Abstract
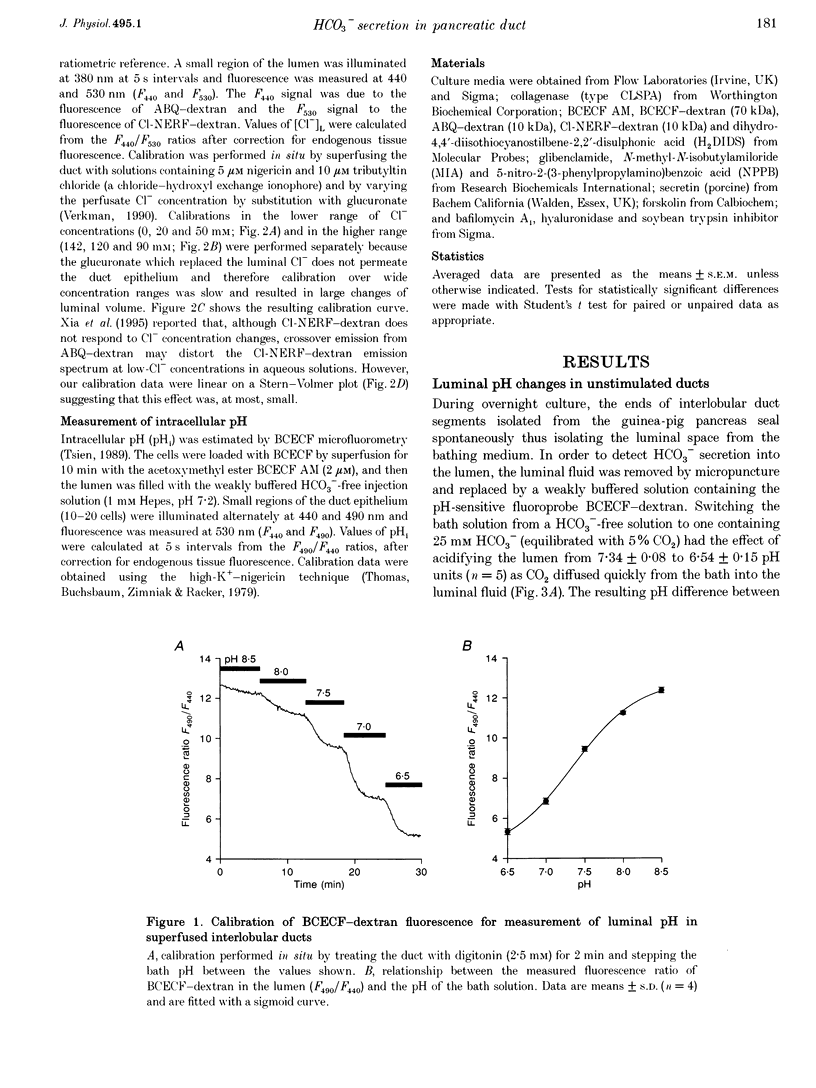
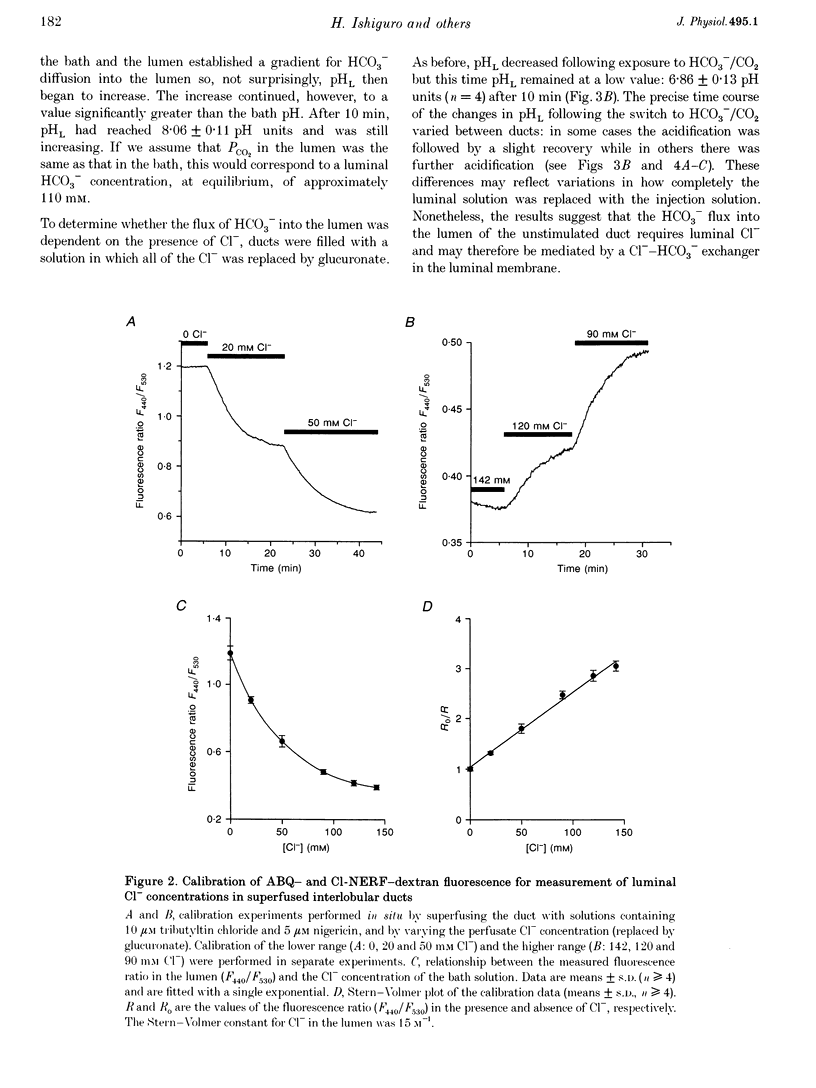
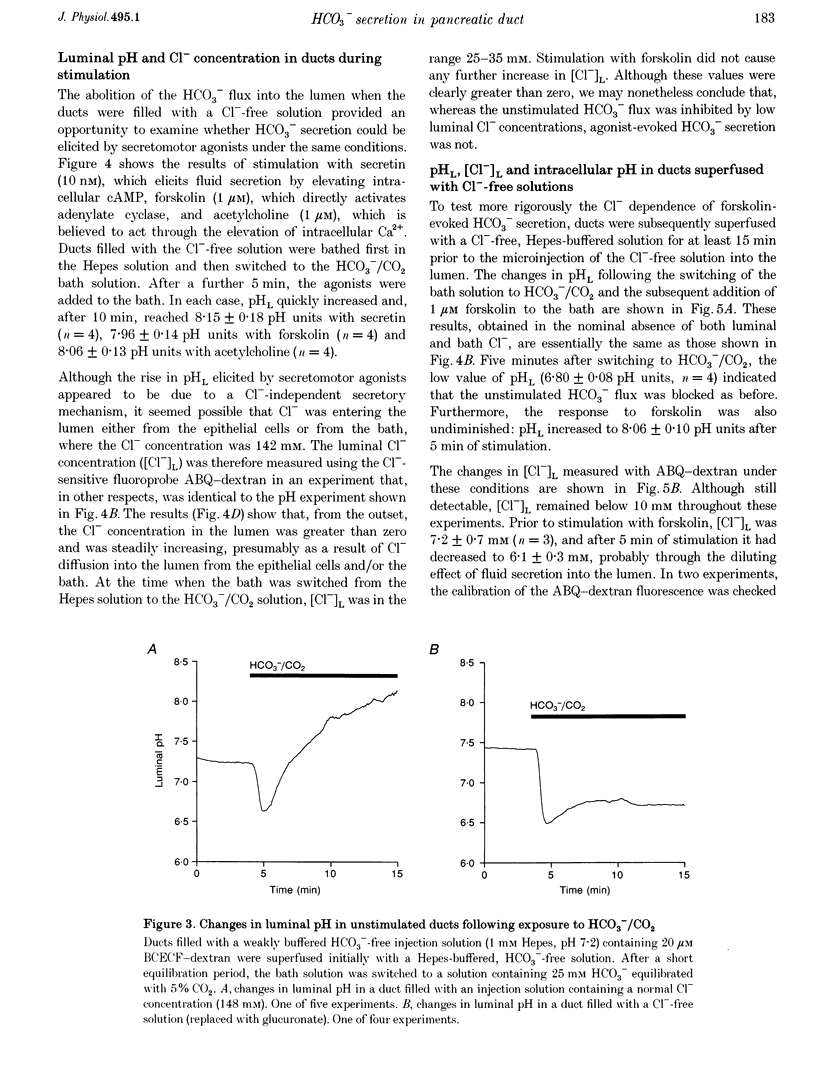
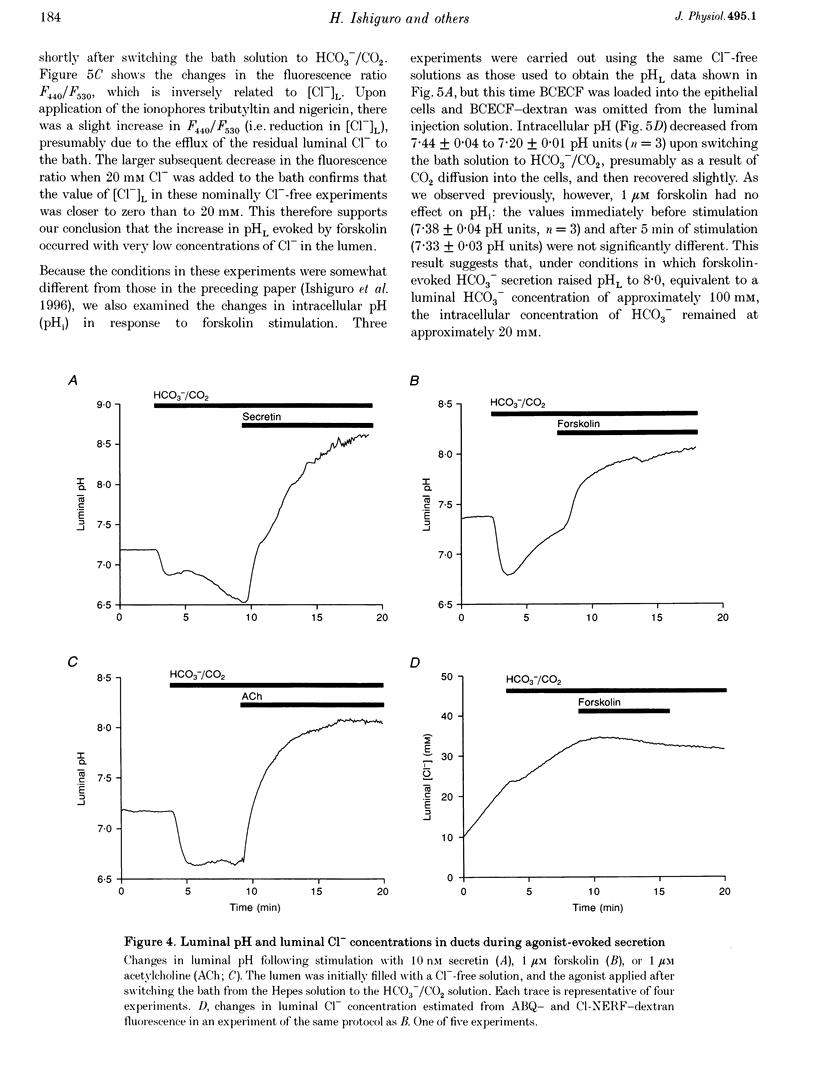
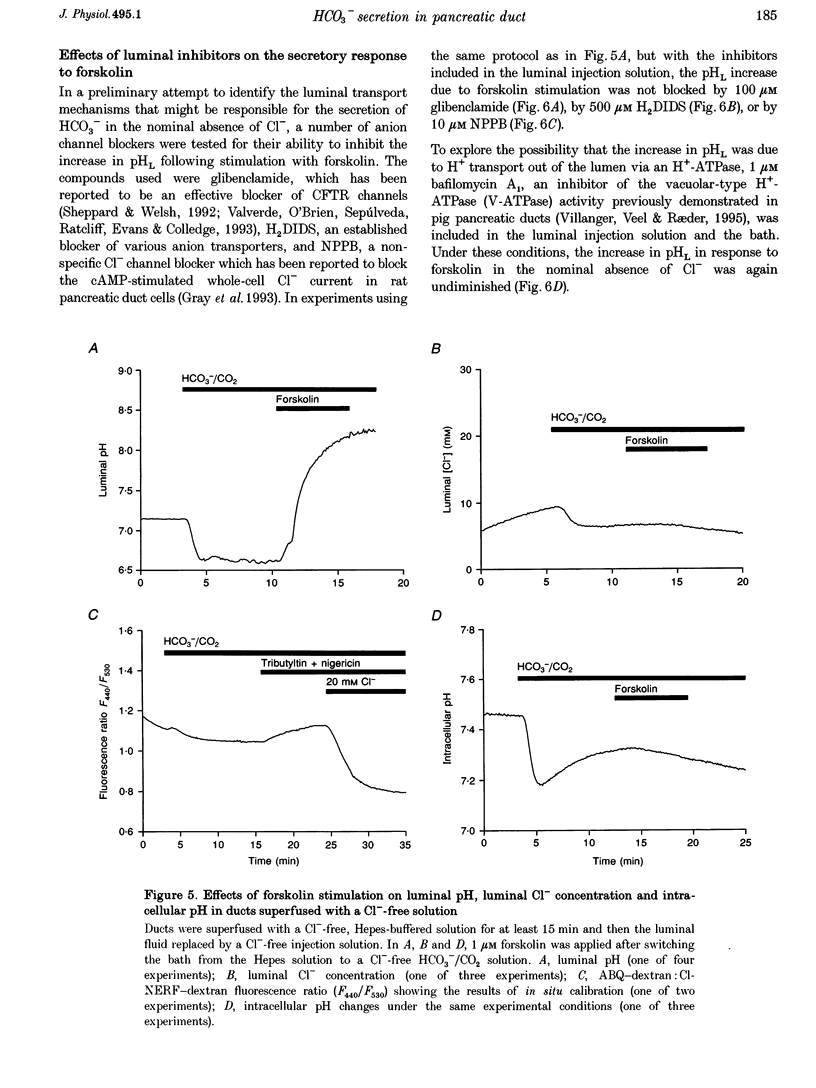
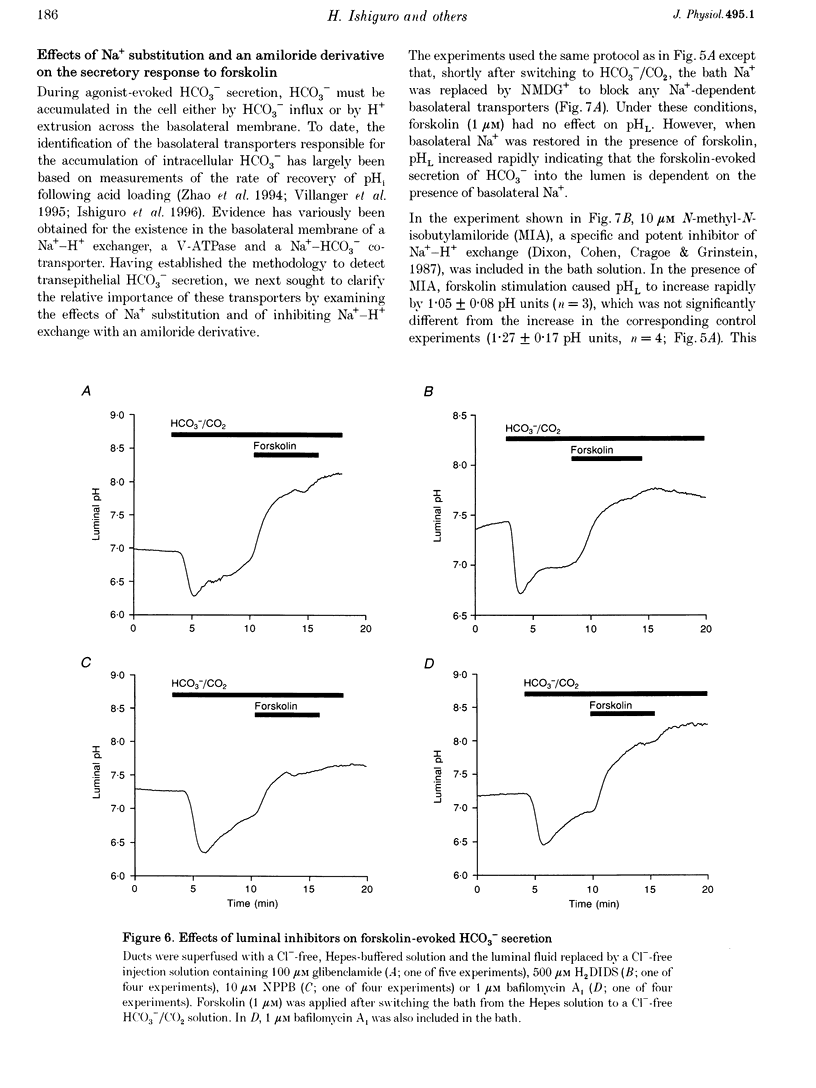
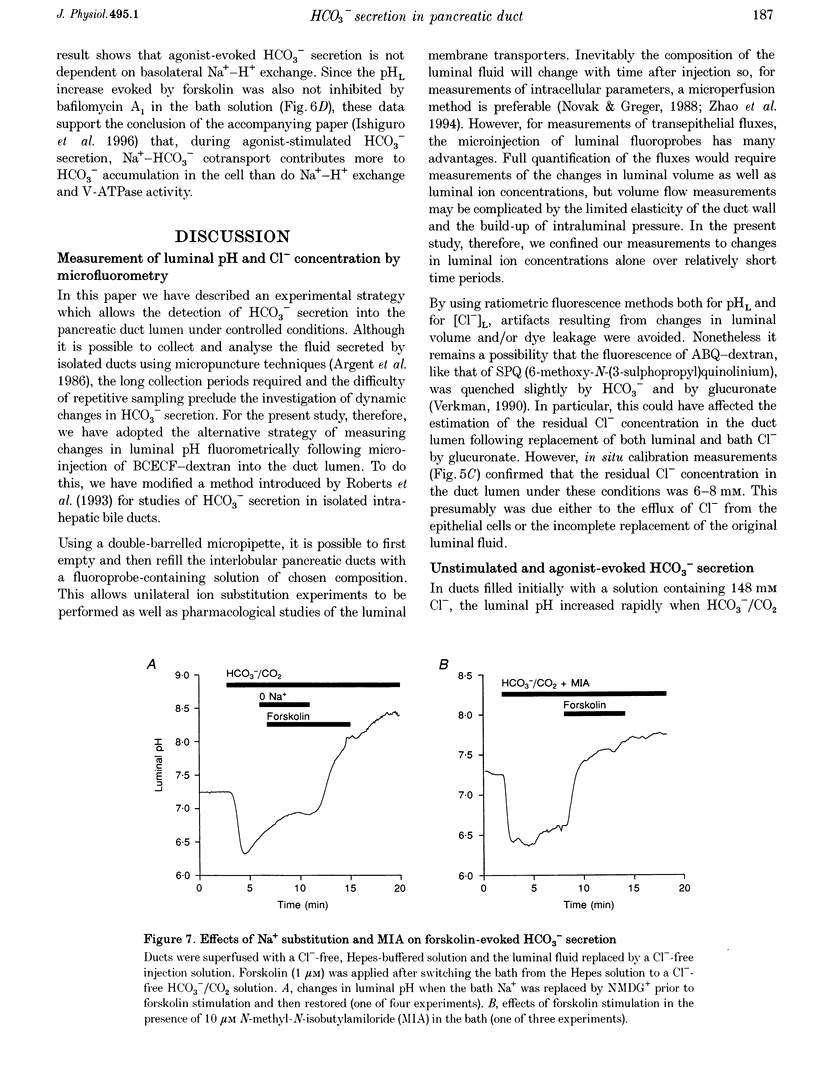
1. The transport of HCO3- across the luminal membrane of pancreatic duct cells was studied by monitoring the luminal pH of isolated guinea-pig interlobular ducts after microinjection of an extracellular fluoroprobe, the dextran conjugate of 2'7'-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF-dextran). Luminal Cl- concentration was also measured by microfluorometry following microinjection of the dextran conjugates of 6-methoxy-N-(4-aminoalkyl)quinolinium bromide (ABQ-dextran) and Cl-NERF (Cl-NERF-dextran). 2. When HCO3-/CO2 was admitted to the bath, a transient acidification of the duct lumen was observed, followed by a marked alkalinization. The latter was abolished when the luminal Cl- concentration was reduced to 25-35 mM by replacement with glucuronate and may, therefore, be attributed to Cl(-)-HCO3- exchange at the luminal membrane. 3. Secretin, forskolin and acetylcholine stimulated HCO3- secretion into the lumen even when the luminal Cl- concentration was reduced to approximately 7 mM. Furthermore, agonist-evoked HCO3- secretion was not inhibited by luminal