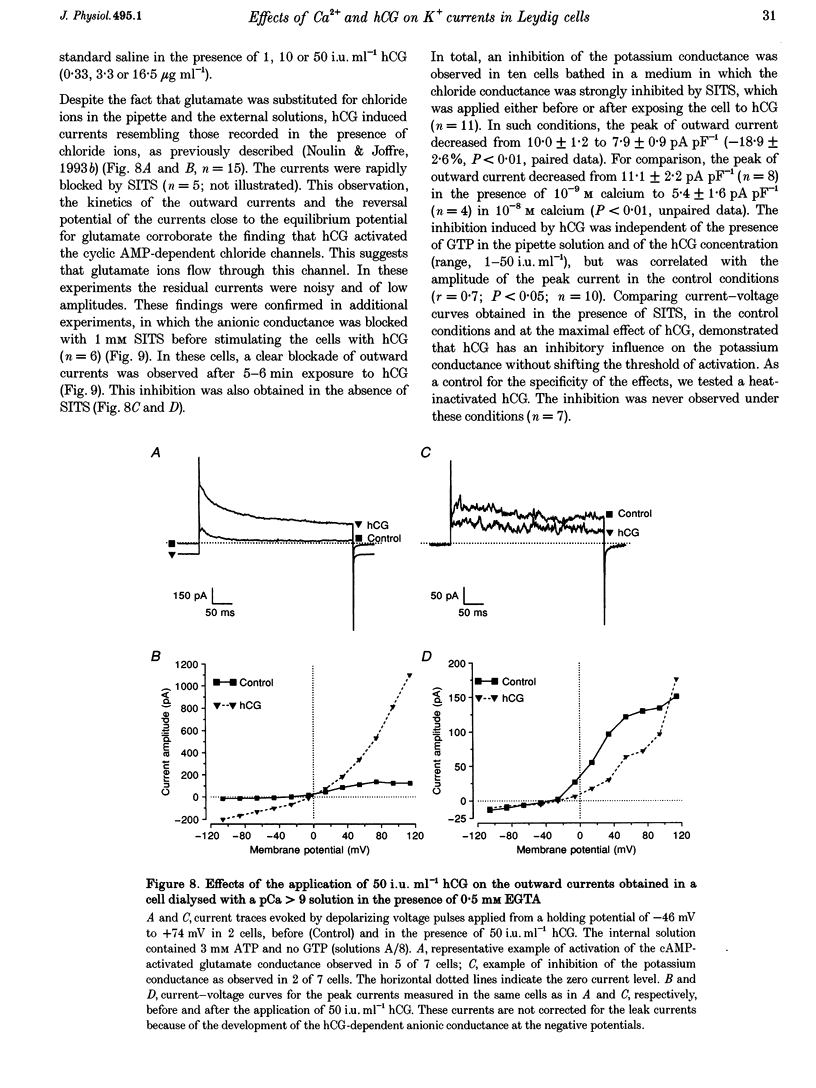
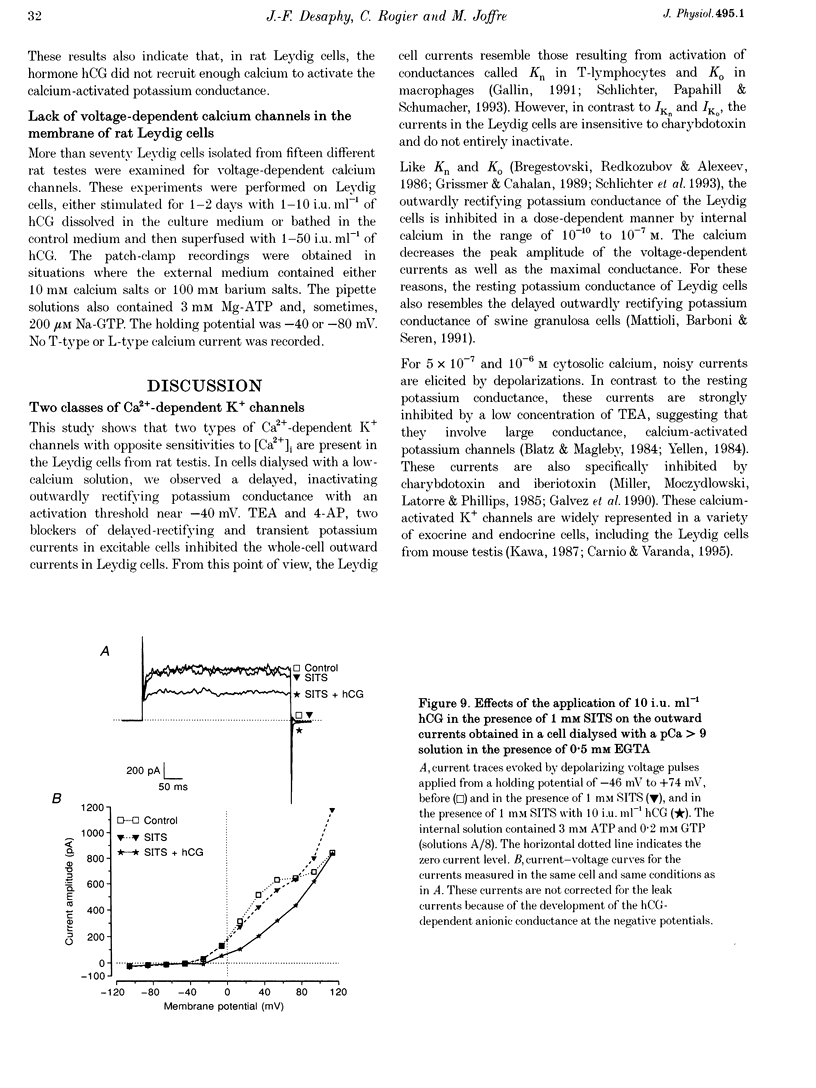
1. Although the control of steroidogenic activity of the Leydig cell by the peptides luteinizing hormone (LH) and human chorionic gonadotrophin (hCG) is clearly mediated by cAMP, the extent to which Ca2+ controls the Leydig cell function is less well defined. In the present study, the whole-cell configuration of the patch-clamp technique was used to investigate the modulation of potassium conductances by calcium and hCG, in the Leydig cells from mature rat testis. 2. In symmetrical glutamate solutions, depolarizations elicited outwardly rectifying currents, which were mainly carried by potassium and were blocked by tetraethylammonium and 4-aminopyridine. For values of [Ca2+]i below 10(-8) M, transient currents of low amplitudes, insensitive to charybdotoxin (CTX) and iberiotoxin (IBTX), were activated above -40 mV. For [Ca2+]i values of 10(-7) M and above, noisy currents with slow activation kinetics were activated above 0 mV. These currents were sustained and were sensitive to CTX and IBTX. 3. Both current types were modulated by intracellular calcium. Ionomycin and a [Ca2+]i elevation in the range from 10(-9) to 10(-7) M, both inhibited the CTX-insensitive currents, whereas a rise in the calcium concentration above 10(-7) M increased the amplitude and shifted the threshold of activation of the CTX-sensitive currents to less positive levels. 4. hCG (1-50 i.u. ml-1), in conditions where the chloride currents were strongly inhibited by 4-acetamido-4'-isothiocyanatostilbene-2,2'-disulphonic acid (SITS), induced a partial inhibition of the CTX-insensitive currents but was unable to increase the CTX-sensitive currents. 5. No voltage-sensitive calcium current was recorded in control or hCG-stimulated cells. 6. The results indicate that hCG inhibits one kind of Ca(2+)-modulated channel, perhaps as a result of a moderate [Ca2+]i rise, but is unable to increase the intracellular Ca2+ concentration to the range in which large conductance Ca(2+)-dependent channels are activated.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascoli M., Pignataro O. P., Segaloff D. L. The inositol phosphate/diacylglycerol pathway in MA-10 Leydig tumor cells. Activation by arginine vasopressin and lack of effect of epidermal growth factor and human choriogonadotropin. J Biol Chem. 1989 Apr 25;264(12):6674–6681. [PubMed] [Google Scholar]
