Abstract
1. We sought to determine whether hypoxic stimulation of neurons of the rostral ventrolateral reticular nucleus (RVL) would elevate regional cerebral blood flow (rCBF) in anaesthetized paralysed rats. 2. Microinjection of sodium cyanide (NaCN; 150-450 pmol) into the RVL rapidly (within 1-2 s), transiently, dose-dependently and site-specifically elevated rCBF1 measured by laser Doppler flowmetry, by 61.3 +/- 22.1% (P < 0.01), increased arterial pressure (AP; +30 +/- 8 mmHg; P < 0.01)1 and triggered a synchronized 6 Hz rhythm of EEG activity. 3. Following cervical spinal cord transection, NaCN and also dinitrophenol (DNP) significantly (P < 0.05) elevated rCBF and synchronized the EEG but did not elevate AP; the response to NaCN was attenuated by hyperoxia and deepening of anaesthesia. 4. Electrical stimulation of NaCN-sensitive sites in the RVL in spinalized rats increased rCBF measured autoradiographically with 14C iodoantipyrine (Kety method) in the mid-line thalamus (by 182.3 +/- 17.2%; P < 0.05) and cerebral cortex (by 172.6 +/- 15.6%; P < 0.05) regions, respectively, directly or indirectly innervated by RVL neurons, and in the remainder of the brain. In contrast regional cerebral glucose utilization (rCGU), measured autoradiographically with 14C-2-deoxyglucose (Sokoloff method), was increased in proportion to rCBF in the mid-line thalamus (165.6 +/- 17.8%, P < 0.05) but was unchanged in the cortex. 5. Bilateral electrolytic lesions of NaCN sensitive sites of RVL, while not altering resting rCBF or the elevation elicited by hypercarbia (arterial CO2 pressure, Pa,CO2, approximately 69 mmHg), reduced the vasodilatation elicited by normocapnic hypoxaemia (arterial O2 pressure, Pa,O2, approximately 27 mmHg) by 67% (P < 0.01) and flattened the slope of the Pa,O2-rCBF response curve. 6. We conclude that the elevation of rCBF produced in the cerebral cortex by hypoxaemia is in large measure neurogenic, mediated trans-synaptically over intrinsic neuronal pathways, and initiated by excitation of oxygen sensitive neurons in the RVL.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benarroch E. E., Granata A. R., Ruggiero D. A., Park D. H., Reis D. J. Neurons of C1 area mediate cardiovascular responses initiated from ventral medullary surface. Am J Physiol. 1986 May;250(5 Pt 2):R932–R945. doi: 10.1152/ajpregu.1986.250.5.R932. [DOI] [PubMed] [Google Scholar]
- Donegan J. H., Traystman R. J., Koehler R. C., Jones M. D., Jr, Rogers M. C. Cerebrovascular hypoxic and autoregulatory responses during reduced brain metabolism. Am J Physiol. 1985 Aug;249(2 Pt 2):H421–H429. doi: 10.1152/ajpheart.1985.249.2.H421. [DOI] [PubMed] [Google Scholar]
- Dóra E., Koller A., Kovách A. G. Effect of topical adenosine deaminase treatment on the functional hyperemic and hypoxic responses of cerebrocortical microcirculation. J Cereb Blood Flow Metab. 1984 Sep;4(3):447–457. doi: 10.1038/jcbfm.1984.64. [DOI] [PubMed] [Google Scholar]
- Goadsby P. J., Shelley S. High-frequency stimulation of the facial nerve results in local cortical release of vasoactive intestinal polypeptide in the anesthetised cat. Neurosci Lett. 1990 May 4;112(2-3):282–289. doi: 10.1016/0304-3940(90)90217-w. [DOI] [PubMed] [Google Scholar]
- Golanov E. V., Reis D. J. Nitric oxide and prostanoids participate in cerebral vasodilation elicited by electrical stimulation of the rostral ventrolateral medulla. J Cereb Blood Flow Metab. 1994 May;14(3):492–502. doi: 10.1038/jcbfm.1994.61. [DOI] [PubMed] [Google Scholar]