glibenclamide, dihydro-4,4'-diisothiocyanostilbene-2,2'-disulphonic acid (H2DIDS) or 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB). These observations are not easily reconciled with HCO3- transport across the luminal membrane being mediated by Cl(-)-HCO3- exchange in parallel with a Cl- conductance. 4. Agonist-stimulated HCO3- secretion was blocked by omitting Na+ from the bath but not by addition of N-methyl-N-isobutylamiloride (MIA) or bafilomycin A1. This supports our previous conclusion that HCO3- entry into duct cells from the extracellular fluid requires Na+ but is not dependent on Na(+)-H+ exchange or vacuolar-type H(+)-ATPase activity. 5. The three actions of secretin on guinea-pig pancreatic duct cells described in this and the accompanying paper - stimulation of a relatively Cl(-)-insensitive luminal HCO3- efflux pathway, stimulation of basolateral Na(+)-HCO3- cotransport, and lack of effect on intracellular pH- require the current model of pancreatic HCO3- secretion to be modified.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammar E. M., Hutson D., Scratcherd T. Absence of a relationship between arterial pH and pancreatic bicarbonate secretion in the isolated perfused cat pancreas. J Physiol. 1987 Jul;388:495–504. doi: 10.1113/jphysiol.1987.sp016627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argent B. E., Arkle S., Cullen M. J., Green R. Morphological, biochemical and secretory studies on rat pancreatic ducts maintained in tissue culture. Q J Exp Physiol. 1986 Oct;71(4):633–648. doi: 10.1113/expphysiol.1986.sp003023. [DOI] [PubMed] [Google Scholar]