- Bhalla V. K., Flasch M. V., Browne E. S., Sohal G. S., Sharawy M. M. Interstitial cell heterogeneity in rat testes. II. Purification of cells by Percoll and metrizamide gradient centrifugation with preferential localization of gonadotropin binding sites in light cell fraction and hormone-induced steroidogenesis in heavier cell fraction. J Biol Chem. 1987 Apr 15;262(11):5322–5332. [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Ion conductance and selectivity of single calcium-activated potassium channels in cultured rat muscle. J Gen Physiol. 1984 Jul;84(1):1–23. doi: 10.1085/jgp.84.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregestovski P., Redkozubov A., Alexeev A. Elevation of intracellular calcium reduces voltage-dependent potassium conductance in human T cells. 1986 Feb 27-Mar 5Nature. 319(6056):776–778. doi: 10.1038/319776a0. [DOI] [PubMed] [Google Scholar]
- Candia S., Garcia M. L., Latorre R. Mode of action of iberiotoxin, a potent blocker of the large conductance Ca(2+)-activated K+ channel. Biophys J. 1992 Aug;63(2):583–590. doi: 10.1016/S0006-3495(92)81630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnio E. C., Varanda W. A. Calcium-activated potassium channels are involved in the response of mouse Leydig cells to human chorionic gonadotropin. Braz J Med Biol Res. 1995 Jul;28(7):813–824. [PubMed] [Google Scholar]
- Duchatelle P., Joffre M. Ca-dependent-chloride and potassium currents in rat Leydig cells. FEBS Lett. 1987 Jun 8;217(1):11–15. doi: 10.1016/0014-5793(87)81232-2. [DOI] [PubMed] [Google Scholar]
- Duchatelle P., Joffre M. Potassium and chloride conductances in rat Leydig cells: effects of gonadotrophins and cyclic adenosine monophosphate. J Physiol. 1990 Sep;428:15–37. doi: 10.1113/jphysiol.1990.sp018198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufau M. L., Baukal A. J., Catt K. J. Hormone-induced guanyl nucleotide binding and activation of adenylate cyclase in the Leydig cell. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5837–5841. doi: 10.1073/pnas.77.10.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez A., Gimenez-Gallego G., Reuben J. P., Roy-Contancin L., Feigenbaum P., Kaczorowski G. J., Garcia M. L. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990 Jul 5;265(19):11083–11090. [PubMed] [Google Scholar]
- Garcia M. L., Galvez A., Garcia-Calvo M., King V. F., Vazquez J., Kaczorowski G. J. Use of toxins to study potassium channels. J Bioenerg Biomembr. 1991 Aug;23(4):615–646. doi: 10.1007/BF00785814. [DOI] [PubMed] [Google Scholar]
- Hall P. F., Osawa S., Mrotek J. The influence of calmodulin on steroid synthesis in leydig cells from rat testis. Endocrinology. 1981 Nov;109(5):1677–1682. doi: 10.1210/endo-109-5-1677. [DOI] [PubMed] [Google Scholar]