- Golanov E. V., Reis D. J. Vasodilation evoked from medulla and cerebellum is coupled to bursts of cortical EEG activity in rats. Am J Physiol. 1995 Feb;268(2 Pt 2):R454–R467. doi: 10.1152/ajpregu.1995.268.2.R454. [DOI] [PubMed] [Google Scholar]
- Golanov E. V., Yamamoto S., Reis D. J. Spontaneous waves of cerebral blood flow associated with a pattern of electrocortical activity. Am J Physiol. 1994 Jan;266(1 Pt 2):R204–R214. doi: 10.1152/ajpregu.1994.266.1.R204. [DOI] [PubMed] [Google Scholar]
- Gonzalez C., Almaraz L., Obeso A., Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994 Oct;74(4):829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Grote J., Siegel G., Zimmer K., Adler A. The influence of oxygen tension on membrane potential and tone of canine carotid artery smooth muscle. Adv Exp Med Biol. 1988;222:481–487. doi: 10.1007/978-1-4615-9510-6_57. [DOI] [PubMed] [Google Scholar]
- Haselton J. R., Guyenet P. G. Ascending collaterals of medullary barosensitive neurons and C1 cells in rats. Am J Physiol. 1990 Apr;258(4 Pt 2):R1051–R1063. doi: 10.1152/ajpregu.1990.258.4.R1051. [DOI] [PubMed] [Google Scholar]
- Iadecola C., Pelligrino D. A., Moskowitz M. A., Lassen N. A. Nitric oxide synthase inhibition and cerebrovascular regulation. J Cereb Blood Flow Metab. 1994 Mar;14(2):175–192. doi: 10.1038/jcbfm.1994.25. [DOI] [PubMed] [Google Scholar]
- Kass I. S., Lipton P. Protection of hippocampal slices from young rats against anoxic transmission damage is due to better maintenance of ATP. J Physiol. 1989 Jun;413:1–11. doi: 10.1113/jphysiol.1989.sp017638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada M., Dampney R. A., Reis D. J. Profound hypotension and abolition of the vasomotor component of the cerebral ischemic response produced by restricted lesions of medulla oblongata in rabbit. Relationship to the so-called tonic vasomotor center. Circ Res. 1979 Jul;45(1):63–70. doi: 10.1161/01.res.45.1.63. [DOI] [PubMed] [Google Scholar]
- Meno J. R., Ngai A. C., Ibayashi S., Winn H. R. Adenosine release and changes in pial arteriolar diameter during transient cerebral ischemia and reperfusion. J Cereb Blood Flow Metab. 1991 Nov;11(6):986–993. doi: 10.1038/jcbfm.1991.165. [DOI] [PubMed] [Google Scholar]
- Nakai M., Tamaki K., Ogata J., Matsui Y., Maeda M. Parasympathetic cerebrovasodilator center of the facial nerve. Circ Res. 1993 Feb;72(2):470–475. doi: 10.1161/01.res.72.2.470. [DOI] [PubMed] [Google Scholar]
- Nehlig A., Vergnes M., Marescaux C., Boyet S., Lannes B. Local cerebral glucose utilization in rats with petit mal-like seizures. Ann Neurol. 1991 Jan;29(1):72–77. doi: 10.1002/ana.410290113. [DOI] [PubMed] [Google Scholar]
- Nolan P. C., Waldrop T. G. In vivo and in vitro responses of neurons in the ventrolateral medulla to hypoxia. Brain Res. 1993 Dec 10;630(1-2):101–114. doi: 10.1016/0006-8993(93)90648-7. [DOI] [PubMed] [Google Scholar]
- Pelligrino D. A., Koenig H. M., Albrecht R. F. Nitric oxide synthesis and regional cerebral blood flow responses to hypercapnia and hypoxia in the rat. J Cereb Blood Flow Metab. 1993 Jan;13(1):80–87. doi: 10.1038/jcbfm.1993.10. [DOI] [PubMed] [Google Scholar]
- Richerson G. B. Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol. 1995 Mar;73(3):933–944. doi: 10.1152/jn.1995.73.3.933. [DOI] [PubMed] [Google Scholar]