- Ashton N., Argent B. E., Green R. Characteristics of fluid secretion from isolated rat pancreatic ducts stimulated with secretin and bombesin. J Physiol. 1991 Apr;435:533–546. doi: 10.1113/jphysiol.1991.sp018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton N., Evans R. L., Elliott A. C., Green R., Argent B. E. Regulation of fluid secretion and intracellular messengers in isolated rat pancreatic ducts by acetylcholine. J Physiol. 1993 Nov;471:549–562. doi: 10.1113/jphysiol.1993.sp019915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. D., Elliott A. C., Lau K. R. Indirect evidence for the presence of non-specific anion channels in rabbit mandibular salivary gland acinar cells. J Physiol. 1989 Jul;414:415–431. doi: 10.1113/jphysiol.1989.sp017696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Hotz J., Hutson D., Scratcherd T., Wynne R. D. Electrolyte secretion by the isolated cat pancreas during replacement of extracellular bicarbonate by organic anions and chloride by inorganic anions. J Physiol. 1979 Jan;286:563–576. doi: 10.1113/jphysiol.1979.sp012637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Scratcherd T., Wynne R. D. The origin and secretion of pancreatic juice bicarbonate. J Physiol. 1970 Sep;210(1):1–15. doi: 10.1113/jphysiol.1970.sp009193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinudom A., Komwatana P., Young J. A., Cook D. I. Control of the amiloride-sensitive Na+ current in mouse salivary ducts by intracellular anions is mediated by a G protein. J Physiol. 1995 Sep 15;487(Pt 3):549–555. doi: 10.1113/jphysiol.1995.sp020899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon S. J., Cohen S., Cragoe E. J., Jr, Grinstein S. Estimation of the number and turnover rate of Na+/H+ exchangers in lymphocytes. Effect of phorbol ester and osmotic shrinking. J Biol Chem. 1987 Mar 15;262(8):3626–3632. [PubMed] [Google Scholar]
- Gray M. A., Greenwell J. R., Argent B. E. Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J Membr Biol. 1988 Oct;105(2):131–142. doi: 10.1007/BF02009166. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Plant S., Argent B. E. cAMP-regulated whole cell chloride currents in pancreatic duct cells. Am J Physiol. 1993 Mar;264(3 Pt 1):C591–C602. doi: 10.1152/ajpcell.1993.264.3.C591. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Pollard C. E., Harris A., Coleman L., Greenwell J. R., Argent B. E. Anion selectivity and block of the small-conductance chloride channel on pancreatic duct cells. Am J Physiol. 1990 Nov;259(5 Pt 1):C752–C761. doi: 10.1152/ajpcell.1990.259.5.C752. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Winpenny J. P., Porteous D. J., Dorin J. R., Argent B. E. CFTR and calcium-activated chloride currents in pancreatic duct cells of a transgenic CF mouse. Am J Physiol. 1994 Jan;266(1 Pt 1):C213–C221. doi: 10.1152/ajpcell.1994.266.1.C213. [DOI] [PubMed] [Google Scholar]
- Ishiguro H., Steward M. C., Lindsay A. R., Case R. M. Accumulation of intracellular HCO3- by Na(+)-HCO3- cotransport in interlobular ducts from guinea-pig pancreas. J Physiol. 1996 Aug 15;495(Pt 1):169–178. doi: 10.1113/jphysiol.1996.sp021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers G. A., Van Nooy I. G., De Pont J. J., Bonting S. L. Anion secretion by the isolated rabbit pancreas. Biochim Biophys Acta. 1984 Jul 25;774(2):269–276. doi: 10.1016/0005-2736(84)90301-8. [DOI] [PubMed] [Google Scholar]
- Kuijpers G. A., Van Nooy I. G., De Pont J. J., Bonting S. L. The mechanism of fluid secretion in the rabbit pancreas studied by means of various inhibitors. Biochim Biophys Acta. 1984 Dec 5;778(2):324–331. doi: 10.1016/0005-2736(84)90376-6. [DOI] [PubMed] [Google Scholar]
- Lee S. I., Turner R. J. Secretagogue-induced 86Rb+ efflux from bovine parotid is HCO3- dependent. Am J Physiol. 1993 Jan;264(1 Pt 2):R162–R168. doi: 10.1152/ajpregu.1993.264.1.R162. [DOI] [PubMed] [Google Scholar]