- Janszen F. H., Cooke B. A., Van Driel M. J., Van Der Molen H. J. The effect of calcium ions on testosterone production in Leydig cells from rat testis. Biochem J. 1976 Dec 15;160(3):433–437. doi: 10.1042/bj1600433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre M., Mollard P., Régondaud P., Gargouïl Y. M. Electrophysiological study of single Leydig cells freshly isolated from rat testis. II. Effects of ionic replacements, inhibitors and human chorionic gonadotropin on a calcium activated potassium permeability. Pflugers Arch. 1984 Jul;401(3):246–253. doi: 10.1007/BF00582591. [DOI] [PubMed] [Google Scholar]
- Kawa K. Existence of calcium channels and intercellular couplings in the testosterone-secreting cells of the mouse. J Physiol. 1987 Dec;393:647–666. doi: 10.1113/jphysiol.1987.sp016846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Blumberg D. L., Canas J. A., Maddaiah V. T. Human chorionic gonadotropin (hCG) increases cytosolic free calcium in adult rat Leydig cells. Cell Calcium. 1994 May;15(5):349–355. doi: 10.1016/0143-4160(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Lin T. The role of calcium/phospholipid-dependent protein kinase in Leydig cell steroidogenesis. Endocrinology. 1985 Jul;117(1):119–126. doi: 10.1210/endo-117-1-119. [DOI] [PubMed] [Google Scholar]
- Lowitt S., Farese R. V., Sabir M. A., Root A. W. Rat Leydig cell phospholipid content is increased by luteinizing hormone and 8-bromo-cyclic AMP. Endocrinology. 1982 Oct;111(4):1415–1417. doi: 10.1210/endo-111-4-1415. [DOI] [PubMed] [Google Scholar]
- Mattioli M., Barboni B., Seren E. Luteinizing hormone inhibits potassium outward currents in swine granulosa cells by intracellular calcium mobilization. Endocrinology. 1991 Nov;129(5):2740–2745. doi: 10.1210/endo-129-5-2740. [DOI] [PubMed] [Google Scholar]
- Mendelson C., Dufau M., Catt K. Gonadotropin binding and stimulation of cyclic adenosine 3':5'-monophosphate and testosterone production in isolated Leydig cells. J Biol Chem. 1975 Nov 25;250(22):8818–8823. [PubMed] [Google Scholar]
- Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985 Jan 24;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Noulin J. F., Joffre M. Cyclic AMP- and calcium-activated chloride currents in Leydig cells isolated from mature rat testis. Arch Int Physiol Biochim Biophys. 1993 Jan-Feb;101(1):35–41. doi: 10.3109/13813459308998126. [DOI] [PubMed] [Google Scholar]
- Saez J. M. Leydig cells: endocrine, paracrine, and autocrine regulation. Endocr Rev. 1994 Oct;15(5):574–626. doi: 10.1210/edrv-15-5-574. [DOI] [PubMed] [Google Scholar]
- Segaloff D. L., Ascoli M. The lutropin/choriogonadotropin receptor ... 4 years later. Endocr Rev. 1993 Jun;14(3):324–347. doi: 10.1210/edrv-14-3-324. [DOI] [PubMed] [Google Scholar]
- Tomić M., Dufau M. L., Catt K. J., Stojilkovic S. S. Calcium signaling in single rat Leydig cells. Endocrinology. 1995 Aug;136(8):3422–3429. doi: 10.1210/endo.136.8.7628378. [DOI] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]