- Ruggiero D. A., Cravo S. L., Golanov E., Gomez R., Anwar M., Reis D. J. Adrenergic and non-adrenergic spinal projections of a cardiovascular-active pressor area of medulla oblongata: quantitative topographic analysis. Brain Res. 1994 Nov 7;663(1):107–120. doi: 10.1016/0006-8993(94)90468-5. [DOI] [PubMed] [Google Scholar]
- Saeki Y., Sato A., Sato Y., Trzebski A. Stimulation of the rostral ventrolateral medullary neurons increases cortical cerebral blood flow via activation of the intracerebral neural pathway. Neurosci Lett. 1989 Dec 15;107(1-3):26–32. doi: 10.1016/0304-3940(89)90785-4. [DOI] [PubMed] [Google Scholar]
- Sato A., Trzebski A., Zhou W. Local cerebral blood flow responses in rats to hypercapnia and hypoxia in the rostral ventrolateral medulla and in the cortex. J Auton Nerv Syst. 1992 Nov;41(1-2):79–86. doi: 10.1016/0165-1838(92)90129-5. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Steriade M., Amzica F. Dynamic coupling among neocortical neurons during evoked and spontaneous spike-wave seizure activity. J Neurophysiol. 1994 Nov;72(5):2051–2069. doi: 10.1152/jn.1994.72.5.2051. [DOI] [PubMed] [Google Scholar]
- Steriade M., Gloor P., Llinás R. R., Lopes de Silva F. H., Mesulam M. M. Report of IFCN Committee on Basic Mechanisms. Basic mechanisms of cerebral rhythmic activities. Electroencephalogr Clin Neurophysiol. 1990 Dec;76(6):481–508. doi: 10.1016/0013-4694(90)90001-z. [DOI] [PubMed] [Google Scholar]
- Sun M. K., Jeske I. T., Reis D. J. Cyanide excites medullary sympathoexcitatory neurons in rats. Am J Physiol. 1992 Feb;262(2 Pt 2):R182–R189. doi: 10.1152/ajpregu.1992.262.2.R182. [DOI] [PubMed] [Google Scholar]
- Sun M. K., Reis D. J. Central neural mechanisms mediating excitation of sympathetic neurons by hypoxia. Prog Neurobiol. 1994 Oct;44(2):197–219. doi: 10.1016/0301-0082(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Taguchi H., Heistad D. D., Kitazono T., Faraci F. M. ATP-sensitive K+ channels mediate dilatation of cerebral arterioles during hypoxia. Circ Res. 1994 May;74(5):1005–1008. doi: 10.1161/01.res.74.5.1005. [DOI] [PubMed] [Google Scholar]
- Tucker D. C., Saper C. B., Ruggiero D. A., Reis D. J. Organization of central adrenergic pathways: I. Relationships of ventrolateral medullary projections to the hypothalamus and spinal cord. J Comp Neurol. 1987 May 22;259(4):591–603. doi: 10.1002/cne.902590408. [DOI] [PubMed] [Google Scholar]
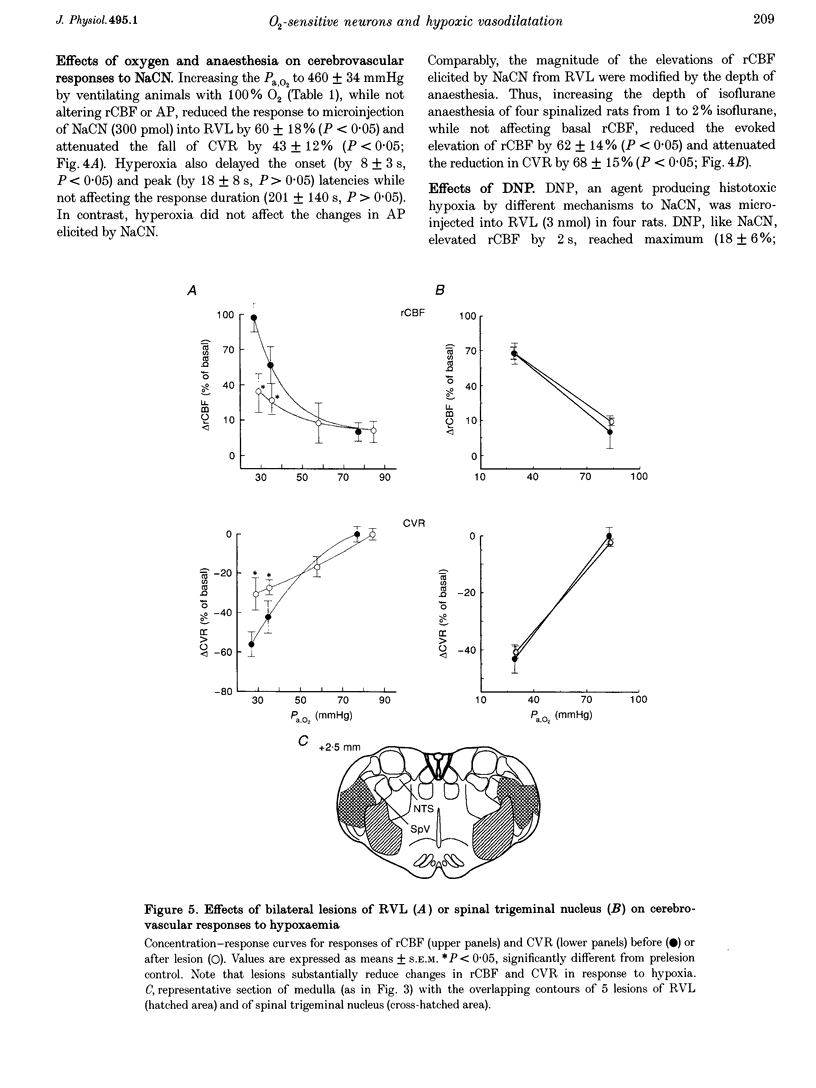
- Underwood M. D., Iadecola C., Reis D. J. Lesions of the rostral ventrolateral medulla reduce the cerebrovascular response to hypoxia. Brain Res. 1994 Jan 28;635(1-2):217–223. doi: 10.1016/0006-8993(94)91442-7. [DOI] [PubMed] [Google Scholar]
- Underwood M. D., Iadecola C., Sved A., Reis D. J. Stimulation of Cl area neurons globally increases regional cerebral blood flow but not metabolism. J Cereb Blood Flow Metab. 1992 Sep;12(5):844–855. doi: 10.1038/jcbfm.1992.116. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Golanov E. V., Reis D. J. Reductions in focal ischemic infarctions elicited from cerebellar fastigial nucleus do not result from elevations in cerebral blood flow. J Cereb Blood Flow Metab. 1993 Nov;13(6):1020–1024. doi: 10.1038/jcbfm.1993.128. [DOI] [PubMed] [Google Scholar]
- Zagon A., Totterdell S., Jones R. S. Direct projections from the ventrolateral medulla oblongata to the limbic forebrain: anterograde and retrograde tract-tracing studies in the rat. J Comp Neurol. 1994 Feb 22;340(4):445–468. doi: 10.1002/cne.903400402. [DOI] [PubMed] [Google Scholar]