- Mahieu I., Becq F., Wolfensberger T., Gola M., Carter N., Hollande E. The expression of carbonic anhydrases II and IV in the human pancreatic cancer cell line (Capan 1) is associated with bicarbonate ion channels. Biol Cell. 1994;81(2):131–141. doi: 10.1016/s0248-4900(94)80004-9. [DOI] [PubMed] [Google Scholar]
- Novak I., Greger R. Properties of the luminal membrane of isolated perfused rat pancreatic ducts. Effect of cyclic AMP and blockers of chloride transport. Pflugers Arch. 1988 May;411(5):546–553. doi: 10.1007/BF00582376. [DOI] [PubMed] [Google Scholar]
- Novak I., Pahl C. Effect of secretin and inhibitors of HCO3-/H+ transport on the membrane voltage of rat pancreatic duct cells. Pflugers Arch. 1993 Nov;425(3-4):272–279. doi: 10.1007/BF00374178. [DOI] [PubMed] [Google Scholar]
- Padfield P. J., Garner A., Case R. M. Patterns of pancreatic secretion in the anaesthetised guinea pig following stimulation with secretin, cholecystokinin octapeptide, or bombesin. Pancreas. 1989;4(2):204–209. doi: 10.1097/00006676-198904000-00009. [DOI] [PubMed] [Google Scholar]
- Pahl C., Novak I. Effect of vasoactive intestinal peptide, carbachol and other agonists on the membrane voltage of pancreatic duct cells. Pflugers Arch. 1993 Aug;424(3-4):315–320. doi: 10.1007/BF00384358. [DOI] [PubMed] [Google Scholar]
- Poronnik P., Schumann S. Y., Cook D. I. HCO3(-)-dependent ACh-activated Na+ influx in sheep parotid secretory endpieces. Pflugers Arch. 1995 Apr;429(6):852–858. doi: 10.1007/BF00374810. [DOI] [PubMed] [Google Scholar]
- Poulsen J. H., Fischer H., Illek B., Machen T. E. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHMAN S. S., BROOKS F. P. ELECTROLYTE SECRETION FROM RABBIT PANCREAS IN VITRO. Am J Physiol. 1965 Jun;208:1171–1176. doi: 10.1152/ajplegacy.1965.208.6.1171. [DOI] [PubMed] [Google Scholar]
- Roberts S. K., Kuntz S. M., Gores G. J., LaRusso N. F. Regulation of bicarbonate-dependent ductular bile secretion assessed by lumenal micropuncture of isolated rodent intrahepatic bile ducts. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9080–9084. doi: 10.1073/pnas.90.19.9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. A., Foskett J. K. Na+ transport pathways in secretory acinar cells: membrane cross talk mediated by [Cl-]i. Am J Physiol. 1994 Jul;267(1 Pt 1):C146–C156. doi: 10.1152/ajpcell.1994.267.1.C146. [DOI] [PubMed] [Google Scholar]
- Sewell W. A., Young J. A. Secretion of electrolytes by the pancreas of the anaestetized rat. J Physiol. 1975 Nov;252(2):379–396. doi: 10.1113/jphysiol.1975.sp011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D. N., Welsh M. J. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol. 1992 Oct;100(4):573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescent indicators of ion concentrations. Methods Cell Biol. 1989;30:127–156. doi: 10.1016/s0091-679x(08)60978-4. [DOI] [PubMed] [Google Scholar]
- Valverde M. A., O'Brien J. A., Sepúlveda F. V., Ratcliff R., Evans M. J., Colledge W. H. Inactivation of the murine cftr gene abolishes cAMP-mediated but not Ca(2+)-mediated secretagogue-induced volume decrease in small-intestinal crypts. Pflugers Arch. 1993 Dec;425(5-6):434–438. doi: 10.1007/BF00374869. [DOI] [PubMed] [Google Scholar]
- Verkman A. S. Development and biological applications of chloride-sensitive fluorescent indicators. Am J Physiol. 1990 Sep;259(3 Pt 1):C375–C388. doi: 10.1152/ajpcell.1990.259.3.C375. [DOI] [PubMed] [Google Scholar]
- Villanger O., Veel T., Raeder M. G. Secretin causes H+/HCO3- secretion from pig pancreatic ductules by vacuolar-type H(+)-adenosine triphosphatase. Gastroenterology. 1995 Mar;108(3):850–859. doi: 10.1016/0016-5085(95)90460-3. [DOI] [PubMed] [Google Scholar]
- Widdas W. F., Baker G. F. The triphasic volume response of human red cells in low ionic strength media: demonstration of a special bicarbonate transport. Cytobios. 1995;81(326):135–158. [PubMed] [Google Scholar]
- Xia P., Persson B. E., Spring K. R. The chloride concentration in the lateral intercellular spaces of MDCK cell monolayers. J Membr Biol. 1995 Mar;144(1):21–30. doi: 10.1007/BF00238413. [DOI] [PubMed] [Google Scholar]
- Zhao H., Star R. A., Muallem S. Membrane localization of H+ and HCO3- transporters in the rat pancreatic duct. J Gen Physiol. 1994 Jul;104(1):57–85. doi: 10.1085/jgp.104.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]


